Abstract
Background
There are two injectable progestogen‐only contraceptives (IPCs) that have been available in many countries in the world since 1983. They are both still extensively used in many developing countries, forming a large proportion of the health system's expenditure on contraception. These are depot medroxyprogesterone acetate (DMPA) and norethisterone oenanthate (NET‐EN). These are both highly effective contraceptives that receive wide acceptance amongst women in their fertile years. They differ in frequency of administration that has implications on patient uptake. They also differ in cost that may significantly affect budgeting in the health system. A systematic comparison will aid to ensure their rational use.
Objectives
To determine if there are differences between depot medroxyprogesterone acetate given at a dose of 150 mg IM every 3 months and norethisterone oenanthate given at a dose of 200mg IM every 2 months, in terms of contraceptive effectiveness, reversibility and discontinuation patterns, minor effects and major effects.
Search methods
We searched the computerized databases MEDLINE using PubMed, Popline, Cochrane Controlled Trials Register, Biblioline, LILACS, EMBASE and PASCAL for randomised controlled trials of DMPA versus NET‐EN for long‐acting progestogenic contraception. Studies were included regardless of language, and all databases were reviewed from the time that injectable progestogens have been in use.
Selection criteria
All randomised controlled comparisons of DMPA acetate given at a dose of 150 mg IM every 3 months versus NET‐EN given at a dose of 200mg IM every 2 months, used for contraception, were included. Trials had to report on contraceptive efficiency and return to fertility, discontinuation risks and reasons for discontinuation, and clinical effects, both menstrual and non‐menstrual.
Data collection and analysis
BD and CM evaluated the titles and abstracts obtained through applying the search strategy and applied the eligibility criteria. BD attempted to contact authors where clarification of the data was required, and contacted all main manufacturers of the contraceptives. After inclusion of the two studies, the data was abstracted and analysed with RevMan 4.2.
Main results
Two trials were included in this review. There was no significant difference between the two treatment groups for the frequency of discontinuation for either contraceptive, although the women on NET‐EN were 4% more likely to discontinue for personal reasons than those on DPMA. Discontinuation because of accidental pregnancy did not differ between the groups. Although the duration of bleeding and spotting events was the same in each group, women on DPMA were 21% more likely to develop amenorrhoea. Mean changes in body weight at 12 and 24 months, and in systolic and diastolic blood pressure at 12 months did not differ significantly between the studies.
Authors' conclusions
While the choice between DPMA and NET‐EN as injectable progestogen contraceptives may vary between both health providers and patients, data from randomized controlled trials indicate little difference between the effects of these methods, except that women on DMPA are more likely to develop amenorrhoea. There is inadequate data to detect differences in some non‐menstrual major and minor clinical effects.
Keywords: Female; Humans; Contraception; Contraception/methods; Contraceptive Agents, Female; Contraceptive Agents, Female/administration & dosage; Delayed‐Action Preparations; Delayed‐Action Preparations/administration & dosage; Drug Administration Schedule; Medroxyprogesterone Acetate; Medroxyprogesterone Acetate/administration & dosage; Norethindrone; Norethindrone/administration & dosage; Norethindrone/analogs & derivatives; Randomized Controlled Trials as Topic
Plain language summary
This review compares two injectable hormonal contraceptives containing only progestogen, namely depot medroxyprogesterone acetate (DMPA) and norethisterone oenanthate (NET‐EN), for the risks and reasons of their discontinuation and for their clinical effects
Injectable hormonal contraceptives remain in extensive use in many developing countries. There are two progestogen‐only injectable contraceptives that have been available in many countries since the 1980's. These are depot medroxyprogesterone acetate (DMPA) and norethisterone oenanthate (NET‐EN). They are both highly effective contraceptives that receive wide acceptance amongst women in their fertile years, and form a sizeable proportion of the health expenditure on contraception. They differ in frequency of administration and cost, and a systematic comparison aids to ensure their rational use. This review seeks to compare DPMA given at a dose of 150 mg IM every 3 months and NET‐EN given at a dose of 200mg IM every 2 months, and determine whether there are differences in contraceptive effectiveness, reversibility and patterns of discontinuation, and their minor and major clinical effects. All databases were reviewed from the time that injectable progestogens have been in use and this review included all randomised controlled comparisons of DMPA and NET‐EN used for contraception. Trials had to report on contraceptive efficiency and return to fertility, the rates and reasons for stopping use, as well as menstrual and non‐menstrual clinical effects. It was found that there was no significant difference between the two treatment groups regarding the time from when women started the contraceptive until they stopped its use. The women on NET‐EN were 4% more likely to stop use for personal reasons than those on DPMA, but this difference was not statistically significant. There was no difference between the groups when an accidental pregnancy was the reason to stop use. The length of time of episodes of vaginal bleeding and spotting was the same in each group. Women who were on DPMA were 21% more likely to stop vaginal bleeding altogether while using the contraceptive. Changes in body weight and changes in blood pressure did not differ between DMPA and NET‐EN. Furthermore, these changes in body weight and blood pressure were relatively small and not clinically relevant. In summary, therefore, data from the trials included in this review indicate little difference between the effects of these methods, except that women on DMPA are more likely to experience cessation of vaginal bleeding during its use. There was inadequate data to detect differences in some non‐menstrual clinical effects, and considering that this contraceptive method remains in use in some countries, further research is indicated.
Background
There are two injectable progestogen‐only contraceptives (IPCs) available for use. These are depot medroxyprogesterone acetate (DMPA) and norethisterone oenanthate (NET‐EN).
