Abstract
Background
Large cerebral infarction has a high case fatality. Despite the use of conventional medical treatments such as hyperventilation, mannitol, diuretics, corticosteroids and barbiturates, the outcome of this condition remains poor. Decompressive surgery to relieve intracranial pressure is performed in some cases, although evidence of any clinical benefits has not been available until recently. This is an update of a Cochrane review first published in 2002.
Objectives
To examine the effects of decompressive surgery in patients with massive acute ischaemic stroke complicated with cerebral oedema, and to judge whether decompressive surgery is effective in improving survival or survival free of severe disability.
Search methods
We searched the Cochrane Stroke Group's Trials Register (last searched October 2010), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 7), MEDLINE (1966 to October 2010), EMBASE (1980 to October 2010) and Science Citation Index (October 2010). We also searched the reference lists of all relevant articles.
Selection criteria
Randomised controlled studies of decompressive surgery plus medical treatment versus medical treatment alone in patients with clinically and radiologically confirmed cerebral infarcts complicated with cerebral oedema.
Data collection and analysis
One author assessed the titles and retrieved the relevant studies. The same author extracted data, with discussion among all authors for clarification. Outcomes were death at the end of follow‐up, death or disability defined as the modified Rankin Scale (mRS) > 3 at the end of follow‐up, death or severe disability defined as mRS > 4 at 12 months and disability defined as mRS 4 or 5 at 12 months. The results are given using the Peto odds ratio (Peto OR) with 95% confidence intervals (CIs).
Main results
We included three trials in this review, involving 134 patients who were 60 years of age or younger. The time window for the intervention was 30 hours from stroke onset in two studies and 96 hours in one study. All trials were stopped early. Surgical decompression reduced the risk of death at the end of follow‐up (OR 0.19, 95% CI 0.09 to 0.37) and the risk of death or disability defined as mRS > 4 at 12 months (OR 0.26, 95% CI 0.13 to 0.51). Death or disability defined as mRS > 3 at the end of follow‐up was no different between the treatment arms (OR 0.56, 95% CI 0.27 to 1.15).
Authors' conclusions
Surgical decompression lowers the risk of death and death or severe disability defined as mRS > 4 in selected patients 60 years of age or younger with a massive hemispheric infarction and oedema. Optimum criteria for patient selection and for timing of decompressive surgery are yet to be defined. Since survival may be at the expense of substantial disability, surgery should be the treatment of choice only when it can be assumed, based on their preferences, that it is in the best interest of patients. Since all the trials were stopped early, an overestimation of the effect size cannot be excluded.
Keywords: Humans; Middle Aged; Decompression, Surgical; Brain Edema; Brain Edema/surgery; Cerebral Infarction; Cerebral Infarction/complications; Prognosis; Randomized Controlled Trials as Topic; Time Factors
Plain language summary
Surgical decompression for cerebral oedema in acute ischaemic stroke
About four‐fifths of strokes are due to blockage of an artery in the brain. When the artery is blocked, part of the brain is damaged, this is called a cerebral infarct. If a large artery is blocked the area of brain damage can be large. About 24 to 48 hours after a large infarct the brain can swell, causing a dangerous rise of pressure inside the head. Surgery to remove some of the skull bone over the swollen area of brain reduces the pressure. Results from recent clinical trials showed that surgery reduced the risk of death. However, survivors were left with moderate to severe disability requiring help in their daily life activities. These results only apply to people 60 years of age or younger.
Background
Patients with a large cerebral infarction generally have a poor prognosis. Approximately 40% of patients with total anterior cerebral infarction (TACI) syndrome deteriorate during the first week, and half of them die during the first month (Bamford 1991; Tei 2000). Poor outcome is mostly explained by the volume of cerebral tissue that is damaged. Early deterioration and death is often the result of oedema in the infarcted tissue (Hacke 1996; King 1951; Ng 1970; Ropper 1984; Saito 1987). Oedema causes mass‐effect with distortion, tissue shift and increased intracranial pressure (Frank 1995; Schwab 1996). Such changes lead to cerebral herniation, further brain damage and death.
Conventional medical treatment aims at reducing oedema and intracranial pressure in stroke patients using hyperventilation, mannitol, diuretics, corticosteroids or barbiturates (Manno 1999; Schwab 1997; Wijdicks 2000). However, once brain swelling produces clinical signs and imaging features of mass effect with tissue shift, case‐fatality becomes higher, despite intensive medical treatment (Hacke 1996; Saito 1987).
Surgical decompression seeks to create space to accommodate the increased volume created by the swollen brain (Cristofori 1998; Ivamoto 1974; Jourdan 1993; Kakita 1976; Kalia 1993; Koh 2000; Kondziolka 1988; Kristensen 1998; Manai 2000; Mracek 1978; Oro 2000; Sakai 1998; Sollid 1999; Ueno 1984; Van Leusen 2001; Young 1982). This can be accomplished by opening the cranial vault and dura (Carter 1997; Delashaw 1990; Ivamoto 1974; Lindegaard 1999; Rengachary 1981; Rieke 1995; Schwab 1998; Wijdicks 2000), or by removing non‐viable or non‐essential brain tissue (Fujita 1982; Martins 1993; Mori 1998; Tsuruno 1993). These strategies are described as 'external' or 'internal' decompression respectively. External decompression involves hemicraniectomy with or without duroplasty. Internal decompression involves the removal of brain tissue, either the infarcted region or non‐eloquent regions of the brain. These techniques are sometimes combined. Despite the possible benefit, surgical therapy involves risks which may include secondary cerebral haemorrhage and brain herniation through the craniectomy defect (Wagner 2001).
Objectives
The aim of this review was to examine the effects of decompressive surgery in patients with massive acute ischaemic stroke complicated with cerebral oedema, and to judge whether decompressive surgery is effective in improving survival or survival free of severe disability.
