Abstract
Bisphenol-A is one of the most studied endocrine-chemicals, which is widely used all over the world in plastic manufacture. Because of its extensive use, it has become one of the most abundant chemical environmental pollutants, especially in aquatic environments. BPA is known to affect fish reproduction via estrogen receptors but many studies advocate that BPA affects almost all aspects of fish physiology. The possible modes of action include genomic, as well as and non-genomic mechanisms, estrogen, androgen, and thyroid receptor-mediated effects. Due to the high detrimental effects of BPA, various analogs of BPA are being used as alternatives. Recent evidence suggests that the analogs of BPA have similar modes of action, with accompanying effects on fish physiology and reproduction. In this review, a detailed comparison of effects produced by BPA and analogs and their mode of action is discussed.
Keywords: Endocrine disrupting compounds, Bisphenol, Fish, Reproduction, oxidative stress, physiology
1. Introduction
Endocrine disrupting chemicals (EDCs) interfere with any aspect of hormone synthesis and action. These compounds have the affinity to bind with the hormone receptors and have the potential to incite or suppress the hormone synthesis /metabolism or their action. EDCs can bind to a variety of receptors including nuclear receptors, non-nuclear steroid and non-steroid receptors (receptors of neurotransmitters e.g. dopamine, serotonin, norepinephrine, etc.) and orphan receptors like aryl hydrocarbon receptor (Diamanti-Kandarakis et al., 2009; Goksøyr, 2006; Segner et al., 2003). EDCs not only act through hormone receptors but also exert their effect through epigenetic mechanisms by altering the expression of key genes involved in reproduction and normal development (Bhandari et al., 2015b).
One of the most studied EDCs is bisphenol A (BPA, 4,4’-iso-propylidene diphenol, CAS No. 80-05-7). BPA is a commercially important and highly produced chemical around the globe (Cantonwine et al., 2013). BPA was synthesized for the first time in 1891 by condensation of phenol and acetone (Vandenberg et al., 2007) and in 1952 the first plastic product was made with BPA (Vom Saal and Welshons, 2006). Because of its cross-linking properties it is an effective plasticizer (Alonso-Magdalena et al., 2006). Approximately around 90% of the total manufactured BPA is used for the production of plastic and plastic derived products including food and beverage containers, water and baby bottles, toys, impact-resistant eyeglass lenses, helmets and compact discs (Eladak et al., 2015). BPA is also used as an antioxidant and stabilizer during the manufacture of polyvinyl chloride (Staples et al., 1998). Other uses of BPA are in dental sealants as dimethacrylate (BIS-DMA), thermal paper (Biedermann et al., 2010; Mendum et al., 2011) and medical equipment (Testai et al., 2016). The first source of BPA exposure is through plastic and plastic made products while the second-largest source of BPA exposure is through thermal paper receipts (Sogorb et al., 2019) and according to “Regulation concerning the Registration, Evaluation, Authorization, and Restriction of Chemicals (REACH)” BPA will no longer be used in the thermal paper after 2020 (Wang et al., 2018a,b).
BPA has been listed as a class 1B reproductive toxicant by European CLP regulation (Sogorb et al., 2019). A significant correlation was observed in serum BPA levels and patients with dilated cardiomyopathy (Xiong et al., 2015) and polycystic ovary syndrome (Hossein Rashidi et al., 2017; Kandaraki et al., 2011). Recent evidence suggests that BPA also acts as a metabolic disrupting chemical, affecting organs such as the pancreas and adipose tissue (Rahmani et al., 2018) as well as causing metabolic syndromes, such as obesity (Santangeli et al., 2018). Given BPA has many negative physiological and behavioral health outcomes, many countries have now banned BPA in consumer products, especially baby bottles (Li et al., 2016). This led to the use of alternatives to BPA in plastic production. The available alternatives are listed in Table 1. The structure of these BPA analogs is similar to parent molecule (Fig. 1).
Table 1.
Name, molecular weight and formula of bisphenol-A and analogs.
| Name | Abbreviation | Chemical name | Linear formula | Molecular weight | CAS # |
|---|---|---|---|---|---|
| Bisphenol-A | BPA | 2,2-bis(4-hydroxyphenyl)propane | 4-(HO)C6H4]2C(CH3)2 | 228.29 | 80-05-7 |
| Bisphenol-F | BPF | 2,2-bis [4-hydroxyphenol] methane | CH2(C6H4OH)2 | 200.23 | 620-92-8 |
| Bisphenol-S | BPS | 2,2-bis [4-hydroxyphenol]sulfone | 4-(HO)C6H4]2SO2 | 250.27 | 80-09-1 |
| Bisphenol-AF | BPAF | 4, 4 ′-hexafluoroisopropylidene]diphenol | (CF3)2C(C6H4OH)2 | 336.23 | 1478-61-1 |
| Bisphenol-AP | BPAP | 4,4’-(1-phenylethylidene) bisphenol | CH3C(C6H5)(C6H4OH)2 | 290.36 | 1571-75-1 |
| Bisphenol-B | BPB | 2,2-bis(4-hydroxyphenyl)butane | C16H18O2 | 242.32 | 77-40-7 |
| Bisphenol-Z | BPZ | [4,4′-(cyclohexane-1,1-diyl)diphenol | C18H20O2 | 268.350 | 843-55-0 |
Fig. 1.
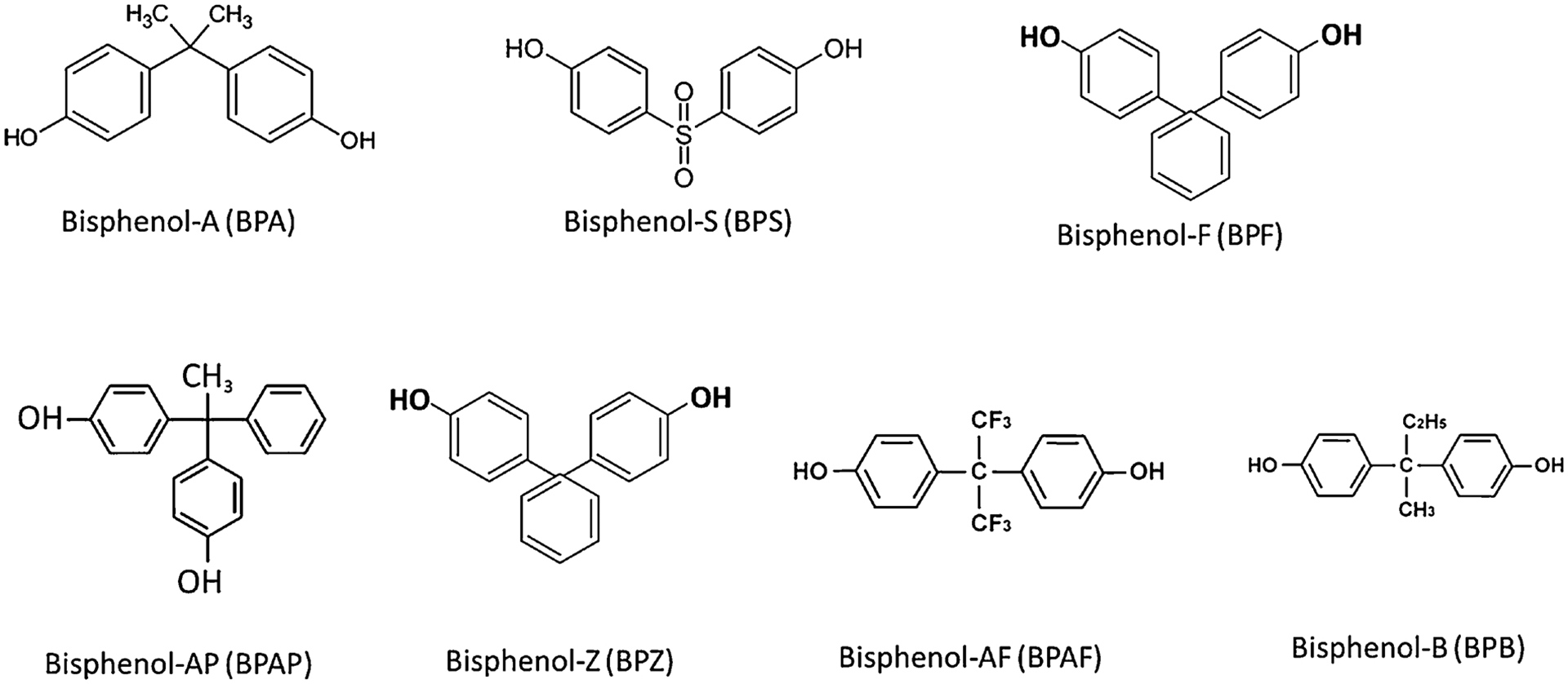
Structure of bisphenol-A and its analogs (Source: Sigma-Aldrich).
2. Detected levels of BPA and its analogs in the water
In the late 1990s researchers started reporting the detection and quantification of bisphenol-A in surface water (Corrales et al., 2015) after the methods were developed to measure them in the select specialized laboratories. Since then, BPA has been detected in various environmental samples, and the findings suggest the ubiquitous nature of its distribution (Bhandari et al., 2015a).
