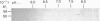Abstract
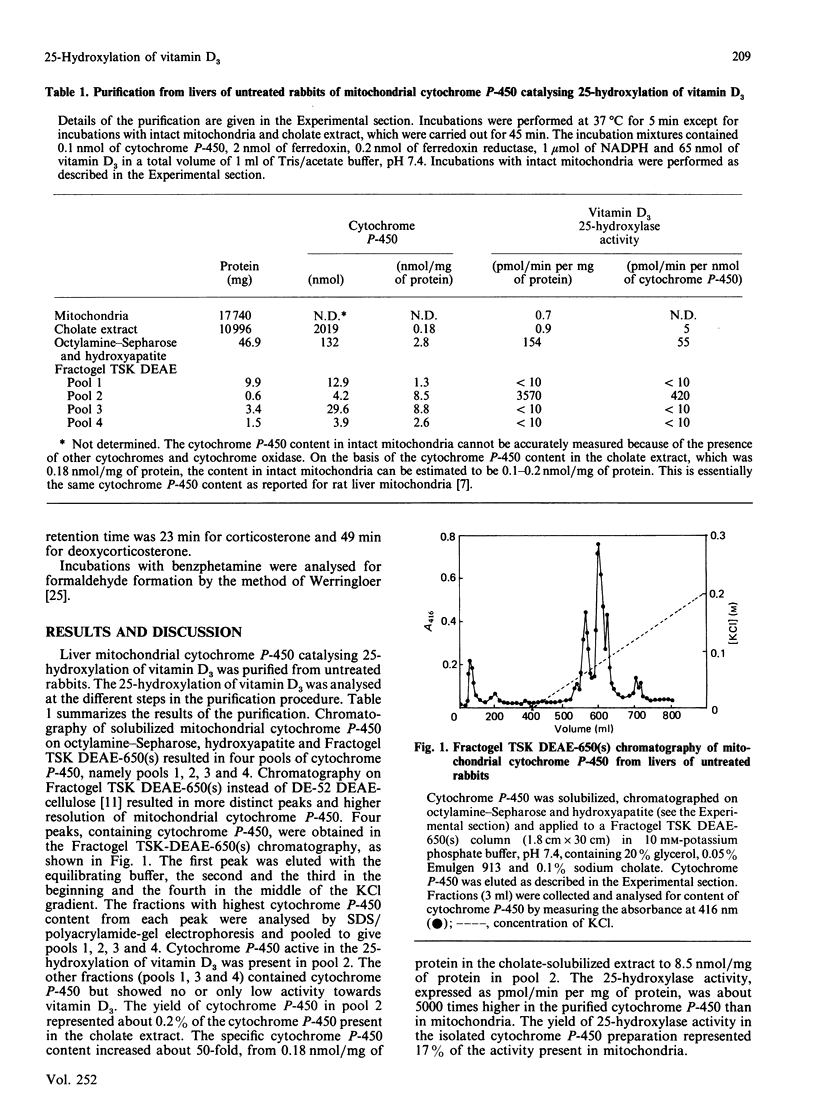
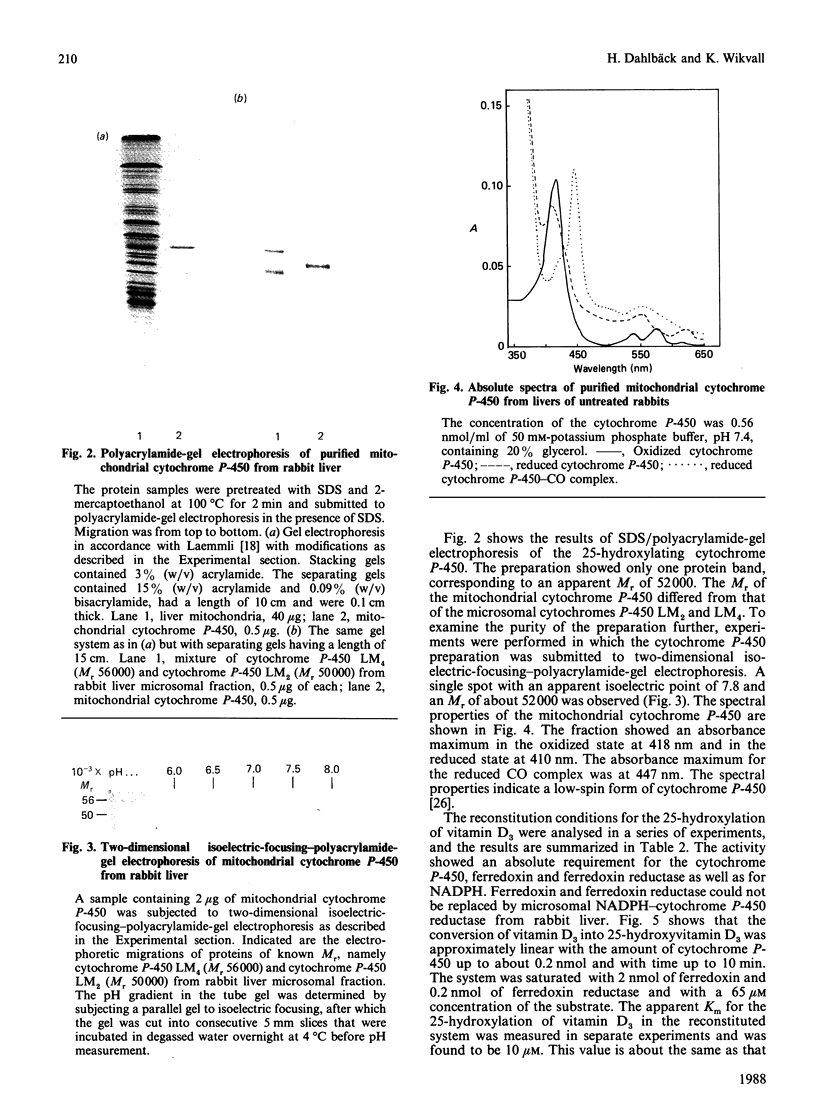
A cytochrome P-450 catalysing 25-hydroxylation of vitamin D3 was purified from liver mitochondria of untreated rabbits. The enzyme fraction contained 9 nmol of cytochrome P-450/mg of protein and showed only one protein band with an apparent Mr of 52,000 upon SDS/polyacrylamide-gel electrophoresis. The preparation showed a single protein spot with an apparent isoelectric point of 7.8 and an Mr of approx. 52,000 upon two-dimensional isoelectric-focusing-polyacrylamide-gel electrophoresis. The purified cytochrome P-450 catalysed 25-hydroxylation of vitamin D3 up to 5000 times more efficiently than did the mitochondria. The cytochrome P-450 required both ferredoxin and ferredoxin reductase for catalytic activity. Microsomal NADPH-cytochrome P-450 reductase could not replace ferredoxin and ferredoxin reductase. The cytochrome P-450 catalysed, in addition to 25-hydroxylation of vitamin D3, the 25-hydroxylation of 1 alpha-hydroxyvitamin D3 and the 26-hydroxylation of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol. The enzyme did not catalyse side-chain cleavage of cholesterol, 11 beta-hydroxylation of deoxycorticosterone, 1 alpha-hydroxylation of 25-hydroxyvitamin D3, hydroxylations of lauric acid and testosterone or demethylation of benzphetamine. The results raise the possibility that the 25-hydroxylation of vitamin D3 and the 26-hydroxylation of C27 steroids are catalysed by the same species of cytochrome P-450 in liver mitochondria. The possible role of the liver mitochondrial cytochrome P-450 in the metabolism of vitamin D3 is discussed.