Methods
Criteria for considering studies for this review
Types of studies
We sought randomised controlled studies comparing decompressive surgery and medical treatment with medical treatment alone as control. To avoid confounding factors, comparison groups had to be comparable for at least the major prognostic factors: age, stroke location, time to admission, time to randomisation, time from stroke onset to clinical appearance of herniation and sex.
Types of participants
We sought trials involving patients with massive acute ischaemic stroke and complicating brain oedema, evident on cerebral computed tomography or magnetic resonance imaging.
Types of interventions
We sought trials of any kind of surgical decompression (external or internal). Surgical decompression refers to hemicraniectomy with duroplasty or resection of neural tissue, or both. We sought studies with an unconfounded comparison of surgery versus no surgery. Such studies should involve 'best' medical treatment (mannitol, other diuretics, corticosteroids, hyperventilation, barbiturates, etc) and nursing care in both the surgical and non‐surgical arms. If the protocol for medical therapy and nursing (including monitoring) was different between arms, then the study was biased and poorly controlled. We examined studies for such discrepancies.
Types of outcome measures
Primary outcomes
Death at the end of follow‐up.
Secondary outcomes
Death or disability defined as a modified Rankin Scale (mRS) > 3.
Death or severe disability defined as mRS > 4.
Disability defined as mRS of 4 or 5 at the end of follow‐up.
Disability was defined as a Glasgow Outcome Scale (GOS) score of 2 or 3 (Jennett 1975), a mRS score of 3, 4 or 5 (Rankin 1957) or a Barthel Index (BI) < 60 (Mahoney 1965). Where no valid scale was given, we interpreted the text description of dependence seeking further detail from authors when necessary. The GOS is often misquoted, with the scale inverted, and we took care to interpret data given in this form.
We evaluated the studies for the presence of observer bias when patients were assessed following treatment. Ideally, outcome or endpoint assessment should be blinded to the type of intervention participants were assigned to. The use of special hats to hide the patient's scalp, thereby 'blinding' the observer during assessment of all patients, was proposed by the HEADDFIRST group as a way of avoiding observer bias.
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module.
Electronic searches
We searched the Cochrane Stroke Group's Trials Register, which was last searched by the Managing Editor in October 2010. In addition, we searched the following electronic bibliographic databases: the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2010, Issue 7), MEDLINE (1966 to October 2010) (Appendix 1) and EMBASE (1980 to October 2010) (Appendix 2). We performed cited reference searches in Science Citation Index (October 2010) using three geographically separated, relevant, high‐profile articles as a prospective search. The articles used were Carter 1997, Mori 1998, and Schwab 1998.
Searching other resources
We searched the reference lists of all relevant articles for references to trials. We contacted a selection of specialists, both medical and surgical, enquiring about relevant published, unpublished, pending or recently commenced trials not already identified. They were: Eric Juettler (Heidelberg, Germany), Jeannette Hofmeijer (Utretch, Netherlands) and Roland Jamora (Manila, Philippines).
Data collection and analysis
One review author (SCF) inspected the titles identified by the searching and examined the abstracts of references with titles of interest to determine relevance. When relevance was not clear from the abstract, or when no abstract was available, we obtained a copy of the article. We sought contact with the study author if further clarification was necessary.
We critically appraised any trial deemed relevant, using the checklist developed by Fowkes and Fulton (Fowkes 1991). One review author (SCF) reviewed and considered the limitations of studies with a view to inclusion (see Criteria for considering studies for this review).
One review author (SCF) extracted data independently from the included studies onto a pro‐forma. Primary outcomes of interest were death at the end of follow‐up, death or moderately severe disability defined as mRS > 3 at 6 and 12 months, and death or severe disability defined as mRS > 4 at 12 months. We also analysed the effect of surgical decompression on survival with severe disability defined as mRS 4 and 5. We calculated relative treatment effects using a fixed‐effect model, expressed as Peto ORs with 95% (CIs). We did not perform subgroup analyses.
Results
Description of studies
In the initial review the database searches from 1966 through to 2002 identified over 9000 references which we screened for relevance. For this update we searched MEDLINE and EMBASE from 2002 to October 2010, and after duplicates were removed we identified 5467 new references. A full search of the Cochrane Central Register of Controlled Trials (CENTRAL) identified 103 references and we received data on 10 trials from the Cochrane Stroke Group's Trials Register for assessment. Seven randomised controlled trials (RCTs) were found (DECIMAL 2007; DEMITUR Trial; DESTINY 2007; DESTINY 2; HAMLET 2009; HEADDFIRST; HeMMI 2004). Five were completed but only three were published and met criteria for inclusion in the analysis (DECIMAL 2007; DESTINY 2007; HAMLET 2009) (see Characteristics of included studies), the other two studies are awaiting assessment (DEMITUR Trial; HEADDFIRST ). Two trials are ongoing (DESTINY 2; HeMMI 2004).
Included studies
See Characteristics of included studies.
The three included studies were carried out in Europe, recruiting a combined total of 134 patients, aged 60 years or younger with a large hemispheric ischaemic stroke. Sixty‐nine patients in total were randomised to surgical decompression that was performed within 30 hours from symptom onset in two studies (DECIMAL 2007; DESTINY 2007), and within 96 hours in one study (HAMLET 2009). A study required the presence of an involvement of ≥ 50% of the middle cerebral artery (MCA) distribution and an infarct volume > 145cm3 in addition to a score ≥ 1 in the National Institutes of Health Stroke Scale (NIHSS) for item 1a (level of consciousness) (DECIMAL 2007). DESTINY 2007 required an involvement of two‐thirds of the MCA territory with NIHSS item 1a > 1 (level of consciousness); while HAMLET 2009 required involvement of two‐thirds of MCA territory associated with mass effect and midline shift. All three trials excluded patients with a pre stroke mRS ≥ 2. They shared the primary effect measure of favourable outcome defined as mRS ≤ 3 measured at 6 months in one study (DECIMAL 2007), at 6 and 12 months in the second (DESTINY 2007) and 12 months in the third study (HAMLET 2009). All three studies were stopped prematurely: two considering that data would be pooled with the other trials (DECIMAL 2007; DESTINY 2007), and one because the data safety committee established that no statistically significant difference would be found considering the sample size (HAMLET 2009).