BPA has been detected in surface water samples collected from Sinos River basin, Rio, Brazil (n.d to 517 ng/L) (Peteffi et al., 2019), Rio das Velhas, Brazil (8.6 to 168.3 ng/L) (Moreira et al., 2011), Iguassu River, Brazil (0.62–12.6 l ng/L) (Froehner et al., 2011), surface water samples from Germany (0.0005–0.41 μg/L)(Fromme et al., 2002a). Up to 330 ng/L of BPA was detected in surface water analyzed from different areas of the Netherlands (Belfroid et al., 2002). Detected levels of BPA in surface water of the Langat River basin, Malaysia was up to 215 ng/L (Santhi et al., 2012). Surface water samples collected different rivers of Portugal has BPA concentration ranging from 0.07 to 4 μg/L (Azevedo et al., 2001). A high level of BPA (7.5 ng/L) has been detected in bottled water stored outdoor from Riyadh, Saudi Arabia (ElAmin et al., 2013) and Malaysia (3.3 ng/L) (Santhi et al., 2012).
Around 10 fold higher BPA concentration than average was detected in surface water sample near industrial sites (Hohenblum et al., 2004) and about 200 fold higher BPA levels were detected in water samples contaminated with municipal waste site seepage (Sánchez-Avila et al., 2009). Bisphenol AP (BPAP), a BPA alternative, has also been detected in food products from China and United States (Liao and Kannan, 2013). Measured concentrations of BPAP in food from China were approximately 12 times higher than in the USA (0.711 ng/g vs 0.059 ng/g) (Liao and Kannan, 2014a). BPAP has also been detected in several personal care products, such as toothpaste, lotions and makeup and indoor dust (Liao et al., 2012). BPAF was detected in the Taihu lake (0.28 ng/L), the Hunhe (2.4 ng/L) and Liaohe Rivers (1.9 ng/L) (Jin and Zhu, 2016). BPF has been detected in sewage effluents from China (3.84 ng/g dw); Bisphenol A (4.69 ng/g dw); Bisphenol S (3.02 ng/g dw) (Song et al., 2014). The measured concentration of BPF in surface water collected from various streams and rivers of Germany ranges from 0.1–180 ng/L (Fromme et al., 2002b). A very high concentration of BPF (7.3 μg/g) was recorded from sediment samples collected from China (Yang et al., 2014). A fairly high concentration of BPF (0.054 mg/g) has also been detected from indoor dust samples collected from various areas of the United States (Liao et al., 2012). BPF has also been detected in canned food and drinks (Tzatzarakis et al., 2017). BPF has been detected in various food products including vegetables, fruits and levels were higher in canned fruits and vegetables (Liao and Kannan, 2014b).
High concentrations of BPS (7200 ng/L) were detected in surface water of the Adyar River, and Buckingham Canal India (1080 ng/L) and from the Korttalaiyar River (8.7 ng/L) (Yamazaki et al., 2015). Relatively low concentrations (0.29 to 19.0 ng/L) of BPS were detected in Hangzhou Bay China (Yang et al., 2014). BPS was also detected in 46 water samples tested from the Liaohe River (14 ng/L), Taihu lake (6.0 ng/L) and Hunhe River (11 ng/L), China (Jin and Zhu, 2016), as well as the Edogawa (3.4 ng/L) and Arakawa Rivers (4.6 ng/L) in Japan (Yamazaki et al., 2015).
3. Mechanism of action
Bisphenol-A has become a chemical of highest concern because it can bind with estrogen receptors. BPA has an affinity for nuclear estrogen receptors (α and β), but also the transmembrane G protein-coupled estrogen receptor (tGPR30) (Eckstrum et al., 2016) and orphan nuclear estrogen receptor-γ (ERRγ) (Bulayeva and Watson, 2004; Gould et al., 1998; Matsushima et al., 2007). BPA not only interacts with estrogen receptors but also with androgen receptors and thyroid receptors (Reif et al., 2010).
Estrogen receptors are nuclear receptors family and have 4 major independent domains (A/B, C, D, E/F) (Zwart et al., 2010). The ligand depended activation of ER is also dependent on the two transactivation factors i.e. Activation function-1 (AF-1) present in 1st domain (A/B) domain and is a hormone-independent factor and Activation function-2 (AF-2) present in 4th E/F domain and it is a hormone-dependent transactivation factor (Hall and McDonnell, 2005). ER-α has both AF-1 and AF-2. Plenty of evidence suggests that BPA acts as an estrogen agonist and has an affinity to bind directly with estrogen receptors of vertebrates (Alonso-Magdalena et al., 2012) but has a higher affinity with ERβ (Routledge et al., 2000). As compared to endogenous estradiol, BPA has a higher affinity (6.6 fold) to bind with estrogen receptor-β (Kuiper et al., 1997). It is considered as a selective estrogen receptor modulator (SERM) as it acts as an ER agonist in some tissues and ER antagonists in other tissues. Similar to BPA, BPAF acts as an agonist of estrogen receptors (Li et al., 2012).
The action of BPA as an ER antagonist or agonist depends various upon the following conditions. 1. Recruitment of co-regulatory factors. These regulatory co-factors are tissue-specific and affect the interactions of BPA with the estrogen receptor and ERE (estrogen response element) on the target genes (Klinge, 2001). These tissue-specific differential interactions lead to different tissue-specific molecular and physiological responses in an organism. The tissue-specific agonistic and antagonistic properties of BPA can be due to ligand-dependent recruitment of co-activator proteins by estrogen receptors as BPA/ ERβ complex is more potent (500-folds) in recruiting co-activator factor TIF2 compared to BPA/ERα complex (Wetherill et al., 2007). 2. Ligand-induced conformational changes in ER. The ligands upon binding to the ERs induce conformational changes specific to the ligands resulting in multiple transcriptional and physiological responses in tissues (Paige et al., 1999). 3. The ratio of ERα and ERβ, and the presence of EREs in the promoter region of a gene. (Pennie et al., 1998). In fish, exposure to BPA results in an elevated level of circulating vitellogenin which is attributed to direct binding of BPA to estrogen receptor (Faheem et al., 2017b). The agonistic mode of action of BPA to the ERs may be due to the two un-substituted phenolic rings in the BPA structure. The substitution of one phenolic ring in BPA-diglydidyl ether makes this derivative as ER antagonist (Letcher et al., 2005). Also, metabolites of BPA have been found to have differential roles in agonism or antagonism of ERs. This may be true for variable results observed in responses of wild populations of fish. Agonistic and antagonistic properties of BPA seems to be species-specific- partly because of ERα- ERβ ratio in the specific target tissue, recruitment of co-activator proteins, presence of EREs, and ligand-induced conformational changes in ERs (Fig.2).
Fig. 2.
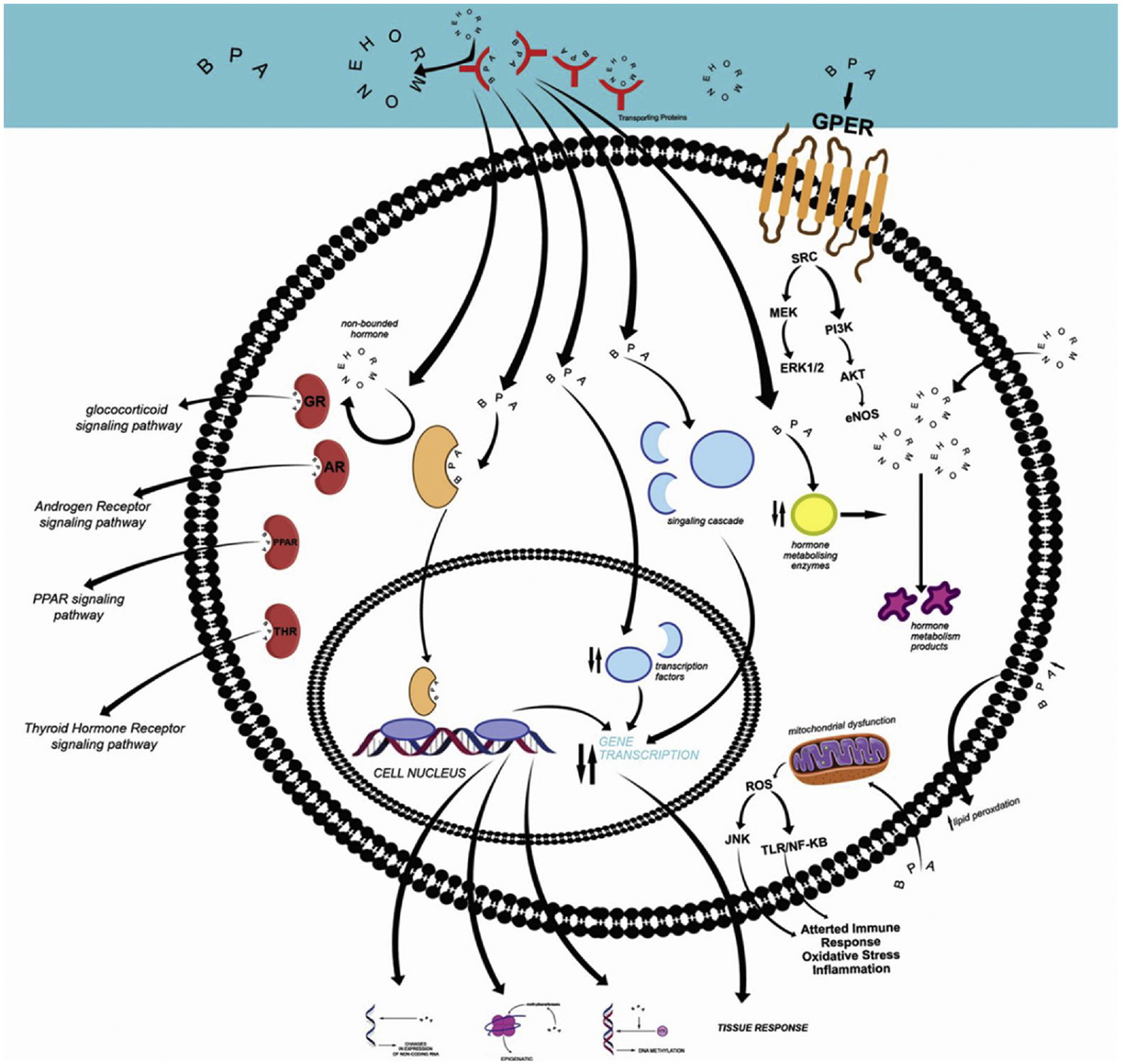
Various mode of actions of BPA.