It was discovered in 1953 that esterifying a progestogen produced a drug with a long lasting effect. Injectable progestogen‐only contraceptives are now available in many countries in the world (Lande 1995) and play an important role in many national family planning and health programs (Sapire 1990). They are extensively used in some developing countries (e.g. Indonesia, Thailand and South Africa) and donor agencies have reported that use of IPCs has increased across the world in the last decade (DoH 1999). For instance, the United Nations Population Fund (UNFPA) provided 12 million doses of injectables in 1992, and about 20 million in 1994 (Lande 1995). In countries where IPCs are widely used, they account for a substantial share of expenditure on drugs (Smit 2000). In South Africa, they are by far the most used contraceptive method (DoH 1999) and provide a safe, convenient, effective and reversible method of fertility regulation (Sapire 1990).
DMPA is a synthetic 17‐hydroxymedroprogesterone derivative with progestational activity, providing contraceptive protection for three months; and NET‐EN is a long chain ester of norethisterone, effective for two months. They are both highly effective contraceptive agents and 12‐month pregnancy rates are generally lower than with oral contraceptives (Sapire 1990). The mechanism of action of the IPCs is primarily the prevention of ovulation, supplemented mainly by contraceptive actions at the endometrial and cervical mucus level (Guillebaud 1993). The commodity cost of DMPA is considerably lower than that of NET‐EN (DoH 1999, Smit 2000). DMPA is the predominant product used world‐wide (Lande 1995), but there appears to be increasing use of norethisterone oenanthate (NET‐EN) in at least one country (South Africa) where IPCs are extensively used (Smit 2000). Given the cost implications of increasing NET‐EN use, a careful and systematic comparison of these preparations is required in order to ensure their rational use, particularly in developing countries.
Objectives
To determine if there are differences between depot medroxyprogesterone acetate (DMPA) given at a dose of 150 mg IM every 3 months and norethisterone oenanthate (NET‐EN) given at a dose of 200mg IM every 2 months, in terms of contraceptive efficacy, reversibility and discontinuation patterns, minor effects and major effects.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled comparisons of DMPA given at a dose of 150 mg IM every 3 months versus NET‐EN given at a dose of 200mg IM every 2 months, used for contraception.
Types of participants
Healthy women of reproductive age, of all ethnic groups who are using either of the IPCs, i.e. DMPA or NET‐EN.
Types of interventions
DMPA given at a dose of 150 mg IM every 3 months versus NET‐EN given at a dose of 200mg IM every 2 months, used for contraception.
Types of outcome measures
Cumulative discontinuation risks: overall risks and risks due to specific menstrual and non‐menstrual effects.
Contraceptive efficacy: Accidental pregnancy as a reason for discontinuation
Minor effects: Menstrual
Amenorrhoea
Menorrhagia
Spotting
Irregular bleeding
Dysmenorrhoea
Non‐menstrual
Headache
Clinically significant weight change of 2 kg
Decreased libido
Mood swings and/or depression
Nausea
Dizziness
Vaginal discharge
Major effects:
Increased HIV vaginal shedding
Susceptibility to HIV and other sexually transmitted infections
Search methods for identification of studies
We searched the computerized databases MEDLINE using PubMed, Popline, Cochrane Controlled Trials Register, Biblioline, LILACS, EMBASE and PASCAL for randomised controlled trials of DMPA versus NET‐EN for long‐acting progestogenic contraception. Studies were included regardless of language, and all databases were reviewed from the time that injectable progestogens have been in use, namely 1963.
We searched PubMed using the search strategy: ((contraceptive agents, female OR contracept*) AND ((medroxyprogesterone acetate‐17 OR medroxyprogesterone acetate OR 17‐medroxyprogesterone acetate OR depot medroxyprogesterone acetate OR depo provera OR depot provera OR DMPA) AND (norethrindrone OR NET‐EN OR NET‐ENT OR NET‐OEN OR nuristerate OR norethisterone oenathate))) AND (clinical trials OR random allocation or random*) NOT (menopaus* OR post menopaus* OR HRT OR "hormone replacement")
We searched POPLINE using the search strategy: ((medroxyprogesterone acetate/depo provera/DMPA) & (NET‐EN/norethindrone/norethindrone acetate/norethindrone enanthate))/ (contraceptive agents progestin & inject*)) & (clinical trial*/random*)
We searched Cochrane Controlled Trial Register using the search strategy: (injectable contraceptives OR contraceptive agents) AND (medroxyprogesterone acetate‐17 OR medroxyprogesterone acetate OR 17‐medroxyprogesterone acetate OR depot medroxyprogesterone acetate OR depo provera OR depot provera OR DMPA) AND (norethrindrone OR NET‐EN OR NET‐ENT OR NET‐OEN OR nuristerate OR norethisterone oenathate.
We searched Biblioline using the search strategy: 1. contraception, hormonal OR contraception, injectable OR family planning, hormonal 2. medroxyprogesterone acetate‐17 OR medroxyprogesterone acetate OR 17‐medroxyprogesterone acetate OR depot medroxyprogesterone acetate OR depo provera OR depot provera OR DMPA 3. NET‐EN OR NET‐ENT OR NET‐OEN OR nuristerate OR norethisterone oenathate 4. clinical trials OR random allocation OR random
We searched LILACS using the search strategy: medroxyprogesterone acetate or depo or depot provera or dmpa or depo provera or ampd or acetado de medroxiprogesteronade de deposito or injectivo de solo progestageno [Words] and norethindrone or noretindrona or noretindrona or net‐en or net‐ent or en‐net or noristerat inyectable or enantato de norestistero or anticonceptiva noristerat inyectable [Words] and (contraceptive or contraceptives or agentes anticonceptivos or anticoncepcionais) [Words] We searched EMBASE using the search strategy: (contraceptive agent or contracept?) AND ((DMPA or depot medroxyprogesterone depot medroxyprogesterone acetate or medroxyprogesterone acetate or depo provera or depot provera) AND (NET EN or norethindrone or norethisterone or noresthisterone enanthate )) AND (clinical trial or random(w)allocation or random)
We searched PASCAL using the search strategy: (Contraceptive OR contraceptive agent OR contracept OR contraception) AND (DMPA OR depo OR depo provera OR depo‐provera OR depo‐provero OR depo‐medroxyprogesterone acetate OR medroxyprogesterone OR medroxyprogesterone acetate OR medroxyprogesteroneacetate OR medroxyprogesterone(17‐0‐acetyl)‐ana OR medroxyprogest ) AND (NET EN OR NET‐EN OR noresthisterone OR noresthisterone enanthate OR noresthindrone OR norethynodrel OR noretisterona) AND (clinical trial OR comparative study)
We searched the reference lists of all identified studies for eligible trials and additional, previously unidentified trials. Relevant book chapters and review articles were searched for all relevant trials. We further attempted to find unpublished randomized controlled trials through personal communication with experts and the manufacturers of both contraceptives.