Excluded studies
There are no excluded studies.
Completed clinical trials with data not yet available
Risk of bias in included studies
DECIMAL 2007: the randomisation method was unclear, it did not have concealment of allocation but blinded outcome assessment by covering patients' heads with a hat.
DESTINY 2007: the study did not have concealment of allocation nor blinded outcome assessment.
HAMLET 2009: the randomisation method was not clear, there was no concealment of allocation, a local investigator provided a narrative of function, and a panel of three blinded investigators adjudicated outcome based on the narrative provided.
Effects of interventions
The three studies with available data for analysis included a total of 134 patients. Surgical decompression reduced the risk of death at end of follow‐up (OR 0.19, 95% CI 0.09 to 0.37) (Analysis 1.1). Death or disability defined as mRS > 3 was not significantly different between the two treatment arms at end of follow‐up (OR 0.56, 95% CI 0.27 to 1.15) (Analysis 2.1). Surgical decompression reduced the risk of death and severe disability defined as mRS > 4 at 12 months (OR 0.26, 95% CI 0.13 to 0.51) (Analysis 2.2). Surgical decompression was associated with a non‐significant trend to survival with disability defined as mRS of 4 or 5 (OR 2.45, 95% CI 0.92 to 6.55) (Analysis 2.3).
1.1. Analysis.
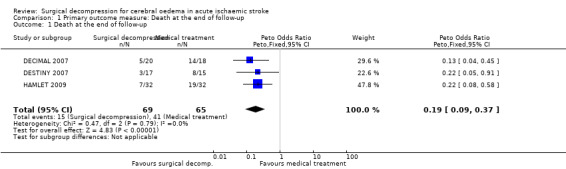
Comparison 1 Primary outcome measure: Death at the end of follow‐up, Outcome 1 Death at the end of follow‐up.
2.1. Analysis.
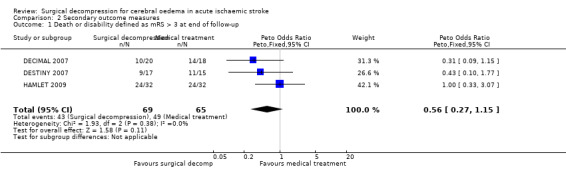
Comparison 2 Secondary outcome measures, Outcome 1 Death or disability defined as mRS > 3 at end of follow‐up.
2.2. Analysis.
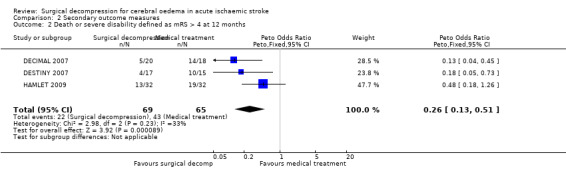
Comparison 2 Secondary outcome measures, Outcome 2 Death or severe disability defined as mRS > 4 at 12 months.
2.3. Analysis.
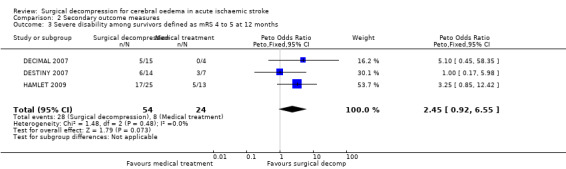
Comparison 2 Secondary outcome measures, Outcome 3 Severe disability among survivors defined as mRS 4 to 5 at 12 months.
Discussion
The pooled analysis of the three completed trials show that surgical decompression reduces the risk of death, and the risk of death or severe disability (mRS > 4) in patients 60 years of age or younger. However, it showed no benefit for disability or death defined as mRS > 3. These findings suggest that the intervention improves survival at the expense of an increased proportion of survivors with severe disability. These results stand in contrast to the results of the pooled analysis of individual patient data of the same clinical trials analysed here (Vahedi 2007), which showed that surgical decompression reduced the risk of death or disability defined as mRS > 3. In the analysis Vahedi 2007 included only those patients operated on within 48 hours from symptom onset; moreover, the mean age in that population was 45 years of age. These two factors may account for the difference between their results and the analyses presented here. In fact, HAMLET 2009, which had a window of intervention of 96 hours, has similar results to those of this review. These differences in findings support the idea of an earlier rather than a later intervention.
These results have two important limitations in terms of their applicability: they cannot be generalised to patients older than 60 years of age and they do not consider patients' values and views on quality of life at different levels of disability after stroke (Berge 2007). In addition, since all three trials were stopped early, an overestimation of the effect size of the intervention cannot be excluded.
Authors' conclusions
Implications for practice.
Decompressive surgery reduces the risk of death and the combined outcome of death or very severe disability. Therefore, it should be the preferred therapy in cases where this can also be assumed to be in the best interest of the patient, given that increased survival may occur at the expense of disability.
Implications for research.
Further research is needed to establish whether this intervention is beneficial in individuals older than 60 years of age. There is also a need for more information about patients' utility values for different levels of disability after stroke.