BPA also has an affinity for estrogen-related receptors (ERRs). These ERRs are members of orphan nuclear receptors and share significant structural similarities with estrogen receptors especially in the ligand-binding domain. These ERR not only bind with endogenous estrogen but have an affinity to bind with estrogen response elements. Therefore, a possible overlap may exist between the action of estrogen receptors and estrogen-related receptors (Acconcia et al., 2015). Very low concentrations of BPA (13.1 nmol/L) can still elicit a strong affinity with human ERR (Takayanagi et al., 2006). Another member of the orphan nuclear receptor having an affinity with BPA is orphan nuclear estrogen receptor-γ (EER-γ). Interaction of BPA with EER-γ in zebrafish resulted in abnormal otolith development (Tohmé et al., 2014). Studies with mammals suggested that EER-γ is involved in the regulation of insulin secretion, muscle metabolism and transcription of genes of gluconeogenesis. Therefore, the binding of BPA with fish EER-ɣ suggests that this agent has greater far-reaching effects in fish than were originally anticipated.
BPA can affect physiological processes by binding with peroxisome proliferator-activated receptors (PPARs) (Fig. 2). These are nuclear receptors involved in many important cellular functions. PPARα and β are involved in fatty acid metabolism, while PPARγ is involved in glucose metabolism (Berger and Moller, 2002). Interaction of BPA and PPAR can lead to the disruption of PPAR regulated genes. Evidence suggested that BPA can modulate mammalian PPAR (Hurst and Waxman, 2003) and its ability to alter these receptors in non-mammalian species is not clearly understood (Riu et al., 2011, 2014). Most of the tissue-specific effects of BPA are through cell signaling pathways. Receptors of rapid signaling pathways are associated with nuclear hormone receptor-like protein (Watson et al., 2007) and transmembrane hepta-helical G protein-coupled receptor e.g. GPR30 (Wong et al., 2003). BPA has a better binding affinity to the membrane G protein-coupled ER (GPER) (Thomas & Dong, 2006). BPA exposure has been found to result in crosstalk between ERα and GPR30 to activate downstream pathways (Sheng and Zhu, 2011). GPR30 binds with estradiol and EDCs with relatively low affinity. The relative binding affinity of BPA with GPR30 is 2.83 nM compared to estradiol which is higher than that of ERs (Quesada et al., 2002). For example, a 30-minute exposure of ER-negative HEK 293 cells to 200 nM of BPA enhanced cAMP activity that is a GPR30 dependent pathway, indicating BPA interactions with GPR30 membrane receptors (Thomas & Dong, 2006). BPA shares a structural resemblance with endogenous thyroid hormones (T3 and T4). Therefore, it can also bind with the receptor of the thyroid hormone (THRs) (Fig.2). Similar to the estrogen receptor, BPA has an antagonistic or agonistic effect on THRs (Hiroi et al., 2006; Jung et al., 2007; Zoeller et al., 2005). The evidence that BPA can bind directly to THRs has come from studies on amphibian models.
BPA can affect the metamorphosis process in amphibians. In a tail cell culture, BPA downregulated the genes responsible for metamorphosis, suggesting that BPA can interact with thyroid hormone receptors (Iwamuro et al., 2003; Zoeller et al., 2005). According to Iwamuro et al. (2006) BPA blocks TH-induced as well as spontaneous metamorphosis in Xenopus larvae. The same author reported the ability of BPA to block thyroid-induced tail resorption in-vitro (Iwamuro et al., 2003). BPA blocked triiodothyronine (T3)-induced apoptotic features in tails of Rana rugosa tadpole (Goto et al., 2006). BPA also suppressed thyrotropin-releasing hormone (TRH)-inducible release of both prolactin and TSH from the pituitary gland and corticotropin-releasing factor (CRF)-inducible release of thyroid-stimulating hormone (TSH) (Kaneko et al., 2008). In tadpoles, 17-β estradiol did not regulate prolactin and TSH release, it confirms that BPA effects also are independent of ER and it may affect the organism by directly binding to thyroid receptors. In rodents, BPA acts as a weak ligand for thyroid receptor and a powerful inhibitor of T3 binding to human TH-binding proteins. In amphibians, BPA inhibits human recombinant TPO activity (Schmutzler et al., 2004) and, accordingly, blocks T3-induced metamorphism of tadpoles (Iwamuro et al., 2003). Furthermore, BPA binds THR (Kudo and Yamauchi, 2005). At the receptor level, BPA binds to the thyroid hormone receptor (TR) as a weak ligand and acts as an antagonist to T3 thus inhibiting TR-mediated transcriptional activity (Freitas et al., 2011; Moriyama et al., 2002; Sun et al., 2009). The derivatives of BPA, TBBPA, and TCBPA, showed an even higher affinity for the receptor (Jean-Baptiste Fini et al., 2007; Kitamura et al., 2005). BPA exposure may also modulate the expression of thyroid-related genes in the brain, although the mechanisms are not clear (Zoeller et al., 2005). Taken together, the TH antagonism could be played not at the receptor level, but through the ability of the chemical to suppress TR-mediated transcription and consequently to reduce TR availability (Mathieu-Denoncourt et al., 2015).
BPA has been found to act as an antagonist of the androgen receptor. Studies with fish and mammals indicate that BPA act as anti-androgen. BPA acted as an androgen receptor antagonist in fish exposed to waterborne BPA (Ekman et al., 2012). In female fathead minnows, BPA at high concentrations (10 and 100 mg/ml) reduced the effect trenbolone (androgen receptor agonists) which has a high affinity for AR (~five times higher than testosterone) (Ankley and Gray, 2013). In another study with female fathead minnows, BPA reduced trenbolone effects on hepatic metabolome (Ekman et al., 2012). In vitro, BPA inhibits the binding of dihydrotestosterone to AR in a dose-dependent manner (Ekman et al., 2012). These studies provide evidence that BPA exerts its toxic effects not only via direct binding with the ER but also through affinity with thyroid hormone receptors and androgen receptors.
4. Effect of BPA and analogs on neuroendocrine system
In fish, reproduction is controlled by a conserved endocrine pathway which includes, pineal, hypothalamus, pituitary and gonads. The neurosecretory system controlling reproduction involves gonadotropin-releasing hormone (GnRH) secreting neurons that secrets GnRH, the primary regulator of reproduction (Millar et al., 2004). GnRH, a decapeptide hormone, synthesized by the hypothalamus which receives the environmental signals through the pineal gland and secrets GnRH to initiate the hormonal cascade. GnRH is critical for normal vertebrate reproductive function as it stimulates the release of gonadotropins (follicle-stimulating hormone; FSH and luteinizing hormone; LH) from the anterior pituitary which signal gonads to release sex steroids (Harrison et al., 2004). This hypothalamus-pituitary-gonadal (HPG) axis is essential for the manifestation of reproduction in all vertebrates (Fig. 3). Any changes in gonadotropin levels may lead to altered production of sex steroids, resulting in steroid imbalance leading to endocrine disruption. Alteration in the sex hormone balance is considered as a reliable biomarker of reproductive disturbance (Kime et al., 1999). BPA not only impacts the proper functioning of the HPG axis but also has several sex-specific effects.
Fig. 3.