We accessed conference proceedings and health organisations including: World Health Organisation Family Health International Population Council U.S. Federal Food and Drug Administration Evidence on adverse effects / Medicines Control Council
Data collection and analysis
Reviewers BD and CM evaluated the titles and abstracts obtained through applying the search strategy as described previously and applied the eligibility criteria. The reviewers performed this independently using a standardised study validity form, and differences were resolved through discussion. Where there was possibility for inclusion, the full article was obtained. We focused on the types of intervention and method of randomization. BD made numerous attempts to contact the authors of trials and centres where they were performed, in studies where randomization was unclear, asking for details about the methods used, but was unable to receive any response, presumably because a considerable length of time has elapsed since these studies were executed. The reasons for excluding studies are stated in the table 'Characteristics of excluded studies'.
After inclusion of the two studies (Salem HT, WHO),BD and CM abstracted the data. When viewing the data of the large WHO study, it was agreed to enter the data for discontinuation rates individually for each of the thirteen centres where the study had been performed, as published in the study results. For all other results we used the total outcome of the study. A typological error was detected and corrected in the Salem et al study. We used RevMan 4.2 to analyze the data.
LvdM collaborated with the statistical analysis of the abstracted data. For the dichotomous outcomes, such as discontinuation rates, episodes of bleeding and spotting, and amenorrhoea, we converted cumulative rates per 100 women to risks and compared these by calculating risk differences with 95% confidence intervals assuming random effects models. Numerical data such as duration of bleeding and spotting episodes, and changes in body weight and blood pressure were summarised using weighted mean differences assuming random effects models. Subgroup analysis included both studies at 12 months, and the WHO study at 24 months.
Results
Description of studies
Four trials were initially included because they were thought to meet the inclusion criteria for the study: WHO 1983, Salem 1988, Swenson 1980 and Janjua 1983 (WHO, Salem HT, Swenson I, Janjua S). However, those of Swenson and Janjua were later excluded. In the study by Swenson, the dose intervals of NET‐EN were not consistent. The second dose of NET‐EN was given 10 weeks after the initial injection, and the third and subsequent injections were given at 12 week intervals. The study that was conducted by Janjua S in Islamabad did not specify whether randomisation took place at the outset of the study, and attempts to contact the researcher to establish whether randomisation was in fact applied, were not successful.
A study by Beksinska 2001 (Beksinska M) compares women aged 40‐49 years using DMPA, NET‐EN or combined oral contraceptives for contraception. However, the users all had at least one year of use on commencement of the study, some of whom had been using an injectable contraceptive method for a number of years. The second part of the trial includes younger women initiating IPCs (Beksinska M part 2) and analysis of the data is still pending. This trial has been listed under ongoing studies.
The current review thus includes two randomised controlled trials with a total of 3572 women after 6 months on treatment, 2776 women that may be compared after 1 year, and 2376 women after two years of treatment. Of these, the main study was the multinational study conducted by the WHO Special Programme of Research, Development and Research Training in Human Reproduction. This was conducted from 1977 ‐ 1982 at thirteen centres throughout the world, with nine from developing countries and four from developed countries. There were in fact three treatment groups: DMPA given at 90 day intervals, NET‐EN given at 60 day intervals, both for the entire period of the study, while a third group were given NET‐EN at 60 day intervals for 6 months and at 84 day intervals thereafter. The study results comparing DMPA given every 90 days and NET‐EN given every 60 days only were included in this review. The objective was to recruit 200 subjects on each drug in each centre, but because of slow recruitment in some centres and premature closure in others, this could not be ultimately attained. The countries in which this study was conducted were Egypt (Alexandria), Thailand (Bangkok), Nigeria (Ibadan), Pakistan (Karachi), Zambia (Lusaka), Phillipines (Manila), Mexico (City), Brazil (Salvador), Chile (Santiago), Yugoslavia (Ljubjana), Luxemborg, Italy (Milan) and the Netherlands (Utrecht). In total 10,331 women participated in this study. There a was variation of some outcomes according to the different centres in which the trial was conducted. The second study (Salem HT) was conducted prior to 1987 in Egypt, and involved 400 participants, 200 in each treatment group, over one year. Details of the included studies are shown in the table of included studies, and detail of the number of participants according to time is shown in additional tables.
The outcomes that were measured in the studies are risks for discontinuation, both total and subdivided according to reasons. The reasons for discontinuation are given as pregnancy, menstrual and non‐menstrual reasons. The menstrual reasons for discontinuation include amenorrhoea as well as bleeding irregularities. The WHO study gives data collected on the duration and proportions of bleeding and spotting episodes occurring in the participants (Table 1). Changes in blood pressure and body weight were also recorded in both studies. The outcomes relating to fertility, as stated in the protocol, namely contraceptive efficacy and reversibility were not specifically included in the included studies, and the only comparison possible was accidental pregnancy as a reason for discontinuation. The minor effects headache, decreased libido, nausea dizziness and vaginal discharge, and the major effects of HIV vaginal shedding and susceptibility to HIV and other sexually transmitted infections were not outcomes of the included studies.