Full text
PDF



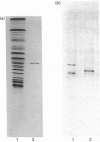
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson S., Holmberg I., Wikvall K. 25-hydroxylation of C27-steroids and vitamin D3 by a constitutive cytochrome P-450 from rat liver microsomes. J Biol Chem. 1983 Jun 10;258(11):6777–6781. [PubMed] [Google Scholar]
- Andersson S., Jörnvall H. Sex differences in cytochrome P-450-dependent 25-hydroxylation of C27-steroids and vitamin D3 in rat liver microsomes. J Biol Chem. 1986 Dec 25;261(36):16932–16936. [PubMed] [Google Scholar]
- Bhattacharyya M. H., DeLuca H. F. Subcellular location of rat liver calciferol-25-hydroxylase. Arch Biochem Biophys. 1974 Jan;160(1):58–62. doi: 10.1016/s0003-9861(74)80008-1. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Holmberg I. Assay and properties of a mitochondrial 25-hydroxylase active on vitamine D3. J Biol Chem. 1978 Feb 10;253(3):842–849. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I., Kristiansen T., Pedersen J. I. Assay of 1,25-dihydroxy vitamin D3 by isotope dilution--mass fragmentography. Clin Chem. 1979 Apr;25(4):584–588. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I., Oftebro H., Pedersen J. I. Properties of a reconstituted vitamin D3 25-hydroxylase from rat liver mitochondria. J Biol Chem. 1980 Jun 10;255(11):5244–5249. [PubMed] [Google Scholar]
- Björkhem I., Holmberg I. On the 25-hydroxylation of vitamin D3 in vitro studied with a mass fragmentographic technique. J Biol Chem. 1979 Oct 10;254(19):9518–9524. [PubMed] [Google Scholar]
- Boström H., Hansson R., Jönsson K. H., Wikvall K. Cytochromes P-450 LM3b and LM4 in biosynthesis of bile acids. Eur J Biochem. 1981 Nov;120(1):29–32. doi: 10.1111/j.1432-1033.1981.tb05665.x. [DOI] [PubMed] [Google Scholar]
- Chu J. W., Kimura T. Studies on adrenal steroid hydroxylases. Molecular and catalytic properties of adrenodoxin reductase (a flavoprotein). J Biol Chem. 1973 Mar 25;248(6):2089–2094. [PubMed] [Google Scholar]
- Cronholm T., Johansson G. Oxidation of 5 beta-cholestane-3alpha, 7alpha, 12alpha-triol by rat liver microsomes. Eur J Biochem. 1970 Oct;16(2):373–381. doi: 10.1111/j.1432-1033.1970.tb01091.x. [DOI] [PubMed] [Google Scholar]
- Cuatrecasas P. Protein purification by affinity chromatography. Derivatizations of agarose and polyacrylamide beads. J Biol Chem. 1970 Jun;245(12):3059–3065. [PubMed] [Google Scholar]
- Dahlbäck H., Wikvall K. 25-hydroxylation of vitamin D3 in rat liver: roles of mitochondrial and microsomal cytochrome P-450. Biochem Biophys Res Commun. 1987 Feb 13;142(3):999–1005. doi: 10.1016/0006-291x(87)91513-0. [DOI] [PubMed] [Google Scholar]
- DeLuca H. F. The transformation of a vitamin into a hormone: the vitamin D story. Harvey Lect. 1979 1980;75:333–379. [PubMed] [Google Scholar]
- Gibson G. G., Orton T. C., Tamburini P. P. Cytochrome P-450 induction by clofibrate. Purification and properties of a hepatic cytochrome P-450 relatively specific for the 12- and 11-hydroxylation of dodecanoic acid (lauric acid). Biochem J. 1982 Apr 1;203(1):161–168. doi: 10.1042/bj2030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson R., Holmberg I., Wikvall K. 25-Hydroxylation vitamin D3 and side chain hydroxylations of 5 beta-cholestane-3 alpha, 7 alpha, 12 alpha-triol by purified rabbit and rat liver microsomal cytochromes P-450. J Biol Chem. 1981 May 10;256(9):4345–4349. [PubMed] [Google Scholar]
- Haugen D. A., Coon M. J. Properties of electrophoretically homogeneous phenobarbital-inducible and beta-naphthoflavone-inducible forms of liver microsomal cytochrome P-450. J Biol Chem. 1976 Dec 25;251(24):7929–7939. [PubMed] [Google Scholar]