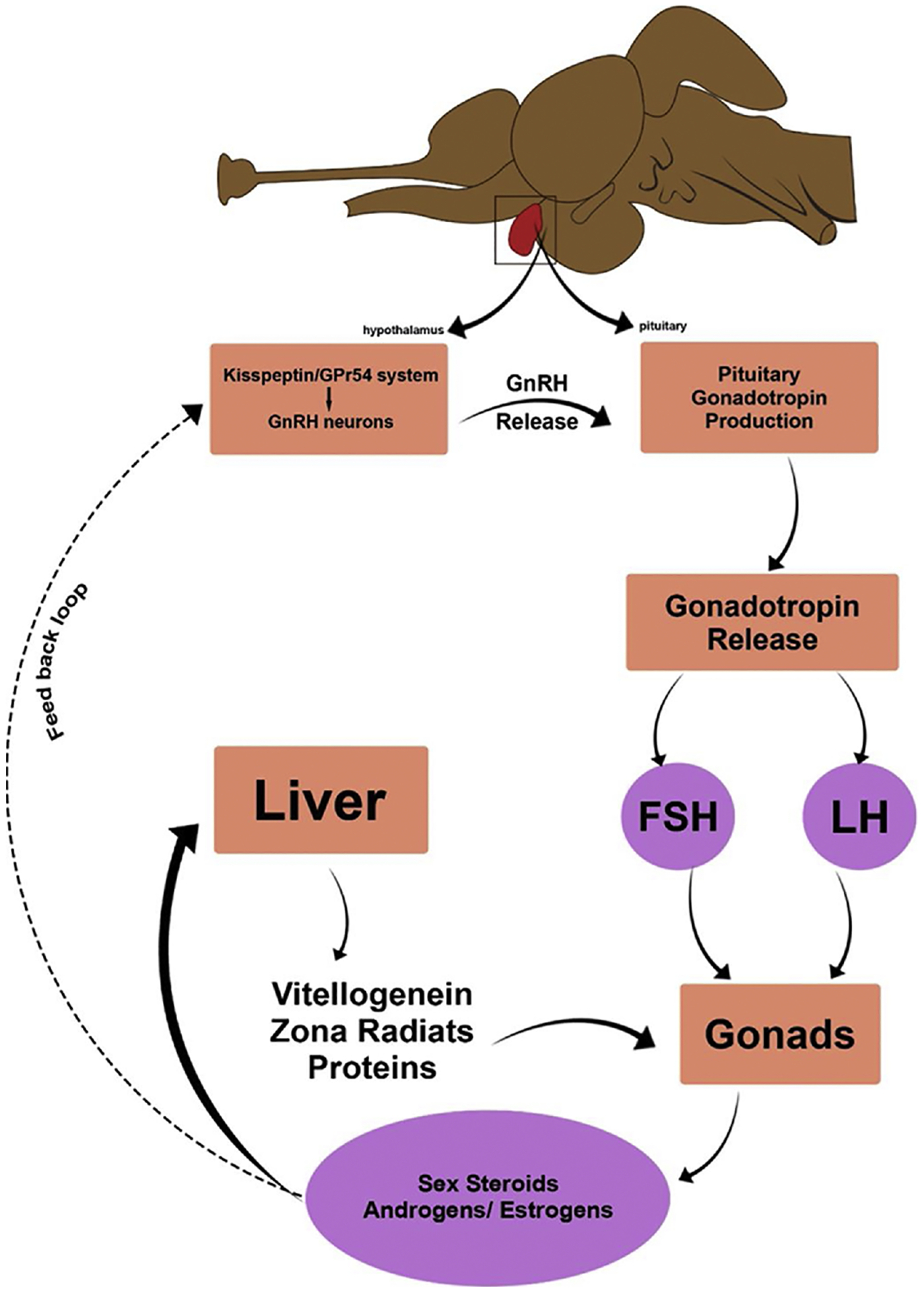
Hypothalamus Pituitary gonadal axis in teleost.
Qin et al. (2013) observed that a 35-day exposure to BPA (5 and 15 μg/L) does not change GnRH2 mRNA levels both in male and female rare minnow (Gobius rarus), while 15 μg/L BPA significantly upregulate transcription of GnRH3 both in the male and female brain of fish. GnRH1 mRNA levels showed an increasing trend in the brain of male and female G. rarus with 15 μg/L BPA exposure (Qin et al., 2013). Female Catla catla exposed to 10 and 100 μg/L BPA showed increased levels of GnRH mRNAs in the brain (Faheem et al., 2019a). Similar responses were observed in zebrafish after exposure to BPS (Qiu et al., 2016a) and BPF (Yang et al., 2017). BPF also induced GnRH3 expression in the zebrafish brain after a 60-day exposure (Qiu et al., 2019). All these reports point to BPA and its analogs causing increased expression of gonadotropin-releasing hormones in the brain. GnRH is regulated by endogenous estrogens through feedback loops (Herbison, 2009), but exact mechanisms are not yet clearly understood.
The recent discovery of the kisspeptin system and their role in fish reproduction led to a new understanding of GnRH regulation of reproduction and behavior in vertebrates (Seminara et al., 2003). Mostly two isoforms of kisspeptins and their respective receptors (gpr54a and gpr54b) have been identified in many fish species (Felip et al., 2009; Kitahashi et al., 2009; Rather et al., 2016; Yang et al., 2010). They regulate GnRH secretion in many species thus affecting the downstream neuroendocrine processes, such as secretion of gonadotropins, oxytocin-vasopressin neuronal excitation, steroid synthesis, and overall reproduction and reproduction-related behaviors (Shahjahan et al., 2010; Zohar et al., 2010). BPA exposure of grandparents during early embryonic development resulted in a significant increase in mRNA levels of kisspeptin and their receptors, but not GnRHs and ERs, in the brain of grand grandchildren, suggesting that BPA can induce transgenerational effects on brain probably affecting sexual behavior of fish (Thayil et al., in press). In a freshwater fish Catla catla, an overexpression of kisspeptin 1 and 2 after exposure to graded concentrations of BPA (10, 100 1000 μg/L) was reported (Faheem et al., 2019a). The expression of kisspeptins showed a non-monotonic response. Similar graded concentrations of BPF and BPS induced expression of kiss1 and its receptor kissr1 in zebrafish however, kiss2 and kiss2r mRNA remained unaffected (Qiu et al., 2019, 2016b). These studies suggest that bisphenols can affect brain kisspeptin system species-specifically. This neural regulation of reproduction by bisphenols is through estrogen-mediated pathways (Weiler and Ramakrishnan, 2019).
Aromatase is an important enzyme that converts androgens to estrogens. Two isoforms of aromatase have been identified in fish (Dong and Willett, 2008) that are responsible for estrogen synthesis in the brain and gonads. Brain aromatase is expressed in progenitor cells of the hypothalamus (Diotel et al., 2011). Estrogen response elements (EREs have been identified in the promotor region of brain aromatase (Kong et al., 2014). Aromatase is a potential target of EDCs especially BPA (Faheem et al., 2019a). BPA has an affinity for both Cyp19a1 and Cyp19a1b genes (encoding ovary and brain aromatase respectively). Exposure to BPA may lead to alteration in local and systemic levels of 17-β estradiol that results in the disruption of estrogen-related biological processes in the body (Cheshenko et al., 2008). In a study, BPA induced significant overexpression of brain aromatase in zebrafish embryos (Chung et al., 2011). Wang et al. (2010) exposed thirty-one days post-fertilization (dpf) rare minnow (Gobiocypris rarus) juveniles to BPA (0.1, 1, 10 nM), for 3 days exposure to both 1 and 10 nM BPA caused 0.7-fold extremely significant decrease of Cyp19a1b gene expression (Wang et al., 2010). cyp19b gene of Rivulus marmoratus adult, and Cyp19a1b of zebrafish embryo was significantly enhanced by BPA (Kishida et al., 2001; Lee et al., 2006). Significant upregulation of cyp19a1b was observed in pubertal Catla catla when exposed to low concentrations (10 μg/l) of BPA, while higher exposure concentrations resulted in a decreased Cyp19a1b mRNA expression (Faheem et al., 2019a). BPA, BPS and BPF (100 μg/l) caused overexpression of brain aromatase in zebrafish (Qiu et al., 2019). Taken together, BPA and its analogs (BPS and BPF) have a similar mode of action and disrupt neuroendocrine regulation of reproduction by acting on hypothalamic gonadotropin, kisspeptins and aromatase mRNA. Exposure of wild fish to these EDCs can change population dynamics in the wild population of fish through disruption of their reproductive abilities.
5. Effect on Reproduction
Reproduction in all vertebrates including fish is hormonally regulated through highly conserved endocrine pathways, such as the brain-pituitary-gonad and brain-pituitary-renal axis. The liver is also an important organ involved in hormonal control of reproduction in fish and other egg-laying species. It does not synthesize any hormone but produces many important chemicals/molecules e.g. vitellogenin under the influence of hormone which is important for the success of reproduction (Fig. 3). Reproduction is initiated when the hypothalamus receives environmental signals and in turn secretes gonadotropin-releasing hormone (GnRH). The GnRH signals pituitary to release gonadotropins mainly (follicle-stimulating hormone and luteinizing hormone). These gonadotropins are released into the bloodstream and act on gonads to produce sex steroids (estrogens and testosterone) through binding to their respective receptors. The release of hormones is controlled by the feedback of gonadotropins (Fig. 3).