What's new
| Date | Event | Description |
|---|---|---|
| 17 September 2011 | New citation required and conclusions have changed | New first author. As a result of the new trials, the review conclusions have changed significantly |
| 17 September 2011 | New search has been performed | Since the publication of the original review in 2002, three clinical trials including a total of 134 patients have been completed. These trials are included in this review |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2002
| Date | Event | Description |
|---|---|---|
| 8 October 2008 | New search has been performed | Converted to new review format. |
Acknowledgements
We thank Nicholas Morley for his work with the first version of this review.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid) and adapted it for CENTRAL
1. cerebrovascular disorders/ or basal ganglia cerebrovascular disease/ or brain ischemia/ or hypoxia‐ischemia, brain/ or carotid artery diseases/ or carotid artery, internal, dissection/ or intracranial arterial diseases/ or cerebral arterial diseases/ or "intracranial embolism and thrombosis"/ or intracranial embolism/ or intracranial thrombosis/ or stroke/ or exp brain infarction/ 2. (stroke$ or cerebral vascular or cerebrovasc$ or cva).tw. 3. ((brain or cerebr$ or hemisph$ or intracranial or mca or anterior circulat$) adj5 (isch?emi$ or infarct$ or emboli$ or thrombo$ or occlus$ or hypoxi$ or apople$)).tw. 4. 1 or 2 or 3 5. decompression, surgical/ or neurosurgical procedures/ or craniotomy/ or trephining/ 6. (decompress$ or craniectom$ or craniotom$ or hemi?craniect$ or trepa$ or treph$).tw. 7. (hippocampectom$ or lobectom$ or strokectom$).tw. 8. 5 or 6 or 7 9. 4 and 8 10. cerebrovascular disorders/su or basal ganglia cerebrovascular disease/su or brain ischemia/su or hypoxia‐ischemia, brain/su or carotid artery diseases/su or carotid artery, internal, dissection/su or intracranial arterial diseases/su or cerebral arterial diseases/su or "intracranial embolism and thrombosis"/su or intracranial embolism/su or intracranial thrombosis/su or stroke/su or exp brain infarction/su 11. exp vascular surgical procedures/ 12. (aneur$ or avm).tw. 13. 10 not (11 or 12) 14. brain edema/ 15. ((brain or cerebr$ or hemispher$ or intracranial or mca or anterior circulat$) adj5 (oedema or odema or edema or swell$ or swollen)).tw. 16. 14 or 15 17. 16 and 8 18. 9 or 13 or 17 19 limit 18 to human
Appendix 2. EMBASE search strategy
We used the following search strategy for EMBASE (Ovid)
1. cerebrovascular disease/ or cerebral artery disease/ or cerebrovascular accident/ or stroke/ or carotid artery disease/ or exp carotid artery obstruction/ or brain infarction/ or brain stem infarction/ or cerebellum infarction/ or brain ischemia/ or occlusive cerebrovascular disease/ or middle cerebral artery occlusion/ 2. (stroke$ or cerebral vascular or cerebrovasc$ or cva).tw. 3. ((brain or cerebr$ or hemisph$ or intracranial or mca or anterior circulat$) adj5 (isch?emi$ or infarct$ or emboli$ or thrombo$ or occlus$ or hypoxi$ or apople$)).tw. 4. 1 or 2 or 3 5. brain decompression/ or decompression surgery/ or decompression/ 6. skull surgery/ or craniectomy/ or cranioplasty/ or craniotomy/ or neurosurgery/ 7. (decompress$ or craniectom$ or craniotom$ or hemi?craniect$ or trepa$ or treph$).tw. 8. (hippocampectom$ or lobectom$ or strokectom$).tw. 9. 5 or 6 or 7 or 8 10. 4 and 9 11. cerebrovascular disease/su or cerebral artery disease/su or cerebrovascular accident/su or stroke/su or carotid artery disease/su or exp carotid artery obstruction/su or brain infarction/su or brain stem infarction/su or cerebellum infarction/su or brain ischemia/su or occlusive cerebrovascular disease/su or middle cerebral artery occlusion/su 12. exp vascular surgery/ 13. (aneurysm$ or avm).tw. 14. 11 not (12 or 13) 15. Brain edema/ 16. ((brain or cerebr$ or hemisper$ or intracranial or mca or anterior circulat$) adj5 (oedema or odema or edema or swell$ or swollen)).tw. 17. 15 or 16 18. 17 and 9 19. 10 or 14 or 18 20. limit 19 to human
Data and analyses
Comparison 1. Primary outcome measure: Death at the end of follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death at the end of follow‐up | 3 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.19 [0.09, 0.37] |
Comparison 2. Secondary outcome measures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or disability defined as mRS > 3 at end of follow‐up | 3 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.56 [0.27, 1.15] |
| 2 Death or severe disability defined as mRS > 4 at 12 months | 3 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.13, 0.51] |
| 3 Severe disability among survivors defined as mRS 4 to 5 at 12 months | 3 | 78 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.45 [0.92, 6.55] |
| 4 Death or moderate to severe disability | 3 | 134 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.53 [0.16, 1.72] |
2.4. Analysis.
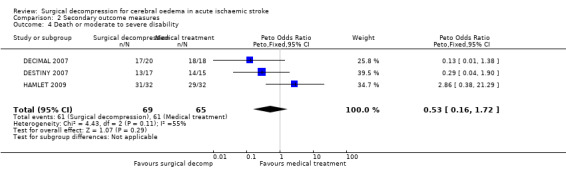
Comparison 2 Secondary outcome measures, Outcome 4 Death or moderate to severe disability.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DECIMAL 2007.