1. Percent of women with bleeding and/or spotting episodes 21 days: WHO trial.
| period | DMPA | NET‐EN |
| 0‐6 months | 10.5 | 4.1 |
| 7‐12 months | 4.6 | 2.4 |
| 13‐18 months | 2.6 | 1.8 |
| 19‐24 months | 1.9 | 0.9 |
Risk of bias in included studies
Participants in the WHO study were randomly allocated to the treatment groups after being recruited into the study. In the Salem study, use was made of a randomisation table prepared by the WHO Special Program of Research, Development and Research Training in Human Reproduction. Women were allocated by picking a sealed envelope which had a random number that assigned her to one of the two methods. The WHO study states simply that sublects were randomly allocated to either the DMPA or the NET‐EN group. The WHO study was conducted by the same WHO Special Program, and after some enquiries, the reviewers concluded that the same method of randomisation was used in both studies.
The time intervals between the administrations of the injections differed between DPMA and NET‐EN. Therefore once the women were allocated into one or other of the study arms, it would not have been possible to blind the participants to the method of contraception. Neither of the studies are clear as to whether there was assessor blinding. The data processing of the larger study was carried out by the WHO in Geneva. In this analysis, the data was analysed according to counts of events and the use of life‐tables procedures to estimate the duration of events.
Loss to follow up in the WHO study is reported as 1% over 2 year follow up. In the study by Salem over one year duration of follow up, the loss to follow up in the DMPA group was 27% and 40% in the NET‐EN group. Patient follow up times correlated with the periods of time that elapsed between each administration of the contraceptive.
Effects of interventions
Two trials comparing DMPA and NET‐EN were included in the review. These are the WHO multinational trial 1983 and Salem 1988 (WHO, Salem HT). The WHO data on discontinuation rates is published only according to the 13 centres, with no single rate for the whole study. For this reason the analysis is by individual sites to provide a more accurate result.
There was no difference between the two treatment groups for the frequency of discontinuation at 12 months. This included both studies.(RD 0.00; 95% CI ‐0.06;0.06 p=0.98). At 24 months the WHO data showed that there was a 3% difference in risk of discontinuation, the DPMA group having higher risk, but this was not statistically significant (RD 0.03; 95% CI ‐0.04;0.09 p=0.42). The reasons for discontinuation were similar for both groups regarding accidental pregnancy (p=0.23), amenorrhoea (p=0.62), bleeding problems (p=0.74) and other medical reasons (p=0.26). However, when discontinuation was for personal reasons, on average there is a 4% difference (95%CI ‐0.07;‐0.01 p=0.008) showing that women on NET‐EN were more inclined to discontinue treatment for personal reasons.
Analysis of the data of both studies of bleeding/spotting events at 12 months indicated a nonsignificant difference of 2% between the groups at 12 months (RD 0.02; 95%CI ‐0.02;0.06 p=0.45) and 1% at 24 months (RD 0.01; 95%CI 0.00;0.02 p=0.11). The weighted mean difference between the groups in the duration of bleeding and spotting episodes similarly showed no significant dissimilarity between the groups at 12 months (p=0.34) and at 24 months (p=0.58).
The analysis of amenorrhoea showed a highly significant difference between groups at both 12 and 24 months , with risk of experiencing amenorrhoea on average 21% higher in women on DPMA at both times (at 12 months p=0.002 and at 24 months p<0.000).
The results for mean changes in body weight showed that on average at 12 months the NET‐EN group gained 0.37 kilograms less than the DMPA group, but this was not statistically significant (95% CI ‐0.33;1.07 p=0.30). By 24 months the WHO study showed equal (nonsignificant) increases in body weight in women in the groups, resulting in no difference between groups (95%CI ‐1.39;1.39 p=1.00). Mean changes in systolic and diastolic blood pressure at 12 months did not differ significantly between the studies. The Salem study did show a significant difference in decrease in systolic blood pressure between the groups, but the larger WHO study carried more weighting, resulting in no significant change.
Discussion
We identified two randomized controlled trials which were included in the review. The WHO study contained a larger sample size of 2376 compared to the study conducted by Salem in Egypt which had a sample size of 400. The Salem study had a loss to follow up of 32.5%, compared to a loss to follow up of 1% in the WHO trial. Blinding after allocation was not possible in trials of this nature, due to the fact that the treatments differed in frequency of administration, and this could introduce information bias to the results. Although indications are that the included studies were well conducted with appropriate randomization and record keeping with menstrual diaries, they were both performed over a decade ago, and all attempts to contact researchers were unsuccessful, so that analysis was performed on the published data alone.
Results showed no difference in the risk of discontinuation between the groups of IPC users after one year. After two years, there is only a 3% difference, the DPMA group being more likely to discontinue. However, there were differences for discontinuation due to personal reasons. These results should be viewed in the context that the WHO study was performed in 13 centres, with differences between the groups according to social and cultural practices. One must consider that discontinuation risks are dependant on many factors, and should be interpreted with caution (Gallo 2008). Deviations from normal menstrual bleeding patterns may exert influence on women's lives in certain cultural and religious groups (Best 1998); and menstrual experiences and beliefs may influence choices of family planning methods (Severy 1993). A difference of menstrual bleeding patterns between the two IPCs would influence the reason for discontinuation due to amenorrhoea. Cessation of menstrual bleeding might be culturally unacceptable in certain groups of women, where menstrual bleeding may be viewed as proof of continued fertility. Personal reasons for discontinuation may also be associated with the demographic profiles of the study subjects from the various centres. This could influence the risk of discontinuation after 2 years.