BPA has adverse effects on fish reproduction. Even at low concentrations, BPA can alter the development of gonads and gamete quality in fish. Declines in sperm density and quality are the main adverse reproductive effects observed in males exposed to low levels of BPA in aquatic organisms (Crain et al., 2007). In female common carp (Cyprinus carpio), zebrafish and Catla catla BPA increased oocyte atresia and increased vitellogenic follicles (Faheem et al., 2017c; Mandich et al., 2007; Migliaccio et al., 2018) and in male common carp BPA alter male gonad structure (Mandich et al., 2007). In brown trout, BPA exposure resulted in reduced semen quality in males and delayed or complete reticence of ovulation in female fish (Lahnsteiner et al., 2005). During the development of gonads, BPA caused changes in germ cell progression in fathead minnow (Sohoni et al., 2001). Environmentally relevant concentrations of BPA caused reduced sperm motility and velocity in male goldfish, the underlying mechanism may involve alteration in sperm maturation pattern or changes in steroidogenic pathways (Hatef et al., 2012). Exposure of 274 and 549 μg/L (1.2 and 2.4 μM) BPA for 21 days caused 40–75 % reduced total sperm count in guppies, Poecilia reticulata (Haubruge et al., 2000). Rahman et al. (2015) reported that BPA (100μM) adversely affects reproduction by altering embryonic development, sperm function and fertilization through phosphorylation or regulation of fertility-related proteins in spermatozoa (Rahman et al., 2015). Alterations in levels of fertility-related protein may be related to BPA mediated reproductive dysfunction. Exposure of male brown trout to environmentally relevant concentrations (1.75, 2.40, 5.00 μg/L) of BPA, during pre-spawning and spawning period affected quantity, quality and maturation of sperm. At low exposure concentrations, BPA (1.75 and 2.40 μg/L) caused a decrease in sperm density, swimming rate, and sperm motility during pre-spawning and spawning males. In female brown trout, exposure of the same concentrations of BPA did not affect the quality of eggs, but it altered the timing of ovulation in females compared to controls. The females exposed to 1.74 μg/l BPA ovulated 2 weeks later, whereas a three-week delay in ovulation was observed in fish exposed to 2.74 μg/L (Lahnsteiner et al., 2005). A 30-day exposure of 1.5 mg/L BPA to Japanese medaka resulted in a significant reduction in the total number of eggs per pair and total number of brood (Li et al., 2016). Hatef et al. (2012) exposed male goldfish to environmentally relevant concentrations (0.6, 4.5 and 11.0 mg/L) of BPA and found the sperm motility to be decreased after 20 or 30 days (Hatef et al., 2012). Exposure of BPA not only affects the development and reproduction of the exposed individuals but also affects the offspring of the exposed individuals. BPA exposure during development induces transgenerational phenotypes of reproductive impairment and increased incidences of embryo mortality in fish of subsequent generations.
Reproduction was compromised in zebrafish when two generations were exposed chronically to BPA (1 nM). Males of both generations had a decreased sperm count. The sperm of the exposed males showed a declined ATP production, were less motile, had a higher rate of lipid peroxidation and sperm swimming velocity (Chen et al., 2015). Bhandari et al. (2015b) reported that exposure of BPA (100 μg/L) to medaka, during the first 7 days of post-fertilization (which overlaps with the critical period of germ cell differentiation) did not produce any morphological abnormality in gonads in F0 and F1 generations, but resulted in a decreased fertilization rate in individuals after 2 generations (F2) (Bhandari et al., 2015a). Taken together, observations suggest that the effects of BPA are persistent and can be transgenerationally inherited by subsequent generations. Similar effects were reported for BPS exposure. Parental exposure of BPS resulted in delayed hatchability in F1 generation (Ji et al., 2013), suggesting similar effects of BPA analogs on fish reproduction. Yokota et al. (2000) reported that exposure of 1.72 μg/L of BPA for 21 days significantly reduced the number of eggs per spawn in medaka and 47 % incidences of testis-ova were observed. A long-term exposure to BPA for 60 days led to an altered sex ratio. Exposure to 355 μg/L (1.6 μM) BPA resulted in a significantly female-biased sex ratio at the 1820 μg/L (8 μM). Short exposure of about 3 weeks to similar concentrations of BPA (837, 1720, 3120 μg/L or 3.7, 7.5, 13.7 μM) resulted in 13–80% testis-ova condition in males (Kang et al., 2002). Similarly, exposure of BPA (1030 and 3406 μg/l) to male Japanese medaka resulted in ova-testis condition and female like characters (Horie et al., 2020).
BPA can alter the somatic growth of the liver. A significantly higher hepatosomatic index was observed in Catla catla after exposure to BPA (Faheem et al., 2019b) and in Zebrafish after exposure to BPF (Yang et al., 2017). Vitellogenin is the egg yolk protein, produced by the liver under the influence of estradiol. The presence of vitellogenin in serum or overexpression of vitellogenin gene in the male and juvenile fish serves as an important biomarker of exposure to EDCs especially EDCs with estrogenic properties (Arukwe, 1998; Kime et al., 1999).
BPF exposure to zebrafish resulted in a decreased number of spermatogonia and spermatocytes in males and enhanced estrogen production and decreased circulating testosterone in male and female zebrafish (Yang et al., 2017). A significant upregulation of vitellogenin was observed in zebrafish exposed to BPF (Yang et al., 2017). Exposure to BPAF induced Vtg transcript in male zebrafish (Yang et al., 2014). Exposure of zebrafish to BPA for 180 days also caused an increased hepatic vitellogenin transcript in F1 males (Keiter et al., 2012). Plasma vitellogenin levels increased significantly in male zebrafish exposed to 1μM BPF for 7 days (Le Fol et al., 2017). BPS also caused a significant increase in plasma vitellogenin after exposure to BPS in zebrafish (Naderi et al., 2014). Vitellogenin expression and protein levels were induced significantly in zebrafish exposed to BPF (Qiu et al., 2019). Higher levels of circulating vitellogenin may be responsible for many reproductive abnormalities like ova-testis condition in male fish as observed in Japanese medaka exposed to BPA (Horie et al., 2020)
The hypothalamus-pituitary-gonadal axis plays a crucial role in fish reproduction and mediates its effects through sex steroids produced by gonads through steroidogenesis. Any changes in the axis and steroidogenic pathway results in altered sex hormone levels, ultimately leading to reproductive dysfunctions. Studies reported that BPA altered the gene expression profile of the enzymes responsible for steroid hormone production. Enzymes affected by BPA in the steroidogenic pathway are shown in Fig. 4.
Fig. 4.
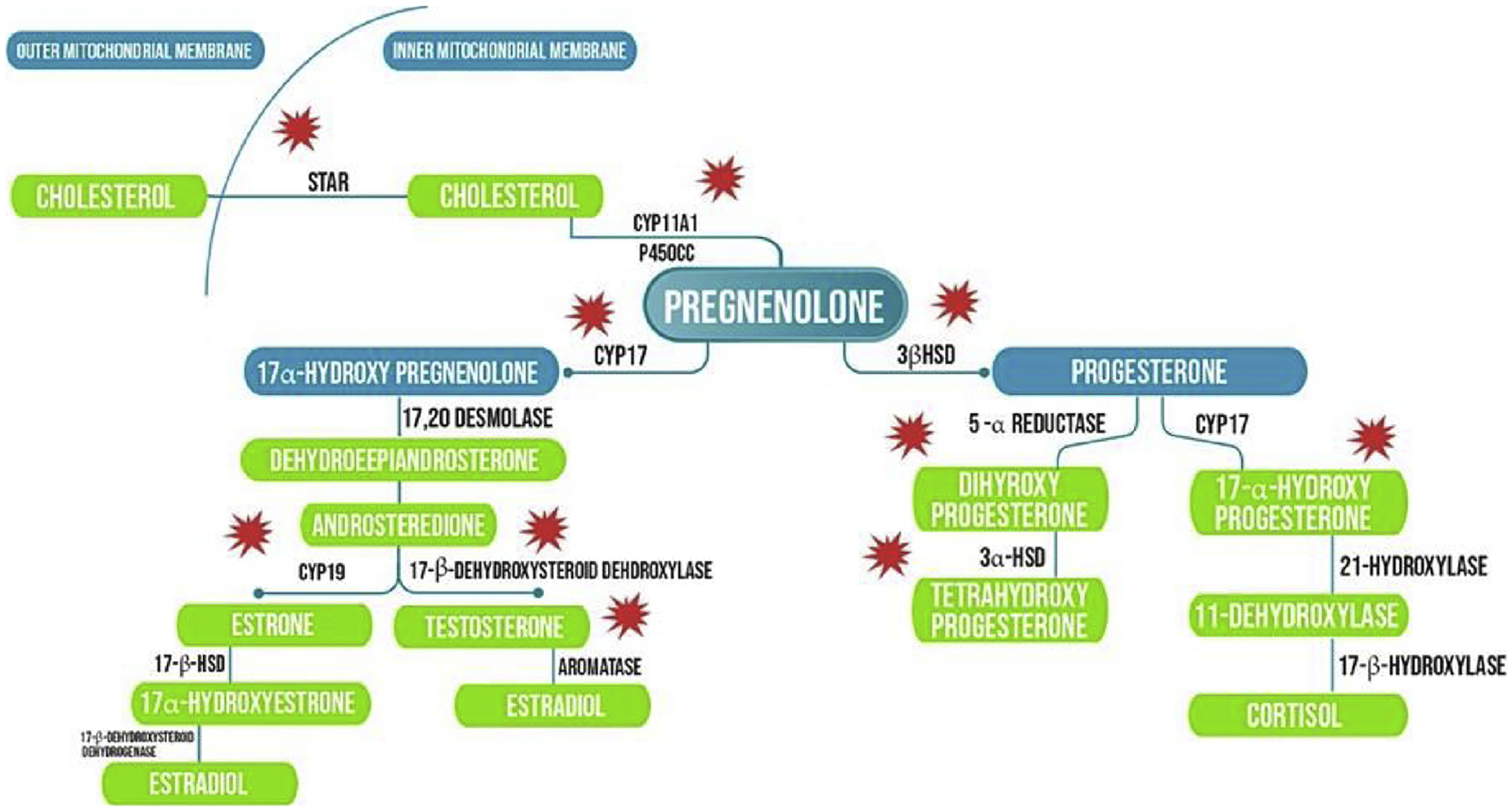
Effect of BPA and analogs on steroidogenic pathway.