| Methods | Prospective, randomised, controlled, open study comparing surgical decompression plus medical treatment versus medical treatment alone The study had blinded evaluation of the primary end point | |
| Participants | 38 patients < 55 years of age All participants must have had involvement of > 50% of the MCA distribution and an infarct volume > 145 cm3 and a score > 1 for item 1a of the NIHSS (level of consciousness) 18 received medical therapy alone and 20 received medical therapy and decompressive craniectomy Patients were excluded if they had pre‐existing disability defined as mRS ≥ 2, a significant contralateral infarction, severe hemorrhagic transformation in > 50% of the MCA territory, coagulopathy, life expectancy < 3 years or any serious illness that could confound treatment assessment | |
| Interventions | Early decompressive craniectomy plus standard medical therapy versus standard medical therapy alone in patients with malignant MCA infarction
The decompressive surgery had to be a large hemicraniectomy that removed a bone flap that included temporal, frontal, parietal and occipital bones
Surgery had to be performed no later that 6 hours from randomisation and < 30 hours from symptom onset The dura had to be opened Medical therapy included blood pressure, glucose control, mechanical ventilation and mannitol with clinical deterioration due to brain edema |
|
| Outcomes | Primary effect measure: favourable functional outcome defined as survival with mRS ≤ 3 at 6 months Secondary effect measures included survival, favourable functional outcome defined as mRS ≤ 3 or BI > 85 at 12 months, NIHSS and quality of life as defined by the Stroke Impact Scale 2.0 at 12 months |
|
| Notes | The study was stopped prematurely due to a slow recruitment and to the fact that data was to be combined with the two other European trials Blinded assessment was accomplished by covering the patients' heads with a surgical cap | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded assessment by covering patients' heads with a surgical cap |
| Selective reporting (reporting bias) | Low risk | |
DESTINY 2007.
| Methods | Prospective, randomised, controlled, open trial Blocked randomisation, stratified for each centre | |
| Participants | 32 patients < 60 years of age Patients included had an infarct involving > 2/3 of the MCA territory with score > 1 in item 1a of NIHSS 15 received medical therapy and 17 received medical therapy and surgical decompression Patients were excluded if they had a mRS ≥ 2, a BI < 95, a GCS < 6, dilated pupils, any other brain lesion that might affect outcome, haemorrhagic transformation, life expectancy < 3 years, coagulopathy or any other serious illness that might affect outcome | |
| Interventions | Medical treatment alone versus medical treatment and surgical decompression Medical treatment included the use of osmotic agents on schedule to a target osmolality 315 to 320 mOsm, mechanical ventilation, blood pressure control, temperature and glucose control maintaining euvolemia Surgical decompression included a bone flap > 12 cm in diameter including frontal, temporal parietal and occipital bones and durotomy Surgery had to be performed > 12 hours but < 30 hours from symptom onset and no more than 6 hours from randomisation | |
| Outcomes | Primary effect measure: favourable functional outcome defined as mRS ≤ 3 at 6 months and 12 months Secondary effect measures: mRS ≤ 4 and BI at 6 and 12 months Mortality at 30 days was also assessed but not as primary outcome | |
| Notes | The study was stopped prematurely given the results of the pooled analysis of the three European trials | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | |
HAMLET 2009.
| Methods | Prospective, randomised, controlled open trial | |
| Participants | 64 patients < 60 years of age, all had an infarct involving > 2/3 of MCA territory with evidence of mass effect and midline shift as well as changes in the level of consciousness as demonstrated by a GCS < 13 32 patients were randomised to each group Patients were excluded if they had an ischaemic stroke of the whole cerebral hemisphere, decrease in consciousness partially because of causes other than the formation of oedema, such as metabolic, disturbances or medication, pupils fixed and dilated, thrombolysis in the 12 hours before randomisation, coagulopathy, pre‐stroke score on the mRS > 1 or < 95 on the BI, life expectancy less than 3 years or other serious illness that might confound treatment assessment | |
| Interventions | Medical treatment alone versus medical treatment plus surgical decompression performed < 96 hours from symptom onset and < 3 hours from randomisation Medical treatment included the use of osmotic agents on schedule to a target osmolality 315 to 320 mOsm, mechanical ventilation, blood pressure control, temperature and glucose control maintaining euvolemia Surgical decompression consisted of removal of a flap of bone of at least 12 cm in diameter including frontal, parietal, temporal and occipital bones The dura was opened and a dural patch was placed Infarcted brain tissue was not resected | |
| Outcomes | Primary effect measure: favourable outcome mRS ≤ 3 at 1 year Secondary effect variable: favourable outcome defined as mRS score of 0 to 4, case fatality, functional dependence expressed as BI, symptoms of depression measured by the Montgomery and Asberg depression rating scale (MADRS), and quality of life measured with the Medical Outcomes Study 36‐item short‐form health survey (SF‐36) and a visual analogue scale (VAS)15 at 1 year | |
| Notes | The study was stopped prematurely as the Data Monitoring Committee established that no statistically significant difference would be found between both arms with the pre‐established sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | An unblinded investigator provided narrative description that was submitted to three blinded investigators to ascertain mRS The final score was obtained by consensus from these blinded observers |
BI: Barthel Index GCS: Glasgow Coma Score MCA: middle cerebral artery mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Characteristics of studies awaiting assessment [ordered by study ID]
DEMITUR Trial.
| Methods | Patients aged 40 to 80 years were prospectively randomised to surgical decompression vs standard medical treatment |
| Participants | 151 patients were included |
| Interventions | Surgical decompression within 48 hours from symptom onset |
| Outcomes | Primary outcome dichotomised mRS score 0 to 3 (good outcome) versus > 3 (poor outcome) at 6 and 12 months Secondary outcome: BI > 85 at 6 and 12 months, NIHSS and quality of life by Turkish version of Stroke Impact Scale 2.0 (SIS) at 6 and 12 months |
| Notes | Principal investigator: Emre Kumral
Trial was carried out between January 2003 and December 2007 ‐ data not yet published It is unclear if outcome assessment was blinded A sample size of 106 was calculated on the assumption that 40% of patients in the surgical group and 70% in each of the medical treatment groups would have a poor outcome Could not contact principal investigator |
HEADDFIRST.
