Abstract
Objective
Dural arteriovenous fistula (dAVF) is generally treated by endovascular therapy, but transarterial embolization (TAE) carries the risk of potential complications, including distal migration of embolic material, brain infarction, and venous congestion. Intracranial hemorrhage is infrequent but remains a considerable concern.
Case Presentation
A man in the seventh decade presented with left hemiparesis. Brain MRI revealed right corona radiata infarction and incidentally identified a left transverse sigmoid sinus dAVF. Under a diagnosis of Borden type III and Cognard type IIb, an endovascular treatment plan was initiated. After an unsuccessful attempt at transvenous embolization, TAE with Onyx (Medtronic, Minneapolis, MN, USA) successfully resolved the dAVF. However, immediate post-treatment CT revealed subarachnoid hemorrhage, leading to decompressive craniotomy. Follow-up DSA showed no residual shunts, and the cause of the bleeding remained unknown.
Conclusion
Despite the unknown cause of bleeding, a thorough evaluation of preoperative hemodynamics and diligent postoperative examination is crucial in managing dAVF cases. Further pathological investigations are needed to gain a comprehensive understanding of such occurrences.
Keywords: subarachnoid hemorrhage, transarterial embolization, transvenous embolization, dural arteriovenous fistula, Onyx
Introduction
Dural arteriovenous fistula (dAVF) is a rare condition, with an estimated detection rate of 0.29 per 100000 persons per year (mean age: 62.7 years) in Japan.1) Despite advancements, dAVF remains shrouded in uncertainties, encompassing aspects such as its cause, natural history, and pathological conditions. The annual mortality rate for patients with dAVF exhibiting cortical venous reflux (CVR) is reported at 10.4%.2) Generally, endovascular therapy is the preferred approach for treating dAVF, with transvenous embolization (TVE) often chosen initially due to its proven safety and efficiency. However, recent developments have introduced transarterial embolization (TAE) with Onyx (Medtronic, Minneapolis, MN, USA, hereinafter called Onyx) as a viable treatment option.3,4) While various complications of TAE are recognized, including distal migration of embolic material, brain infarction, and venous congestion, intracranial hemorrhage is a rare occurrence, and subarachnoid hemorrhage (SAH) post-treatment has not been reported. In this study, we present a case involving a patient with transverse sigmoid sinus (TSS) dAVF who underwent unsuccessful TVE and experienced SAH leading to hematoma removal and decompressive craniectomy after a second treatment with TAE for the residual shunt.
Case Presentation
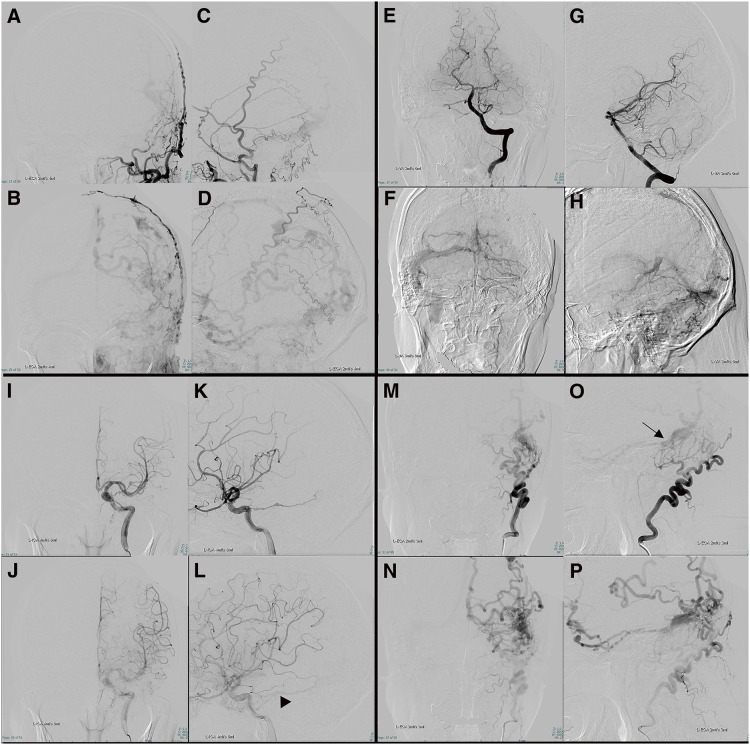
A man in the seventh decade suffered left hemiparesis and dysarthria. MRI showed acute brain infarction in the right corona radiata. dAVF was also detected incidentally in the left TSS. DSA demonstrated the left occipital artery (OA), left posterior auricular artery, left tentorial artery, left middle meningeal artery, and right OA as the feeders, and isolation of the left TSS. CVR was present through the sphenopetrosal vein and superficial middle cerebral vein to the superior sagittal sinus (SSS), and through the parietal cortical vein with venous ectasia to the SSS (Fig. 1). No aneurysms, pial supply feeder, arteriovenous malformation, or other dAVF was detected. The dAVF was diagnosed as Borden type Ⅲ5) and Cognard type Ⅱb.6) Venous echography of the lower extremities revealed deep venous thrombosis, leading to the administration of direct oral anticoagulation. The patient was discharged with improving physical function and a modified Rankin Scale (mRS) score of 1.