Significantly higher levels of circulating estrone and lower testosterone were observed in juvenile turbot exposed to BPA (Labadie and Budzinski, 2006). A similar higher estradiol level was observed in female Catla catla after exposure to BPA (Faheem et al., 2017a). A decreasing trend in testosterone levels was observed in male goldfish and female Catla catla after exposure to BPA (Faheem et al., 2017a; Hatef et al., 2012). Alteration of sex steroids in fish after exposure to bisphenols may be due to the over- and underexpression of genes of the steroidogenic pathway.
The 1st step in the steroidogenic pathway is the movement of cholesterol (precursor molecule) across the mitochondrial membrane. This movement is governed by steroidogenic acute regulatory protein (StAR). Any changes in the activity of this enzyme will lead to altered levels of circulating steroid hormones. BPA is known to affect StAR mRNA expression in fish. In self-fertilizing Kryptolebias marmoratus (Rhee et al., 2011) and rare minnow ovaries, BPA up-regulated StAR expression (Liu et al., 2012; zhang et al., 2014). This effect seemed to be gender-dependent. A different result came from a study with male rare minnow where similar concentrations of BPA downregulated expression of StAR in the ovary (Liu et al., 2012). Rare minnow exposed to BPA for 21 and 63 days (short and long term exposure) resulted in the upregulation of StAR expression in the ovary (Liu et al., 2020).
The next step in the steroidogenic pathway is governed by Cyp11a1 (a side-chain cleavage enzyme) that converts cholesterol to pregnenolone. cyp11a1 mRNA increased significantly in rare minnow ovaries and testis in fish exposed to 15 μg/L BPA. However the response was nonmonotonic (Liu et al., 2012). A similar increased expression of cyp11a1 was observed in rare minnow ovaries after long term (63-day) exposure to BPA (Liu et al., 2020). BPA also upregulated the expression of 3β-HSD (an enzyme responsible for conversion of pregnenolone to progesterone, the 1st step towards cortisol synthesis) in both male and female minnow gonads (Zhang et al., 2014). A possible mechanism behind the disruption of key genes of steroidogenic pathway may be the DNA/histone-methylation. Significant decrease in important histone methylation markers (H3K4me3, H3K9me3, and H3K27me3) was recorded in rare minnow ovaries after exposure to BPA (Liu et al., 2020)
6. Effect of BPA on growth and development
The direct effect of BPA is on reproduction, but this chemical has also been found to negatively impact the development of the fish embryo and growth both at the larval and juvenile stage.
Exposure of 200 μg/L of BPA to Oryzias melastigma embryos for the whole embryonic stage, which started at 2 days postfertilization (dpf), resulted in reduced body length and width in larvae (Huang et al., 2012). Exposure of F0 generation of zebrafish to 10, 200 and 400 μg/L of BPA shows its effects in F2 generation. 90 dpf male and females of F2 generation had reduced body weight and body length compared to F2 individuals from the control group. These growth-related defects were pronounced in groups exposed to the highest BPA concentration (Keiter et al., 2012). In rainbow trout, three-hour acute exposure of eggs to 30 and 100 μg/L BPA did not alter fertilization rate but resulted in delayed hatching, a longer time for yolk absorption, reduced larval growth and delayed first feeding (approximately 7 days) of larvae. The level of growth hormone was significantly high in both treatment groups compared to controls at 65dpf. (Aluru et al., 2010a). Birceanu et al. (2015) exposed rainbow trout eggs for 3 hours with a low concentration of BPA 0.3, 3, and 30 μg/ ml and then fertilized with untreated milt, mimicking the maternal transfer of BPA. After 42dpf all the BPA treated fish has approximately 16 % significantly higher whole-body water content and these levels were maintained even at 65 days post-fertilization. In all BPA treated groups, a specific growth rate decreased approximately 15 % compared to control. This suggests that maternally transferred BPA has a long-term impact on the metabolism and growth of the offspring (Birceanu et al., 2015). BPA has been shown to alter metabolism rate and appetite. Rainbow trout eggs treated with 0.3, 3 and 30 μg/ml - of BPA followed by fertilization with clean milt showed the BPA-treated group with altered appetite. The food conversion ratio was high in BPA-treated groups. Approximately it took ~20% more food by the BPA groups (Birceanu et al., 2015). Whether this increased appetite in treated fish leads to lean and obese phenotypes is currently unknown. BPA is weakly obesogenic and induces genes involved in adipogenesis (Sun et al., 2019). Developmental BPA exposure can exert latent effects that remain undetected during the mid-age but elicit as obesogenic effects in adulthood via modulation of pathways involved in differentiation of adipocytes from mesenchymal cells (Angle et al., 2013).
Growth hormone(GH) and Insulin-like growth factors (IGFs) are key mediators of somatic growth in teleosts (Björnsson et al., 2002; Shepherd et al., 2007). IGF signaling plays a key role in regulating somatic growth and post-hatch development in fishes (Aluru et al., 2010b; Reinecke, 2010; Wood et al., 2005), and it is also a target for environmental contaminants impact (Aksakal et al., 2010; Aluru et al., 2010b; Hanson et al., 2014; Vandenberg et al., 2012). It has been recently shown that BPA accumulation in eggs disrupts IGF transcript levels in the trout larvae (Aluru et al., 2010a,b). The reduction in IGF-1 transcript levels in the BPA groups at 112 dpf may be playing a role in the growth reduction seen in the contaminant groups. Aluru et al. (2010a,b) exposed juvenile rainbow trout to 0, 30, and 100 μg/mL BPA for 3 h in ovarian fluid followed by fertilization and showed that BPA decreased the expression of genes involved in the endocrine regulation of growth and development, including the insulin-like growth factor 1 and 2 (igf-1 and igf-2), IGF-1 receptor alpha and beta (igf-1rα and igf-1rβ), and growth hormone receptors 1 and 2 (ghr1 and ghr2).
Tail length, total length, and body weight were all reduced in Japanese medaka and swordtail fish chronically treated with BPA at concentrations as low as 2 μg/L (Kwak et al., 2001; Yokota et al., 2000). BPA delayed hatching in zebrafish (13.8 mg/L BPA; Duan et al., 2008) and caused the delayed hatching, yolk absorption, and first feeding by about seven days in juvenile rainbow trout (30–100 μg/mL BPA; Aluru et al., 2010a,b). The authors suggested that BPA may have increased vitellogenin (vtg) mRNA level, decreased growth hormone (GH)-related gene expression and/or shifted the energy allocation from somatic growth to vitellogenesis. Furthermore, delayed hatching was observed in zebrafish exposed to 13.81 mg/L BPA at 72 hours post fertilization (Duan et al., 2008). Altogether, BPA was shown to delay the development and to reduce offspring weight and size by affecting the transcription of TH-related genes in vertebrates. BPA, BPS, BPF and BPAF resulted in developmental deformities in larval zebrafish, including cardiac edema, spinal malformation, and craniofacial deformities (Moreman et al., 2017). Literature suggests that bisphenols affect multiple developmental pathways leading to direct developmental abnormalities and abnormalities related to growth (Faheem et al., 2020).