| Methods | Patients were randomised to surgical decompression versus standard medical care |
| Participants | 75 patients were included in this study |
| Interventions | Surgical decompression carried out within 96 hours from symptom onset |
| Outcomes | Case fatality, functional outcome, quality of life, caregiver burden, patient perceptions of survivorship, acute health care utilisation measured all at 21, 90 and 180 days |
| Notes | Principal investigator: Jeffrey I Frank Trial carried out from February 2000 through to January 2002 ‐ data not yet published |
BI: Barthel Index mRS: modified Rankin Scale NIHSS: National Institutes of Health Stroke Scale
Characteristics of ongoing studies [ordered by study ID]
DESTINY 2.
| Trial name or title | Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery 2 |
| Methods | Prospective randomised open controlled multicentre phase III comparative trial |
| Participants | Participants older than 60 years of age with a large MCA infarction to be treated within 48 hours from symptom onset Sample size 160 |
| Interventions | Surgical decompression plus medical treatment versus medical treatment alone |
| Outcomes | mRS score, dichotomised 0 to 4 versus 5 to 6 after 6 months |
| Starting date | 2009 |
| Contact information | Eric Juettler MD eric.juettler@med.uni‐heidelberg.de |
| Notes | ISRCTN# 21702227 |
HeMMI 2004.
| Trial name or title | Hemicraniectomy for Malignant Middle cerebral artery Infarcts |
| Methods | Randomised controlled trial |
| Participants | Participants with MCA infarction, aged 18 to 65 years to be treated within 72 hours from onset Sample size 56 |
| Interventions | Surgical decompression versus medical treatment |
| Outcomes | Primary effect measure: mRS < 4 or BI > 60 |
| Starting date | 2004 |
| Contact information | Principal investigator: Roland D Jamora dominicjamora@gmail.com |
| Notes |
BI: Barthel Index MCA: middle cerebral artery mRS: modified Rankin Scale
Contributions of authors
Dr S Cruz‐Flores: literature searching, data collection and data extraction and writing the first draft of the present review. Dr E Berge: participated in writing the protocol and writing the first version of the review. In the second version of the review he participated in the assessment of data and writing the review. Prof I Whittle: reviewed both versions of the review.
Sources of support
Internal sources
University of Edinburgh, UK.
External sources
No sources of support supplied
Declarations of interest
Dr S Cruz‐Flores was a site principal investigator for the HEADDFIRST trial supported by the National Institute of Neurological Diseases and Stroke. The trial is mentioned in this review. However, its results have not been published and therefore are not included in the review.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
DECIMAL 2007 {published data only}
- Vahedi K, Vicaut E, Mateo J, Kurtz A, Orabi M, Guichard JP, et al. DECIMAL investigators. Sequential‐design, multicenter, randomized, controlled trial of early decompressive craniectomy in malignant middle cerebral artery infarction (DECIMAL Trial). Stroke 2007;38(9):2506‐17. [DOI: 10.1161/STROKEAHA.107.485235] [DOI] [PubMed] [Google Scholar]
DESTINY 2007 {published data only}
- Juttler E, Schwab S, Schmiedek P, Unterberg A, Hennerici M, Woitzik J, et al. DESTINY Study Group. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery (DESTINY): a randomized, controlled trial. Stroke 2007;38(9):2518‐25. [DOI: 10.1161/STROKEAHA.107.485649] [DOI] [PubMed] [Google Scholar]
HAMLET 2009 {published data only}
- Hofmeijer J, Kappelle LJ, Algra A, Amelink GJ, Gijn J, Worp HB, et al. HAMLET investigators. Surgical decompression for space‐occupying cerebral infarction (the Hemicraniectomy After Middle Cerebral Artery infarction with Life‐threatening Edema Trial [HAMLET]): a multicentre, open, randomised trial. Lancet Neurology 2009;8(4):326‐33. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
DEMITUR Trial {published data only}
- Kumral E. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery: a randomised, controlled trial in a Turkish population. http://www.strokecenter.org/trials/clinicalstudies/decompressive‐surgery‐for‐the‐treatment‐of‐malignant‐infarction‐of‐the‐middle‐cerebral‐artery‐a‐randomized‐controlled‐trial‐in‐a‐turkish‐population/results 2009.
HEADDFIRST {published data only}
- Frank JI. A randomised multi‐center trial of hemicraniectomy and durotomy for deterioration from infarction related swelling: HEADDFIRST. http://www.strokecenter.org/trials/clinicalstudies/hemicraniectomy‐and‐durotomy‐for‐deterioration‐from‐infarction‐relating‐swelling‐trial 2000. [DOI] [PMC free article] [PubMed]
- Frank JI, Krieger D, Chyatte DM, Cancian S. HEADDFIRST: Hemicraniectomy and durotomy upon deterioration from massive hemispheric infarctions. A proposed multicenter, prospective randomized study. Stroke 1999;30:243. [Google Scholar]
References to ongoing studies
DESTINY 2 {published data only}
- Juettler E. Decompressive surgery for the treatment of malignant infarction of the middle cerebral artery 2: a prospective open randomised multicenter comparative trial. ControlIed‐trials.com/ISRCTN21702227/stroke 2009.
HeMMI 2004 {published data only}
- Jamora RD, Nigos J, Collantes ME, Chua A, Gan R. Hemicraniectomy for malignant middle cerebral artery infarcts (HeMMI). www.strokecenter.org/trials/TrialDetail.aspx?tid=575. 2004.