Both intervention groups experienced episodes of menstrual bleeding and spotting. There is no significant difference between the groups in the proportion of women experiencing these after one or two years. There was no difference between the groups in the mean duration of bleeding and spotting episodes. However, the DPMA group were found to have significantly more amenorrhoea than the NET‐EN group after one and two years, with little change from one year to two years. It must be made clear that women in both groups equally experienced some bleeding and spotting and did not differ in the actual duration of these episodes. However, women on DPMA were more likely to eventually become amenorhhoeic than those in the NET‐EN group. This finding regarding amenorrhoea is in fact the only clinical difference that was found on analysis.
In response to the demand for injectable contraceptives with less menstrual bleeding disturbances, once‐a‐month injectable contraceptives containing a combination of progesterone and oestrogen have been developed, which offer a higher risk of regular bleeding patterns. However, it is interesting to note that in these combination contraceptives, menstrual disturbances remain a leading medical reason for discontinuation (Newton 1994). It has also been documented that disruption of patterns of vaginal bleeding with implantable progesterone contraceptives is almost inevitable (Hickey 2002), and irregular and prolonged bleeding, and amenorrhoea are common. Therefore if injectable progesterone contraceptives are the method of choice, then the choice between DPMA and NET‐EN in terms of probable menstrual bleeding effects becomes important.
While changes in changes of mean body weight and blood pressure are often considered as factors in hormonal contraception, analysis showed no statistically significant differences between the groups after one year of use, and the WHO study shows that even after two years there was no difference at all in weight gain between the groups, and the weight change that was recorded did not exceed 3.5 kilograms. Further, there were no significant differences between the groups regarding changes in either systolic or diastolic blood pressure, and the size of the weighted mean differences measured in mm Hg were 2.31 for systolic and 0.58 for diastolic blood pressure. It is important to note that there is no differences between the groups, and further that the mean changes in blood pressure are very small when applied to the clinical situation and do not constitute a risk to the patient.
Authors' conclusions
Implications for practice.
The difference in discontinuation risks between DPMA and NET‐EN is very small and the users differ in their discontinuation of these injectable contraceptives for personal reasons alone. It is further important to note that there is no difference at all between the discontinuation of the contraceptive because of accidental pregnancy. Although episodes of spotting or bleeding are the same for both groups, DPMA carries a higher risk of amenorrhoea than NET‐EN and may be recommended to women who prefer minimal menstrual bleeding. Changes in body weight and blood pressure do not differ between the two groups, and are small and therefore not clinically significant. There is not sufficient data to compare the groups regarding other minor effects including headache, nausea, dizziness or loss of libido; neither is there data to compare the major effects of vaginal shedding or increased susceptibility to HIV and other sexually transmitted infections.
Implications for research.
There are no recent trials comparing the clinical effects of the injectable progesterone contraceptives, although they remain in wide use in some developing countries. Considering their continued use in these countries, further research on this method of contraception and its relation to the HIV epidemic is needed. Further research to address health provider attitudes towards the use of either DPMA or NET‐EN as a reason for variation of use would assist in health systems planning.
What's new
| Date | Event | Description |
|---|---|---|
| 7 March 2012 | Amended | Additional table linked to the text |
History
Protocol first published: Issue 2, 2005 Review first published: Issue 3, 2006
| Date | Event | Description |
|---|---|---|
| 14 April 2008 | Amended | Converted to new review format. |
| 24 May 2006 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Carol Manion of Family Health International assisted with the literature search.
Data and analyses
Comparison 1. DMPA vs NET‐EN.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Discontinuation rates | 1 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 Discontinuation at 12 months | 1 | 400 | Risk Difference (M‐H, Random, 95% CI) | ‐0.12 [‐0.21, ‐0.03] |
| 1.2 discontinuation at 24 months | 0 | 0 | Risk Difference (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Reasons for discontinuation at 12 months | 2 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Accidental pregnancy | 2 | 2776 | Risk Difference (M‐H, Random, 95% CI) | ‐0.00 [‐0.01, 0.00] |
| 2.2 Amenorrhoea | 2 | 2776 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.06, 0.09] |
| 2.3 Bleeding problems | 2 | 2776 | Risk Difference (M‐H, Random, 95% CI) | ‐0.01 [‐0.07, 0.05] |
| 2.4 Other medical reasons | 2 | 2776 | Risk Difference (M‐H, Random, 95% CI) | ‐0.02 [‐0.05, 0.01] |
| 2.5 Personal reasons | 2 | 2676 | Risk Difference (M‐H, Random, 95% CI) | ‐0.04 [‐0.07, ‐0.01] |
| 3 Proportion of women with bleeding / spotting | 2 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 3.1 Bleeding / spotting at 12 months | 2 | 2577 | Risk Difference (M‐H, Random, 95% CI) | 0.02 [‐0.02, 0.06] |
| 3.2 Bleeding / spotting at 24 months | 1 | 1618 | Risk Difference (M‐H, Random, 95% CI) | 0.01 [‐0.00, 0.02] |
| 4 Duration of bleeding and spotting episodes | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 duration of bleeding and spotting episodes at 12 months | 1 | 1162 | Mean Difference (IV, Random, 95% CI) | 0.40 [‐0.43, 1.23] |
| 4.2 Duration of bleeding and spotting episodes at 24 months | 1 | 604 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.51, 0.91] |
| 5 Amenorrhoea | 2 | Risk Difference (M‐H, Random, 95% CI) | Subtotals only | |
| 5.1 Amenorrhoea at 12 months | 2 | 1498 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.08, 0.35] |
| 5.2 Amenorrhoea at 24 months | 1 | 698 | Risk Difference (M‐H, Random, 95% CI) | 0.21 [0.14, 0.29] |
| 6 Mean increase in body weight | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Increase in weight at 12 months | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.33, 1.07] |
| 6.2 Increase in weight at 24 months | 1 | 604 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐1.39, 1.39] |
| 7 Mean decrease in blood pressure | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Decrease in systolic blood pressure | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐2.31 [‐8.79, 4.16] |
| 7.2 Decrease in diastolic blood pressure | 2 | 1363 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.82, 0.66] |