Fig. 1. Left external carotid (A–D), left vertebral (E–H), left internal carotid (I–L), and left OA (M–P) DSA before TVE (frontal and lateral view). Feeders are the OA, posterior auricular artery, middle meningeal artery, and tentorial artery. The left transverse sinus to the sigmoid sinus is isolated. CVR is present through the sphenopetrosal vein to the superficial middle cerebral vein, the Labbé vein to the Trolard vein with venous ectasia, and through the parietal cortical vein to the SSS. Internal carotid DSA also shows outflow from the tentorial artery to the sphenopetrosal vein (L: black arrowhead). Both internal carotid and vertebral artery DSA show veins with CVR not utilized for normal venous return and no shunt. The shunt point appeared diffuse in the left sigmoid sinus to the transverse sinus (O: black arrow). Internal carotid artery DSA shows a pseudophlebitic pattern and no avascular area. Right internal carotid, right external carotid, and right vertebral artery DSA depicted no shunt. CVR, cortical venous reflux; OA, occipital artery; SSS, superior sagittal sinus; TVE, transvenous embolization.
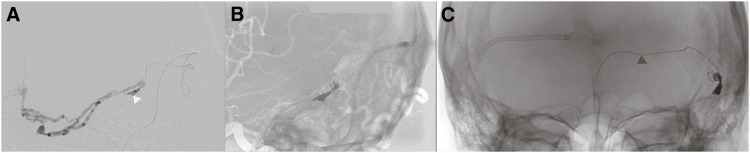
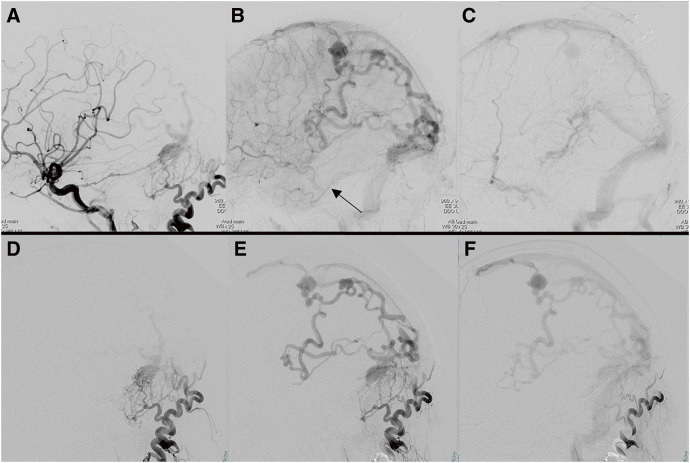
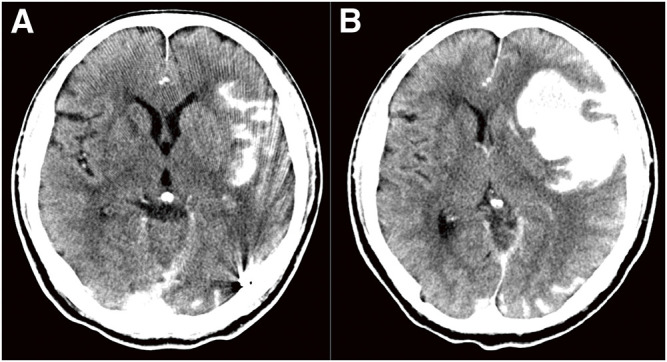
We planned to perform TVE penetrating the occluded left transverse sinus from the contralateral internal jugular vein via the confluence. A microcatheter was successfully inserted into the isolated left TSS, and several coils were placed (Fig. 2A and 2B). However, attempts to reposition the microcatheter led to a fracture of the microguidewire (Synchro SELECT Guidewire Soft; Stryker, Portage, MI, USA), resulting in a 10-cm long piece of broken wire left behind in the transverse sinus. Due to this complication, TVE could not proceed via the same route (Fig. 2C). Subsequent angiography post-TVE showed decreased shunt flow and a significant reduction in drainage through the sphenopetrosal vein (Fig. 3). Two weeks later, TAE was planned. A microcatheter was inserted through the left petrosquamous branch into the isolated sinus through the shunt point (Fig. 4A and 4B). Onyx 18 was injected under flow control with occlusion of the left OA using a balloon catheter. The isolated sinus was filled with Onyx without minimum