Additional references
Bamford 1991
- Bamford J, Sandercock P, Dennis M, Burn J, Warlow C. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet 1991;337(8756):1521‐6. [DOI] [PubMed] [Google Scholar]
Berge 2007
- Berge E. Decompressive surgery for cerebral oedema after stroke: evidence at last. Lancet Neurology 2007;6(3):200‐1. [DOI] [PubMed] [Google Scholar]
Carter 1997
- Carter BS, Ogilvy CS, Candia GJ, Rosas HD, Buonanno F, Solomon RA, et al. One‐year outcome after decompressive surgery for massive nondominant hemispharic infarction. Neurosurgery 1997;40:1168‐76. [DOI] [PubMed] [Google Scholar]
Cristofori 1998
- Cristofori L, Musumeci A, Turazzini M, Gambin R, Vivenza C, Silvestri M, et al. The role of surgery for acute ischemic hemispheric swelling. Report of two cases. Rivista di Neurobiologia 1998;44(6):589‐94. [Google Scholar]
Delashaw 1990
- Delashaw JB, Broaddus WC, Kassell NF, Haley EC, Pendleton GA, Vollmer DG, et al. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke 1990;21(6):874‐81. [DOI] [PubMed] [Google Scholar]
Fowkes 1991
- Fowkes FG, Fulton PM. Critical appraisal of published research: introductory guidelines. BMJ 1991;302(6785):1136‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frank 1995
- Frank JI. Large hemispheric infarction, deterioration, and intracranial pressure. Neurology 1995;45(7):1286‐90. [DOI] [PubMed] [Google Scholar]
Fujita 1982
- Fujita K, Tamaki N, Matsumoto S, Nagao T. [Unilateral inferior temporal lobectomy with hippocampectomy for acute ischemic brain edema] [Japanese]. No Shinkei Geka ‐ Neurological Surgery 1982;10(8):849‐55. [PubMed] [Google Scholar]
Hacke 1996
- Hacke W, Schwab S, Horn M, Spranger M, Georgia M, Kummer R. 'Malignant' middle cerebral artery territory infarction: clinical course and prognostic signs. Archives of Neurology 1996;53(4):309‐15. [DOI] [PubMed] [Google Scholar]
Ivamoto 1974
- Ivamoto HS, Numoto M, Donaghy RM. Surgical decompression for cerebral and cerebellar infarcts. Stroke 1974;5(3):365‐70. [DOI] [PubMed] [Google Scholar]
Jennett 1975
- Jennett B, Bond M. Assessment of outcome after severe brain damage. Lancet 1975;1:480‐4. [DOI] [PubMed] [Google Scholar]
Jourdan 1993
- Jourdan C, Convert J, Mottolese C, Bachour E, Gharbi S, Artru F. [Evaluation of the clinical benefit of decompression hemicraniectomy in intracranial hypertension not controlled by medical treatment] [French]. NeuroChirurgie 1993;39(5):304‐10. [PubMed] [Google Scholar]
Kakita 1976
- Kakita K, Miyazaki T, Kadowaki H, Izawa M, Kubota S. A trial of surgical management of brain edema in cerebral infarction ‐ a review with our own experiences in 31 cases. No Shinkei Geka ‐ Neurological Surgery 1976;4(3):277‐83. [PubMed] [Google Scholar]
Kalia 1993
- Kalia KK, Yonas H. An aggressive approach to massive middle cerebral artery infarction. Archives of Neurology 1993;50(12):1293‐7. [DOI] [PubMed] [Google Scholar]
King 1951
- King AB. Massive cerebral infarction producing ventriculographic changes suggesting a brain tumor. Journal of Neurosurgery 1951;8:536‐9. [DOI] [PubMed] [Google Scholar]
Koh 2000
- Koh MS, Goh KY, Tung MY, Chan C. Is decompressive craniectomy for acute cerebral infarction of any benefit?. Surgical Neurology 2000;53(3):225‐30. [DOI] [PubMed] [Google Scholar]
Kondziolka 1988
- Kondziolka D, Fazl M. Functional recovery after decompressive craniectomy for cerebral infarction. Neurosurgery 1988;23(2):143‐7. [DOI] [PubMed] [Google Scholar]
Kristensen 1998
- Kristensen BO, Lindsten H, Malm J, Shamsgovara P, Ridderheim PA, A'Roch Fagerlund M. Hemicraniectomy in malignant mid‐cerebral infarction. Further trials needed before its acceptance in clinical practice. Lakartidningen 1998;95(11):1145‐8. [PubMed] [Google Scholar]
Lindegaard 1999
- Lindegaard KF, Roste GK. Life‐saving hemicraniectomy in acute massive brain infarction. Tidsskrift for Den Norske Laegeforening 1999;119(28):4190‐2. [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Manai 2000
- Manai R, Rancurel G. Treatment by craniectomy in patients with malignant MCA infarction. Sang Thrombose Vaisseaux 2000;12(1):12‐8. [Google Scholar]
Manno 1999
- Manno EM, Adams RE, Derdeyn CP, Powers WJ, Diringer MN. The effects of mannitol on cerebral edema after large hemispheric cerebral infarct. Neurology 1999;52(3):583‐7. [DOI] [PubMed] [Google Scholar]
Martins 1993
- Martins LF, Costa V, Costa J, Melo‐Souza SE. [Temporal lobectomy in cerebral infarction with mass effect] [Portuguese]. Arquivos de Neuro‐Psiquiatria 1993;51(1):118‐24. [DOI] [PubMed] [Google Scholar]
Mori 1998
- Mori K, Ishimaru S, Maeda M. Unco‐parahippocampectomy for direct surgical treatment of downward transtentorial herniation. Acta Neurochirurgica 1998;140(12):1239‐44. [DOI] [PubMed] [Google Scholar]
Mracek 1978