1.1. Analysis.
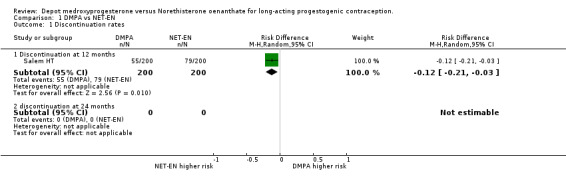
Comparison 1 DMPA vs NET‐EN, Outcome 1 Discontinuation rates.
1.2. Analysis.
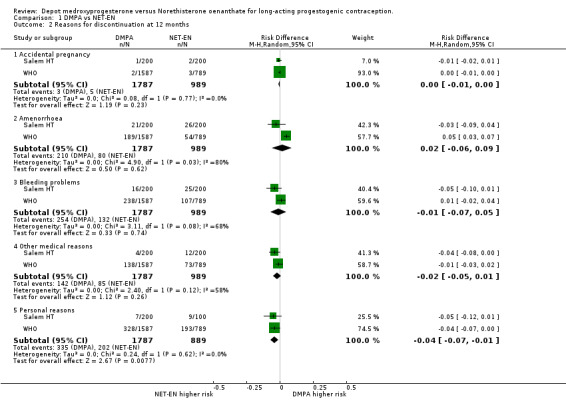
Comparison 1 DMPA vs NET‐EN, Outcome 2 Reasons for discontinuation at 12 months.
1.3. Analysis.
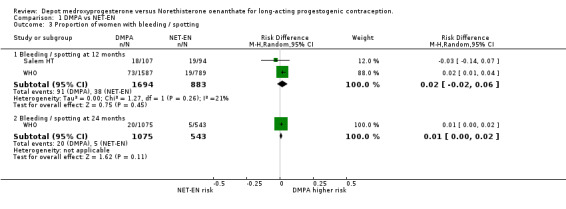
Comparison 1 DMPA vs NET‐EN, Outcome 3 Proportion of women with bleeding / spotting.
1.4. Analysis.
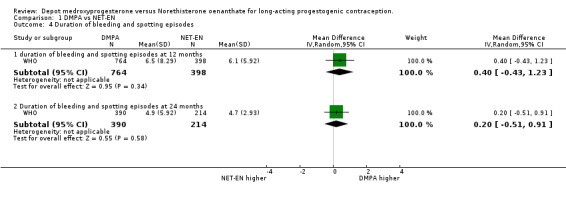
Comparison 1 DMPA vs NET‐EN, Outcome 4 Duration of bleeding and spotting episodes.
1.5. Analysis.
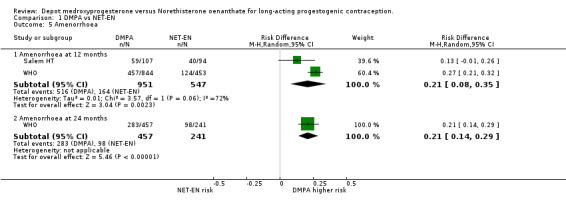
Comparison 1 DMPA vs NET‐EN, Outcome 5 Amenorrhoea.
1.6. Analysis.
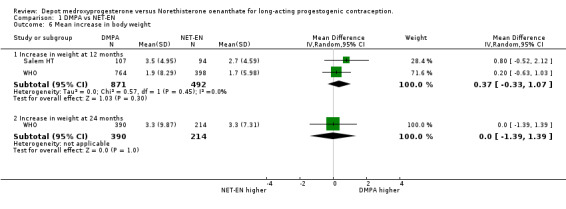
Comparison 1 DMPA vs NET‐EN, Outcome 6 Mean increase in body weight.
1.7. Analysis.
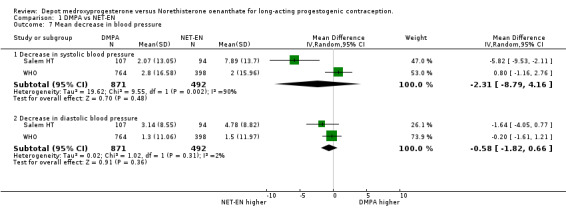
Comparison 1 DMPA vs NET‐EN, Outcome 7 Mean decrease in blood pressure.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Salem HT.