migration into the cortical veins (Fig. 4C and 4D), resulting in the disappearance of the dAVF (Fig. 5). The final activated clotting time was 197 seconds, so heparinization was naturally reversed. The patient recovered well from general anesthesia. However, post-treatment CT revealed SAH (Fig. 6A). Protamine 50 mg was administered to reverse the effects of heparin. Despite this, an increase in SAH was observed (Fig. 6B). Emergency craniotomy was performed for hematoma removal with no obvious bleeding points detected. The patient was transferred to a rehabilitation hospital with an mRS score 4. Eight days after TAE, follow-up DSA showed no residual shunts.
Fig. 2. Intraoperative DSA during TVE. (A) The lateral view shows the microcatheter inserted into the left sigmoid sinus through the tortuous confluence (white arrowhead). (B) The lateral view shows a partially packed left sigmoid sinus with several coils. (C) Frontal view shows the fractured microguidewire (black arrowhead). TVE, transvenous embolization.
Fig. 3. Left common carotid (A–C) and left OA (D–F) DSA after TVE (lateral view). Drainage through the sphenopetrosal vein is markedly reduced (B: black arrow). Left OA DSA did not show the sphenopetrosal vein. OA, occipital artery; TVE, transvenous embolization.
Fig. 4. (A–D) Intraoperative DSA via microcatheter during TAE, frontal view (A and C), and lateral view (B and D). Microcatheter is inserted via the petrosquamous branch of the middle meningeal artery into the left transverse sinus through the shunt point (A and B, black arrowhead). Sphenopetrosal vein (A and B, black arrow). Microguidewire remnant after fracture (A and B, white arrowhead). Onyx cast front end (C and D, black arrow). (E) Illustration of the treatment progress. A: left transverse sinus, B: left sigmoid sinus, black arrow: approach direction during TVE, dotted line arrow: approach direction during TVE. CVR, cortical venous reflux; OA, occipital artery; PA, posterior auricular artery; TAE, transarterial embolization; TVE, transvenous embolization.
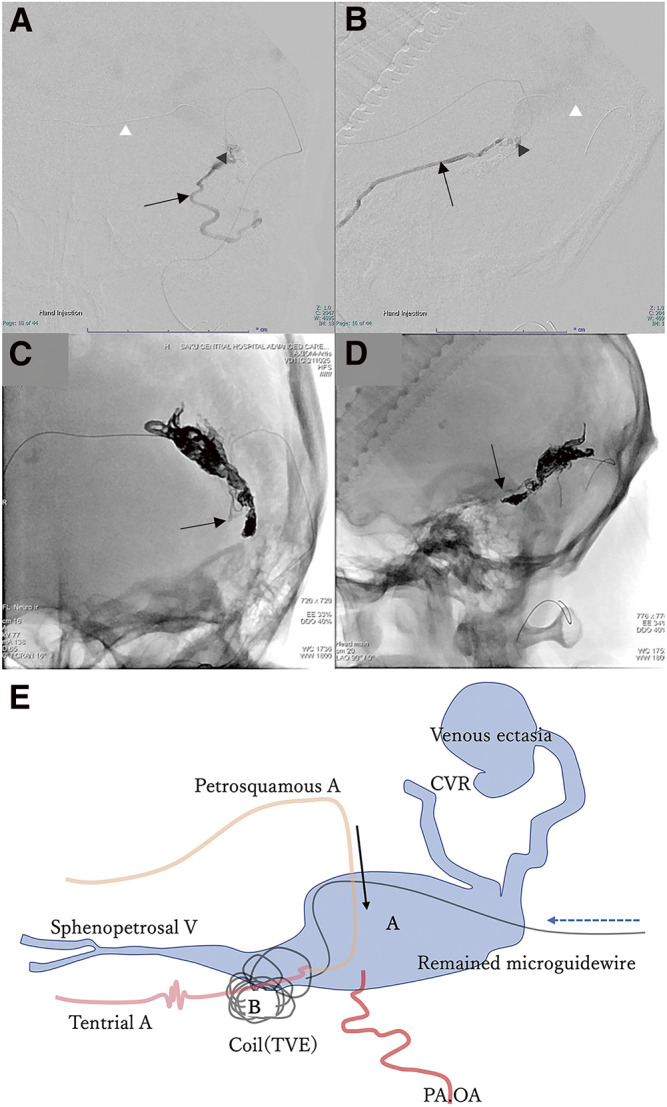
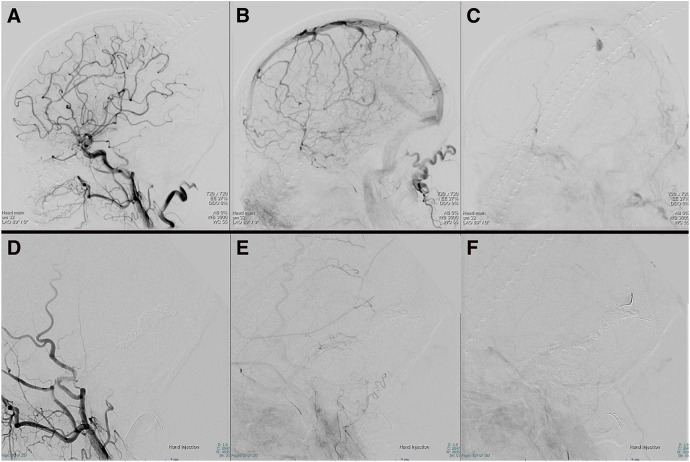