- Hayashi S., Noshiro M., Okuda K. Isolation of a cytochrome P-450 that catalyzes the 25-hydroxylation of vitamin D3 from rat liver microsomes. J Biochem. 1986 Jun;99(6):1753–1763. doi: 10.1093/oxfordjournals.jbchem.a135653. [DOI] [PubMed] [Google Scholar]
- Holmberg I., Berlin T., Ewerth S., Björkhem I. 25-Hydroxylase activity in subcellular fractions from human liver. Evidence for different rates of mitochondrial hydroxylation of vitamin D2 and D3. Scand J Clin Lab Invest. 1986 Dec;46(8):785–790. doi: 10.3109/00365518609084051. [DOI] [PubMed] [Google Scholar]
- Holmberg I., Kristiansen T., Sturén M. Determination of 25-hydroxyvitamin D3 in serum by high performance liquid chromatography and isotope dilution-mass spectrometry. Scand J Clin Lab Invest. 1984 Jun;44(4):275–282. doi: 10.3109/00365518409083808. [DOI] [PubMed] [Google Scholar]
- Holmes R. P., Kummerow F. A. The relationship of adequate and excessive intake of vitamin D to health and disease. J Am Coll Nutr. 1983;2(2):173–199. doi: 10.1080/07315724.1983.10719923. [DOI] [PubMed] [Google Scholar]
- Huang J. J., Kimura T. Studies on adrenal steroid hydroxylases. Oxidation-reduction properties of adrenal iron-sulfur protein (adrenodoxin). Biochemistry. 1973 Jan 30;12(3):406–409. doi: 10.1021/bi00727a007. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lu A. Y., Junk K. W., Coon M. J. Resolution of the cytochrome P-450-containing omega-hydroxylation system of liver microsomes into three components. J Biol Chem. 1969 Jul 10;244(13):3714–3721. [PubMed] [Google Scholar]
- Madhok T. C., DeLuca H. F. Characteristics of the rat liver microsomal enzyme system converting cholecalciferol into 25-hydroxycholecalciferol. Evidence for the participation of cytochrome p-450. Biochem J. 1979 Dec 15;184(3):491–499. doi: 10.1042/bj1840491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. II. SOLUBILIZATION, PURIFICATION, AND PROPERTIES. J Biol Chem. 1964 Jul;239:2379–2385. [PubMed] [Google Scholar]
- Oftebro H., Saarem K., Björkhem I., Pedersen J. I. Side chain hydroxylation of C27-steroids and vitamin D3 by a cytochrome P-450 enzyme system isolated from human liver mitochondria. J Lipid Res. 1981 Nov;22(8):1254–1264. [PubMed] [Google Scholar]
- Pedersen J. I., Holmberg I., Björkhem I. Reconstitution of vitamin D3 25-hydroxylase activity with a cytochrome P-450 preparation from rat liver mitochondria. FEBS Lett. 1979 Feb 15;98(2):394–398. doi: 10.1016/0014-5793(79)80225-2. [DOI] [PubMed] [Google Scholar]
- Ryan D. E., Thomas P. E., Korzeniowski D., Levin W. Separation and characterization of highly purified forms of liver microsomal cytochrome P-450 from rats treated with polychlorinated biphenyls, phenobarbital, and 3-methylcholanthrene. J Biol Chem. 1979 Feb 25;254(4):1365–1374. [PubMed] [Google Scholar]
- Saarem K., Bergseth S., Oftebro H., Pedersen J. I. Subcellular localization of vitamin D3 25-hydroxylase in human liver. J Biol Chem. 1984 Sep 10;259(17):10936–10940. [PubMed] [Google Scholar]
- Saarem K., Pedersen J. I. 25-Hydroxylation of 1 alpha-hydroxyvitamin D-3 in rat and human liver. Biochim Biophys Acta. 1985 May 29;840(1):117–126. doi: 10.1016/0304-4165(85)90168-0. [DOI] [PubMed] [Google Scholar]
- Werringloer J. Assay of formaldehyde generated during microsomal oxidation reactions. Methods Enzymol. 1978;52:297–302. doi: 10.1016/s0076-6879(78)52031-4. [DOI] [PubMed] [Google Scholar]
- Wikvall K. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem. 1984 Mar 25;259(6):3800–3804. [PubMed] [Google Scholar]
- Wray W., Boulikas T., Wray V. P., Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981 Nov 15;118(1):197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]