| Methods | Women were recruited from among those attending a Family Planning centre. A randomisation table was prepared by the WHO Special Program for Research & Development in Human reproduction. Women were allocated by picking a sealed envelope that contained a number assigning her one of the two methods Loss to follow up was fairly large at 32.5% | |
| Participants | Women who attended a family planning clinic. Their mean age was 32.4 +/‐ 5.7 (DPMA) and 33.1 +/‐ 5.0 (NET‐EN) Mean parity was 6.2 +/‐ 2.9 (DPMA) and 6.6 +/‐ 2.3 (NET‐EN) Religion of the sample was 78.5% Moslem and 21.5% Christian. Residential distribution was 52% urban and 48% rural Inclusion criteria for the study were: ‐ 18 ‐ 40 years ‐ proven fertility ‐ regular menstrual cycles ‐ willingness to comply to use and follow up Exclusion criteria for the study: ‐ breast feeding ‐ past cardiovascular disease ‐ past liver disease ‐ breast or genital malignancy ‐ uterine fibroids ‐ suspected pregnancy All participants underwent a physical examination and were given information about the contraceptive method prior to acceptance. Pregnancy was excluded by pelvic examination, pregnancy test or ultrasonography. | |
| Interventions | There were two treatment groups: (1) DPMA 150 mg every 3 months comprising of 200 women and (2) NET‐EN 200 mg every 2 months comprising of 200 women. The intervention was continued and followed up for one year. | |
| Outcomes | *Discontinuation rates Total rates and reasons for discontinuation (1) Pregnancy (2) Bleeding (3) Amenorrhoea (3) Other medical reasons (4) Other personal reasons (5) Planned pregnancy * Menstrual complaints (1) None (2) Amenorhhoea (3) Irregular bleeding (4) Spotting (5) Heavy bleeding (6) Oligo‐ / hypomenorrhoea * Changes in blood pressure * Changes in body weight | |
| Notes | The pregnancy rate was influenced by women supplying false data about the date of their last menstruation, and would have been lower. The most important causes of discontinuation were amenorrhoea and bleeding problems The study was upported by, but not part of the WHO multicentre trial. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
WHO.
| Methods | Trial conducted by the WHO Special Programme of Research, Development & Research Training in Human Reproduction. It was a clinic based multicentred Phase III trial of 3 regimens at 13 centres, in 9 developing and 4 developed countries. The participants were non‐breastfeeding women who were randomly allocated and followed up for 2 years. Loss to follow up is listed as 1% of the study sample | |
| Participants | Women who were not breastfeeding and chose IPC as contraceptive of choice. Their mean age was 27.4 +/‐ 5.2 years; mean parity was 3.3; mean interval since last birth was 4.4 +/‐ 1.7 months; mean body weight 55.5 +/‐ 12.1 kg. All participants underwent a medical history and examination, and cervical cytology on entry into the trial | |
| Interventions | There were 3 treatment groups: 1. DPMA every 90 days 2. NET‐EN every 60 days 3. NET‐EN every 60 days for 6 months, then every 84 days for the rest of the 2 years of follow up. Only the results for 1. & 2. are included in this review. Injection was given within 5 days of the menstrual cycle. Follow up was done over two years The DPMA group consisted of 1587 participants, and the NET‐EN group of 789 participants. | |
| Outcomes | * Discontinuation rates: Total rates and reasons for discontinuation: (1) Accidental pregnancy (2) Amenorrhoea (3) Bleeding problems (Table 1) (4) Other medical reasons, including ‐ abdominal distention or discomfort ‐ weight gain ‐ anxiety/depression ‐ fatigue ‐ dizziness ‐ headaches ‐ decreased libido ‐ hypertension (5) Cervical neoplasia discontinuation rates * Number & duration of menstrual bleeding / spotting * Changes in blood pressure * Changes in body weight | |
| Notes | The sample comprised a total of 3429 women‐years There was local variability of discontinuation rates, according to different centres, which may have been related to culturally determined tolerance of menstrual disturbances, and attitudes of clinic staff towards injectable contraception. It is stated that logistic and economic advantage must be considered when viewing the results of the individual centres. There were 2 cases of cervical neoplasia and 4 deaths, that could not be related to drug use | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdel‐Sayed WS | Randomisation is not specified. The outcomes are metabolic and hormonal changes that are not the outcomes for this review. Gonadotrophin inhibition is discussed as the mechanism for ovulation inhibition, but no conclusions are drawn. |
| Aly FA | The study subjects were not randomised into the two treatment groups. A random sample was selected of two groups who used either depot mederoxyprogesterone acetate or norethisterone oenanthate, and data collected from each group. Efforts to contact the researchers were unsuccessful. |
| Beksinska M | The study subjects were not randomised on recruitment. The users all had at least one year of use of contraceptive on commencement of the study. |
| Fotherby K | It is not clear whether randomisation took place. The authors were contacted with no success. Furthermore, the outcomes were not applicable to the review. Return to ovuation function was recorded but did not apply to discontinuation, because pregnancy as a reason for discontinuation did not necessarily coincide with return to ovulation. |
| Gray RH | It does not specify in which country this study was conducted. Publication was in 1981 around the time of the WHO study to which it refers. The interventions are not the same as the review for Norethisterone. This study looks at Norethisterone every 12 weeks, the same time interval as the Depotmedroxyprogesterone intervention, instead of every 2 months as specified by this review. |
| Janjua S | It is unclear whether randomisation took place. Mean variables of the two study groups indicate that this was not accomplished. |
| Swenson I | The intervention in the Norethisterone group differed from the type of intervention stipulated in this review. The second Norethisterone dose was given at 10 weeks after the first, and the subsequent doses were given at 12 week intervals. |
Characteristics of ongoing studies [ordered by study ID]
Beksinska M part 2.
| Trial name or title | Bone mineral density in women using depot‐medroxyprogesterine acetate, norethisterone enenthate or combines oral contraceptives for contraception |
| Methods | |
| Participants | |
| Interventions | Oral contraception |
| Outcomes | |
| Starting date | |
| Contact information | |
| Notes |
Contributions of authors
Beverly Draper has written the protocol for the review, performed the search and identification of studies and written up the final review
Chelsea Morroni has served in an advisory capacity in writing the data analysis for the protocol and for changes to the protocol after the first distribution for comment. She has assisted in identifying outcomes for analysis.
Margaret Hoffman has initiated the review from her current research, compiled the review team and advised on protocol and review changes. She supplied the information on local research and findings on the use of injectable contraceptives that has provoked interest in the research.
Jennifer Smit was the original initiator of the review during the writing of her PhD on this topic and contacted Professor Hoffman in this regard. She supplied some of the original references for the protocol background.
Mags Beksinska has served in an advisory capacity on the research question, on the strength of her research on injectable contraceptives. She has performed a study including injectable contraceptives.