- Mracek Z. Significance of decompression craniotomy in acute occlusion of the middle cerebral artery with brain‐stem symptomatology due to pressure by the edematous cerebral hemisphere. Ceskoslovenska Neurologie a Neurochirurgie 1978;41(6):390‐3. [PubMed] [Google Scholar]
Ng 1970
- Ng LK, Nimmannitya J. Massive cerebral infarction with severe brain swelling: a clinicopathological study. Stroke 1970;1(3):158‐63. [DOI] [PubMed] [Google Scholar]
Oro 2000
- Oro J, Amiridze N, Boyer R. Decompressive craniotomy in medically uncontrollable malignant infarction. Missouri Medicine 2000;97(1):17‐20. [PubMed] [Google Scholar]
Rankin 1957
- Rankin J. Cerebral vascular accidents in patients over the age of 60: prognosis. Scottish Medical Journal 1957;2:200‐15. [DOI] [PubMed] [Google Scholar]
Rengachary 1981
- Rengachary SS, Batnitzky S, Morantz RA, Arjunan K, Jeffries B. Treatment of right hemispheric cerebral infarction by hemicraniectomy. Stroke 1981;8:874‐81. [DOI] [PubMed] [Google Scholar]
Rieke 1995
- Rieke K, Schwab S, Krieger D, Kummer R, Aschoff A, Schuchardt V, et al. Decompressive surgery in space‐occupying hemispheric infarction: results of an open, prospective trial. Critical Care Medicine 1995;23(9):1576‐87. [DOI] [PubMed] [Google Scholar]
Ropper 1984
- Ropper AH, Shafran B. Brain edema after stroke. Clinical syndrome and intracranial pressure. Archives of Neurology 1984;41(1):26‐9. [DOI] [PubMed] [Google Scholar]
Saito 1987
- Saito I, Segawa H, Shiokawa Y, Taniguchi M, Tsutsumi K. Middle cerebral artery occlusion: correlation of computed tomography and angiography with clinical outcome. Stroke 1987;18(5):863‐8. [DOI] [PubMed] [Google Scholar]
Sakai 1998
- Sakai K, Iwahashi K, Terada K, Gohda Y, Sakurai M, Matsumoto Y. Outcome after external decompression for massive cerebral infarction. Neurologia Medico‐Chirurgica 1998;38(3):131‐5. [DOI] [PubMed] [Google Scholar]
Schwab 1996
- Schwab S, Aschoff A, Spranger M, Albert F, Hacke W. The value of intracranial pressure monitoring in acute hemispheric stroke. Neurology 1996;47(2):393‐8. [DOI] [PubMed] [Google Scholar]
Schwab 1997
- Schwab S, Spranger M, Schwarz S, Hacke W. Barbiturate coma in severe hemispheric stroke: useful or obsolete?. Neurology 1997;48(6):1608‐13. [DOI] [PubMed] [Google Scholar]
Schwab 1998
- Schwab S, Steiner T, Aschoff A, Schwarz S, Steiner KK, Jansen O, et al. Early hemicraniectomy in patients with complete middle cerebral artery infarction. Stroke 1998;29(9):1888‐93. [DOI] [PubMed] [Google Scholar]
Sollid 1999
- Sollid S, Kloster R, Ingebrigtsen T. Decompression craniectomy ‐ life‐saving treatment in acute cerebral infarction. Tidsskrift for Den Norske Laegeforening 1999;119(28):4199‐201. [PubMed] [Google Scholar]
Tei 2000
- Tei H, Uchiyama S, Ohara K, Kobayashi M, Uchiyama Y, Fukuzawa M. Deteriorating ischemic stroke in 4 clinical categories classified by the Oxfordshire Community Stroke Project. Stroke 2000;31(9):2049‐54. [DOI] [PubMed] [Google Scholar]
Tsuruno 1993
- Tsuruno T, Takeda M, Imaizumi T, Tanooka A. Internal decompression with hippocampectomy for massive cerebral infarction. No Shinkei Geka ‐ Neurological Surgery 1993;21(9):823‐7. [PubMed] [Google Scholar]
Ueno 1984
- Ueno K, Oosato T, Sasaki H, Nomura M. Prophylactic external decompression for massive cerebral infarction. No Shinkei Geka ‐ Neurological Surgery 1984;12(3 Suppl):261‐7. [PubMed] [Google Scholar]
Vahedi 2007
- Vahedi K, Hofmeijer J, Juettler E, Vicaut E. Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurology 2007;6:215‐22. [DOI] [PubMed] [Google Scholar]
Van Leusen 2001
- Leusen HJ, Tans JT, Wurzer JA. Hemicraniectomy for treatment of malignant medial cerebral artery infarction in 3 patients. Nederlands Tijdschrift voor Geneeskunde 2001;145(13):639‐43. [PubMed] [Google Scholar]
Wagner 2001
- Wagner S, Schnippering H, Aschoff A, Koziol JA, Schwab S, Steiner T. Suboptimum hemicraniectomy as a cause of additional cerebral lesions in patients with malignant infarction of the middle cerebral artery. Journal of Neurosurgery 2001;94(5):693‐6. [DOI] [PubMed] [Google Scholar]
Wijdicks 2000
- Wijdicks EF. Management of massive hemispheric cerebral infarct: is there a ray of hope?. Mayo Clinic Proceedings 2000;75(9):945‐52. [DOI] [PubMed] [Google Scholar]
Young 1982
- Young PH, Smith KR Jr, Dunn RC. Surgical decompression after cerebral hemispheric stroke: indications and patient selection. Southern Medical Journal 1982;75(4):473‐5. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Morley 2002
- Morley NCD, Berge E, Cruz‐Flores S, Whittle IR. Surgical decompression for cerebral oedema in acute ischaemic stroke. Cochrane Database of Systematic Reviews 2002, Issue 3. [DOI: 10.1002/14651858.CD003435] [DOI] [PubMed] [Google Scholar]