Fig. 5. Left common carotid artery DSA (A–C) and external carotid artery DSA without OA (D–F) after TAE (lateral view) show the disappearance of the CVR, sphenopetrosal vein, and shunt point. The contrast agent in the parietal venous ectasia was stagnant, and the blood flow in the superficial middle cerebral vein remained stagnant as before TAE. CVR, cortical venous reflux; OA, occipital artery; TAE, transarterial embolization.
Fig. 6. (A) CT scan revealing SAH in the Sylvian fissure, without any bleeding near the parietal venous ectasia. (B) The second CT scan 1 hour after (A) and injection of protamine 50 mg showed increasing hemorrhage and exacerbating brain shift. SAH, subarachnoid hemorrhage.
Discussion
Intracranial hemorrhage following endovascular therapy for dAVF is a rare occurrence, and its etiology remains challenging to ascertain. Multiple dAVFs are reported to occur in 8% of cases and have a high risk of hemorrhage.7) Similarly, dAVF with pial supply is reported to carry a high risk of hemorrhage during treatment.8,9) In our case, pial supply was unlikely to be the cause of SAH because of no pial supply and no bleeding near the sinus. The absence of a visible Onyx cast in the distal pathway and improved flow stasis in the cortical veins suggested that distal migration of embolic material or venous congestion did not account for the hemorrhage. Furthermore, potential causes such as partial obliteration of drainage pathways, residual venous drainage, or elevated intra-sinus pressure during the procedure were considered.10–12)
In this patient, postoperative angiography of the OA and internal carotid artery was not performed, so incomplete occlusion of the shunt may not have been identified. However, postoperative external carotid artery angiography, excluding the OA, did not reveal the sphenopetrosal vein connected to the sylvian vein (Fig. 5), which was observed before Onyx embolization and was suspected to be the source of bleeding. In addition, angiography of the OA after coil embolization did not detect the sphenopetrosal vein (Fig. 3). Furthermore, postoperative angiography of the common carotid artery showed the disappearance of the sphenopetrosal vein and tentorial artery, which had been observed preoperatively, and no significant change in Sylvian vein stagnation occurred before and after surgery. Considering the above findings, residual shunting is unlikely.