Janet Hapgood has provided the biochemical information on the differences between the injectable progestogen contraceptives, and thereby aided the identification of major and minor effects to be included in the protocol.
Lize van der Merwe assisted with the extraction of data, statistical analysis of the outcomes of the identified studies, both in the conversion of the published data and in the meta‐analyses, and commented and assisted with the final draft of the review.
Sources of support
Internal sources
School of Public Health, University of Cape Town, South Africa.
South African Cochrane Centre, SA Medical Research Council, South Africa.
External sources
Africa Centre for Population Studies and Reproductive Health, South Africa.
Declarations of interest
No conflict of interest exists in the research for this review.
Edited (no change to conclusions)
References
References to studies included in this review
Salem HT {published data only}
- Salem HT, Salah M, Aly MY, Thabet AI, Shabaan MM, Fathalla MF. Acceptability of injectable contraceptives in Assuit, Egypt. Contraception 1988;38:697‐710. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
WHO {published data only}
- World Health Organisation. Multinational comparative clinical trial of long‐acting injectable contraceptives. Contraception 1983;28:1‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdel‐Sayed WS {published data only}
- Abdel‐Sayed WS, Toppozoda HK, Said SA, El‐Sayed OK. Some metabolic and hormonal changes in women using long acting injectable contraceptives. Alexandria Journal of Pharmaceutical Sciences 1989;3:29‐32. [MEDLINE: ] [PubMed] [Google Scholar]
Aly FA {published data only}
- Aly FA, El‐genedi MM, Toppozoda HK, El‐abd M, Loutfi I. Acceptability study of the two versus three monthly injectable contraceptives. Population Studies 1984;11:27‐39. [MEDLINE: ] [PubMed] [Google Scholar]
Beksinska M {published data only}
- Beksinska M, Rees HV. Vaginal discharge: a perceived side‐effect and minor reason for discontinuation in hormone injectable users in South Africa. African Journal of Reproductive Health 2001;5:84‐88. [MEDLINE: ] [PubMed] [Google Scholar]
Fotherby K {published data only}
- Fotherby K, Saxena B N, Shrimanker K, Hingorani V, Taker D, Diczfaulsy E, Landgren BM. A preliminary pharmacokinetic and pharmacodynamic evaluation of depot‐medroxyprogesterone acetate and norethisterone oenathate. Fertility and Sterility 1980;34:131‐139. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gray RH {published data only}
- Gray RH, Parker RA, Diethelm P. Vaginal bleeding disturbances associated with the discontinuation of long‐acting injectable contraceptives. British Journal of Obstetrics and Gynaecology 1981;88:317‐321. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Janjua S {published data only}
- Janjua S. Comparative trials of Depo‐provera vs Norigest. National Research Institute of Fertility Control, Eleventh Seminar on Research in Population Planning. Karachi: NRIFC, 1983:116‐31. [MEDLINE: ]
Swenson I {published data only}
- Swenson I, Khan AR, Jahan FA. A randomised, single blind comparative trial of norethindrone anethate and depot‐medroxyprogesterone acetate in Bangladesh. Contraception 1980;21:207‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Beksinska M part 2 {published data only}
- Bone mineral density in women using depot‐medroxyprogesterine acetate, norethisterone enenthate or combines oral contraceptives for contraception. Ongoing study Starting date of trial not provided. Contact author for more information.
Additional references
Best 1998
- Best K. Menstrual changes influence method use. Network 1998;19:4‐9. [PubMed] [Google Scholar]
DoH 1999
- Department of Health of South Africa. Demographic and Health Survey 1998. Medical Research Council, and Macro International. South Africa Demographic and Health Survey 1998 Preliminary Report. 1999..
Gallo 2008
- Gallo M, Grimes D, Schulz K, d'Arcangues C, Lopez L. Combination injectable contraceptives for contraception. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858] [DOI] [PubMed] [Google Scholar]
Guillebaud 1993
- Guillebaud J. Contraception: Your questions answered. Contraception: Your questions answered. Second Edition. New York: Churchill Livingstone, 1993:261‐285. [Google Scholar]
Hickey 2002
- Hickey M, d'Arcangues C. Vaginal bleeding disturbances and implantable contraceptives. Contraception 2002;65:75‐84. [DOI] [PubMed] [Google Scholar]
Lande 1995
- Lande RE. New Era for Injectables. Population Information Program, Center for Communication Programs, Johns Hopkins School of Hygiene and Public Health 1995; Vol. Population Reports, Series K, No 5.
Newton 1994
- Newton JR, D'arcangues C, Hall PE. A review of "once‐a‐month" combined injectable contraceptives. Journal of Obstetrics & Gynaecology 1994;4:S1‐34. [DOI] [PubMed] [Google Scholar]
Sapire 1990
- Sapire KE. Contraception and sexuality in Health and Disease. In: Revised, adapted by Belfield T, Guillebaud J editor(s). Contraception and Sexuality in Health and Disease. First Edition. Johannesburg: McGraw‐Hill, 1990:139‐164. [Google Scholar]
Severy 1993
- Severy LJ, Thapa S, Askew I, Glor J. Menstrual experiences and beliefs: a multicountry study of relationships with fertility and fertility regulating methods. Womens Health 1993;20:1‐20. [DOI] [PubMed] [Google Scholar]
Smit 2000
- Smit J, Gray A, McFadyen L. Cost implications of injectable contraceptive utilisation patterns in South Africa.. Abstracts of the Joint Meeting of VII World Conference on Clinical Pharmacology and Therapeutics IUPHAR ‐ Division of Clinical Pharmacology & 4th Congress of the European Association for Clinical Pharmacology and Therapeutics (EACPT).. Glasgow: Blackwell Science, 2003:93.