7. Neurobehavioral effects and learning
Exposure of human-relevant concentrations of BPA during ontogenetic development resulted in learning deficits and behavioral abnormalities in rodents (Jones et al., 2011; Palanza et al., 2008). In zebrafish, exposure of low-dose BPA during the development of central nervous system (CNS) resulted in hyperactivity in larvae and learning delays in adults (Saili et al., 2012). Parental exposure of BPA resulted in altered sexually dimorphic behaviors in offspring (Jones et al., 2011; Negishi et al., 2004; Palanza et al., 2008). BPA affects learning and memory by altering synaptic transmission and expression of neurotransmitter receptor (Ishido et al., 2004; Xu et al., 2010). The learning dysfunction in BPA-exposed fish may be due to inappropriate activation of estrogen receptors (Ben-Jonathan and Steinmetz, 1998). Alternatively, alteration in hypothalamic neurogenesis by BPA/BPS is accompanied by upregulation of aromatase which is androgen receptor-dependent (Kinch et al., 2015).
Exposure to BPA and BPS to zebrafish larvae resulted in precocious neurogenesis and hyperactive behavior (Kinch et al., 2015). Exposure to BPS altered retinal physiology which resulted in impaired vision in male zebrafish (Liu et al., 2017). BPS (3 mg/L) exposure to larval zebrafish altered the structure of the retina, affected locomotor behavior and down-regulated genes required for normal neural development (Gu et al., 2019). In a recent study, exposure of BPA to zebrafish larvae revealed that BPA can cross the blood-brain barrier and affect dopaminergic, GABAergic and cholinergic pathway that resulted in disruption of behavior involving altered color preference, decrease in distance traveled and slow movement (Kim et al., 2020). All these reports suggest that BPA and its analog BPS can cause a damage to the nervous system and impaired motor functions in aquatic animals, similar to the effects seen in mammals and humans. The ability of BPA to cross the blood-brain barrier and disrupt neurotransmitter systems is the underlying cause of hyperactivity and altered behavioral pattern as observed in the above-mentioned studies.
8. Effect of BPA and analogs on metabolism
Bisphenol analogs induce metabolic disruption, hyper-insulinemia, and obesity in mammals (Alonso-Magdalena et al., 2015, 2006). However, little is known about the role of these compounds in disruption of metabolic health of aquatic animal models, particularly fish. Exposure of BPS to zebrafish led to insulin resistance and altered glucose homeostasis (Zhao et al., 2018). BPA exposure resulted in increased weight, hepatic triglyceride level, and lipid accumulation in male zebrafish (Sun et al., 2019). Low concentration BPS (10,100 μg/L) impaired glucose homeostasis in adult male zebrafish by altering higher fasting glucose and lower insulin levels (Zhao et al., 2018). The exposure to 10 and 100 μg/L BPS decreased glycogen content of liver, while 1000 μg/L BPS exposure resulted in increased hepatic glycogen content after 28 days of exposure (Zhao et al., 2018). Insulin resistance and high glucose level are highly correlated with obesity (Algoblan et al., 2014), indicating that BPS has obesogenic properties. BPS acts on this pathway in a similar mode of action to BPA by effecting the liver’s glucose homeostasis and vianon-monotonic dose responses. Peroxisome proliferator-activated receptor ɣ (PPARɣ) which is a member of the nuclear receptor super-family has significant role in adipogenesis regulation and is the most common target of almost all the obesogens. PPARγ combined with retinoic acid X receptor α are involved in lipid storage in zebrafish (Den Broeder et al., 2015). Zebrafish (Danio rerio) embryos exposed to BPA developed lipid accumulation via PPARɣ activation leading to early onset of weight gain in juveniles (Riu et al., 2014). Exposure to low concentration BPA (5 μg/L) increased the storage of triglycerides and promoted fatty acid synthesis, while the higher concentrations (i.e., 20 μg/L) promoted de novo lipogenesis and cholesterolosis in adult female zebrafish (Santangeli et al., 2018). Similarly, adult male zebrafish when exposed to BPA resulted in the upregulation of genes responsible for lipogenesis (Sun et al., 2019).
Fish liver and intestine are the main organs for the production of fats. The liver is the main site to convert glucose into glycogen/fatty acids which are ultimately converted into triglycerides. These TAGs are either stored in hepatocytes as fat droplets or released into the bloodstream as VLDL (Coleman and Lee, 2004). In the bloodstream, this VLDL is converted into high- and low-density lipoproteins which are responsible for lipid transport to the liver (for energy production) and adipose tissues (for storage). Alterations in these pathways results in excessive storage of TAG. Exposure to BPA resulted in a higher hepatic TAG level in zebrafish (Sun et al., 2019). BPS exposure resulted in increased hepatic glycogen content in zebrafish (Wang et al., 2019; Zhao et al., 2018) indicating that glucose is converted into glycogen but not fatty acids which may lead to increased TAG content. In another study by Guan et al. (2016), BPA exposure at 15 μg/L for 27 days resulted in increased triglyceride content in male zebrafish suggesting that BPA caused metabolic disorders. Chronic exposure to BPS resulted in fat accumulation, higher plasma triglyceride levels in male zebrafish (Wang et al., 2019). Zebrafish larvae (2hpf) exposed to BPS had higher TAG and visceral fat content after 15 days (Wang et al., 2018a,b). Similarly, an increased TAG level was observed in male zebrafish exposed to BPS for 120 days (Wang et al., 2019). The obesogenic effect caused by BPA and analogs may be due to their direct effect on genes involved in lipogenesis and metabolism. Both BPA and BPS increased the expression of genes responsible for lipid synthesis (fasn, acc1, and agpat4) and also altered the expression of retinoic acid X receptor α (Santangeli et al., 2018; Wang et al., 2019, 2018a,b). Taken together, literature suggests that the bisphenols can induce obesity and lipid accumulation by upregulating expression of genes in the lipogeneic pathways.
9. Effect of BPA and analogs on the immune system
Chemicals released into the waterbodies influence the immune competence of aquatic organisms. The endocrine and immune systems are intricately linked in vertebrates. Macrophages in fish have estrogen receptors. Effects of BPA and related bisphenols on immune regulation of macrophages may be due to interaction with nuclear factor −κB signaling and estrogen receptor α (Yang et al., 2015). Cytokines and chemokines are the pro-inflammatory mediators and effector molecules of the innate immune system, secreted by immune cells and are critical for inflammatory response. The proinflammatory activity of cytokines and chemokines are mediated through upstream signal transduction pathways (Xu et al., 2013). BPA induces the state of inflammation in the body which can be detected by the biomarkers of inflammation. Xu et al. (2013) observed that cytokines, chemokines and interleukins were significantly induced in zebrafish exposed to 0.1, 1 and 1000 μg/L of BPA (Xu et al., 2013). BPA also act as a kinase inhibitor or activator especially for mitogen-activated protein kinase, regulating signal transduction and transcription activation which in turn control cytokines and chemokines expression (Canesi et al., 2005, 2004). In goldfish (Carassius auratus) BPA at a concentration of 5–50 μg/L increased proliferation of lymphocytes. Higher doses of BPA (500–1000 μg/L) inhibited proliferation of macrophages (Yin et al., 2007). In yellow perch, exposure to environmentally relevant concentrations of BPA (2–8 μg/L) caused a significant increase in leukocyte count (Rogers and Mirza, 2013) Yang et al. (2015) observed that in Cyprinus carpio head kidney, low doses of BPA (0.1, 1, and 10 μg/L) resulted in antimicrobial activity, but the higher doses (100–1000 μg/L) induced apoptosis (Yang et al., 2015). The proinflammatory effects of BPA were associated with increased mRNA of NF-kB and other NF-kB-associated immune genes, interleukin 1β and increase in reactive oxygen species (ROS) and nitric oxide (NO). Transcription of iNOS gene leads to production of iNOS. NO is a byproduct of oxidative metabolism and acts as a pro-inflammatory mediator. Appropriate levels of NO generated by iNOS in cells facilitate the mounting of an effective defense against invading microbes. However, excess NO is toxic and proinflammatory to cells (Guzik et al., 2003). Inflammatory cytokines e.g. IFNɣ, IL-1β and TNF-α can stimulate iNOS gene transcription leading to overproduction of NO. BPA induces a state of inflammation in the body. A wide variety of cells including neutrophils, monocytes, T cells and epithelial cells secrete inflammatory chemokines (CC-chemokine and CXCL-clc) (Hermann and Kim, 2005). These chemokines activate leukocytes and recruit them to mount an immune response. Xu et al. (2013) observed increased expression of these chemokines after the exposure of zebrafish embryos to graded concentrations of BPA. BPA exposure resulted in the upregulation of pro-inflammatory genes leading to a state of inflammation (Sun et al., 2019). Similarly, long term30-days exposure to BPA activated toll-like receptor (TLRs) signaling pathway in gills of common carp leading to inflammation and immune disturbance (Gu et al., 2020). A little is known about effects of BPS and BPF on immune function in fish. A recent study demonstrated an up-regulation of immune genes (il-1β, il-6, il-10, il-11α, il-12α, infγ and tnfα) in zebrafish embryo similar to BPA (Qiu et al., 2018), suggesting that bisphenols can alter immune function in fish. It is important to explore the biomarkers of immunotoxicity to monitor and predict the toxicological risk of various aquatic pollutants in fish (Segner et al., 2012; Zelikoff et al., 2002).