Hyperperfusion was considered, but none provided a definitive explanation. Moreover, delayed venous thrombosis is also conceivable; since heparinization was not reversed, and the level of consciousness gradually worsened after awakening from general anesthesia, the progression was acute, and the cause of the SAH remains unclear. Other possibilities include middle meningeal artery injury during catheter insertion or injury when withdrawing the catheter after Onyx injection. However, due to the absence of extravasation during and after the procedure and the lack of resistance during Onyx removal, these possibilities can be excluded.
This case underscores the necessity for further pathological investigation and emphasizes the importance of preoperative hemodynamic evaluation and meticulous postoperative monitoring for early diagnosis and intervention. Furthermore, the microguidewire (Synchro SELECT Guidewire Soft) fractured in this case. The cause of the wire breaking is likely to originate in the tortuous access route, which made it difficult to transmit torque to the microguidewire. As a result, excessive torque was applied, causing the microguidewire fracture. In addition, the structure of this type of microguidewire uses different materials for the tip and hand parts, which may have contributed to the fracture.
Conclusion
In conclusion, this case of TSS dAVF was effectively managed with TVE followed by TAE. However, post-treatment, CT revealed SAH of unknown origin, despite the disappearance of CVR and the shunt, and the absence of exacerbated venous congestion. Although the bleeding cause was unknown, evaluation of the preoperative hemodynamics of dAVF and postoperative close examination should be considered crucial. Further pathological investigations are needed for a comprehensive understanding of such cases.
Disclosure Statement
The authors report no conflicts of interest concerning the materials or methods used in this study or the findings specified in this paper.
References
- 1).Kuwayama N. Epidemiologic survey of dural arteriovenous fistulas in Japan: clinical frequency and present status of treatment. Acta Neurochir Suppl 2016; 123: 185–188. [DOI] [PubMed] [Google Scholar]
- 2).van Dijk JM, terBrugge KG, Willinsky RA, et al. Clinical course of cranial dural arteriovenous fistulas with long-term persistent cortical venous reflux. Stroke 2002; 33: 1233–1236. [DOI] [PubMed] [Google Scholar]
- 3).Cognard C, Januel AC, Silva NA, Jr, et al. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: new management using Onyx. AJNR Am J Neuroradiol 2008; 29: 235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4).Natarajan SK, Ghodke B, Kim LJ, et al. Multimodality treatment of intracranial dural arteriovenous fistulas in the Onyx era: a single center experience. World Neurosurg 2010; 73: 365–379. [DOI] [PubMed] [Google Scholar]
- 5).Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995; 82: 166–179. [DOI] [PubMed] [Google Scholar]
- 6).Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995; 194: 671–680. [DOI] [PubMed] [Google Scholar]
- 7).van Dijk JM, TerBrugge KG, Willinsky RA, et al. Multiplicity of dural arteriovenous fistulas. J Neurosurg 2002; 96: 76–78. [DOI] [PubMed] [Google Scholar]
- 8).Sato K, Matsumoto Y, Endo H, et al. A hemorrhagic complication after Onyx embolization of a tentorial dural arteriovenous fistula: a caution about subdural extension with pial arterial supply. Interv Neuroradiol 2017; 23: 307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9).Brinjikji W, Cloft HJ, Lanzino G. Clinical, angiographic, and treatment characteristics of cranial dural arteriovenous fistulas with pial arterial supply. J Neurointerv Surg 2021; 13: 331–335. [DOI] [PubMed] [Google Scholar]
- 10).Mochizuki Y, Iihoshi S, Tsukagoshi E, et al. A rare brainstem hemorrhage due to incomplete transvenous embolization of the cavernous sinus dural arteriovenous fistula: a case report. Radiol Case Rep 2021; 16: 2526–2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11).Lee RJ, Chen CF, Hsu SW, et al. Cerebellar hemorrhage and subsequent venous infarction followed by incomplete transvenous embolization of dural carotid cavernous fistulas: a rare complication. J Neurosurg 2008; 108: 1245–1248. [DOI] [PubMed] [Google Scholar]
- 12).Nakagawa I, Wada T, Nakagawa H, et al. A rare brainstem hemorrhage during transvenous embolization of a cavernous dural arteriovenous fistula. J Clin Neurosci 2012; 19: 589–592. [DOI] [PubMed] [Google Scholar]