10. Effect on the Thyroid Axis
Thyroid hormones are important for the maintenance of the physiology and metabolism of vertebrates including energy balance, growth and metabolism (Zhang et al., 2018). Thyroid hormones influence a wide variety of tissues and biological functions than any other hormones (Janz, 2000). In fish, thyroid hormones provide assistance to control osmoregulation, somatic growth, post-hatch metamorphosis and development. Thyroid hormones also regulate uptake of vitellogenin by the oocytes (Shibata et al., 1993) and act in cooperation with gonado-trophins to stimulate early ovarian follicle growth (Cyr and Eales, 1996). Chemicals that cause thyroid disruption act either by disturbing synthesis, secretion, and transport of thyroid hormone or by altering their binding to receptors or transactivation of target genes containing thyroid response elements (Brent et al., 2007; Raldúa and Babin, 2009) and elimination (Kloas and Lutz, 2006).
The thyroid gland in fish is variable in location and form. It is either present as compact or connective tissue encapsulated, but it is present around vascular tissue in a diffused form (Eales, 1979; Wanderlaar Bonga, 1993). Thyroid follicles are present next to the ventral aorta in medaka (Oryzias latipes) (Raine et al., 2001), dispersed in connective tissue near the pharyngeal region in fathead minnow (Wabuke-Bunoti and Firling, 1983; Wanderlaar Bonga, 1993) and some are present in association with kidney as seen in poecilids and some freshwater cyprinids. In fish, T4 is produced by thyroid follicles which is converted to T3 by the action of deiodinases. T4 is converted into biologically active T3 form by outer ring deiodination (ORD). T3 production from T4 occurs in peripheral tissues, mainly the liver (Darras et al., 1998; Eales et al., 1999). Therefore, in the piscine thyroid gland, increased production of T4 does not mean increased production of T3 and any adverse effects on T4 may not directly affect the function of T3. Less association is present between alteration in circulating T4 levels and change in biologically active tissue level of T3 and this is important in consideration of methods for measurement of thyroidal status in fish (Balton and Specker, 2007). Mol et al. (1998) reported that T3 present in blood-stream is mainly released from the liver after conversion, other peripheral tissues responsible for T4 to T3 conversion e.g. brain and gill will bind this T3 to their receptors and prevent it to enter in plasma. In teleosts e.g. in Cod, the liver manufactures the bulk of the circulating T3 (Eales & Brown, 1993; Cyr et al., 1988). The activity of thyroid-stimulating hormone (TSH) is only limited to iodide uptake by thyroid follicles and regulating T4 release (Eales et al., 1999). In the teleosts, both T3 and T4 exert a negative feedback mechanism to secretion of TSH from pituitary (Yoshiura et al., 1999).
Data from in vitro and in vivo studies showed that BPA and analogs have the potential to bind with thyroid hormone receptors (THRα and β) (Zhang et al., 2019) and inhibit TR mediated transcription (Moriyama et al., 2002; Sun et al., 2009; Freitas et al., 2010). Plasma levels of thyroid hormones (T3 and T4) were significantly decreased in male and female zebrafish exposed to 10 and 100 μg/L BPS (Naderi et al., 2014). Similarly, 10 and 30 μg/L BPS significantly lowered the whole body T3 and T4 levels in larval zebrafish while inducing significant TSH levels (Zhang et al., 2017). Similarly, BPAF exposure to zebrafish larvae also resulted in an insignificant decrease in whole-body level of T3 and T4 (Tang et al., 2015). A similar decrease in T4 levels was reported in zebrafish exposed to BPF, but the T3 levels increased in zebrafish larvae after exposure to 200 μg/L BPF (Huang et al., 2016). In Catla catla females, BPA exposure (1000 μg/L) resulted in significant reduction in T3 and T4 levels (Faheem et al., 2017a,b,c). A decrease in circulating levels of thyroid hormones may be associated with the increased transcription of deiodinases enzymes. In fish, T4 is produced by thyroid follicles which is converted to T3 by action of peripheral deiodinases. Three isoforms of deiodinases are reported in fish, namely Dio1, Dio2, and Dio3 (Orozco and Valverde, 2005). Dio1 and Dio2 are responsible for iodine recovery and conversion of T4 to T3, respectively, while Dio3 inactivates enzyme (Van der Geyten et al., 2005; Orozco and Valverde, 2005). Zebrafish larvae exposed to 10 and 30 μg/L BPS had significantly higher transcripts of both Dio1 and Dio 2 and significantly lower whole-body T3 and T4 levels (Zhang et al., 2014). The mRNA level of Dio1 and Dio2 were significantly higher in zebrafish larvae treated with BPAF (Tang et al., 2015). A similar increase in Dio2 transcript was observed in zebrafish after exposure to BPF (Huang et al., 2016). Collectively, BPA and its analogs including BPS, BPF and BPAF exert thyroid disrupting effects by altering circulating levels of thyroid hormones and gene expression of deiodinases, which will impact the growth, metabolism and reproduction of fish.
11. Bisphenols and Cellular stress
Reactive oxygen species (ROS) are produced by normal metabolic products and they are normalized by enzymatic and non-enzymatic antioxidants. Oxidative stress arises when the balance between production and depletion of ROS is disturbed. ROS cause lipid peroxidation and damage cellular biomolecules (Valavanidis et al., 2006). Lipid peroxidation is the direct measure of tissue membrane damage due to reactive oxygen species (Blokhina et al., 2003). Enzymes in the body prevent oxidative stress. Catalases and SOD are vital first-line defenses against oxygen toxicity (Yu, 1994). The glutathione system is a key nonenzymatic radical scavenger and antioxidant that scavenges residual free radicals generated from oxidative metabolism and those not decomposed by antioxidant enzymes (El-Shenawy, 2010). Inhibition of these enzymes may lead to the accumulation of hydrogen peroxide or its decomposition products (Halliwell, 1994). Studies in rats suggest that BPA induces oxidative stress by decreasing antioxidant enzyme activities, resulting in liver abnormalities (Bindhumol et al., 2003).
A concentration-dependent increase in ROS, NO, MDA and a significant decrease in GSH, CAT, and GR were recorded in zebrafish embryos exposed to graded concentration (0.1, 1, 10, 100, 1000 μg/L) of BPA suggesting that BPA acts as a strong inducer of oxidative stress (Wu et al., 2011). BPA induced generation of ROS in fish embryos in a dose-dependent manner. Exposure to BPA at concentration of 100 and 1000 μg/L caused significant induction of NO and NOS (Xu et al., 2013). Long-term exposure to BPA was found to induce CAT and SOD activities in the livers of adult Japanese medaka fish (Wu et al., 2011). Dietary exposure of 50 and 100 mg/kg of BPA to juvenile seabream (Sparus aurata) for 21 days resulted in a significant increase in catalase activity, while the level of GST remained unaffected (Maradonna et al., 2014). This increase could be a defense mechanism of the body to cope with the ROS produced after BPA exposure. In eukaryotic cells, GSH is the most abundant free thiol and is important to maintain optimal redox balance in cells for the proper function of cellular proteins (Circu and Aw, 2010). Exposure to 1, 15 and 225 μg/L of BPA caused a reduction in the GSH levels in rare minnow ovaries leading to oxidative stress (Zhang et al., 2016). Li et al. (2016) observed that 60 days of exposure of 1.5 mg/L of BPA to juvenile Japanese medaka resulted in reduced catalase activity in the ovary. GST activity was significantly enhanced in the liver and gill tissues, while MDA activity was increased in liver gills testes, and ovaries of the BPA exposed fish compared to control (Li et al., 2015). The sub-lethal concentration of BPA caused oxidative stress in liver and kidney of grass carp (Faheem and Lone, 2017). Exposure to the graded concentrations of BPA (0.01–2 mg/l) resulted in a significant decrease of antioxidants (Cat, GST, GSH) in gills of common carp inducing a state of oxidative stress (Gu et al., 2020). A similar increase in ROS level was reported in zebrafish embryos exposed to BPA, BPS and BPF (Qiu et al., 2018). Taken together, BPA and bisphenol analogs can induce a state of oxidative stress in fish. Oxidative stress can cause damage to lipids, carbohydrates, and nucleic acids and ultimately disrupt cellular homeostasis.
12. Conclusions
Bisphenols are ubiquitous chemicals and have been detected in aquatic environments across the globe. All the literature discussed here suggests that BPA and analogs have a similar mode of action. The mechanisms of action are estrogen-, androgen-, and thyroid-mediated. These bisphenols also exert their effects through epigenetic and rapid signaling pathways. Fish physiological systems are severely affected by bisphenols. Reproduction is adversely affected in fish after exposure to BPA and its analogs. Bisphenols also exert their harmful effects on the immune system, cellular homeostasis, and metabolism. The BPA-induced changes in the normal profile of endogenous hormones and disruption of neuroendocrine genes may lead to adverse health effects and reproductive disorders, such as a shift in spawning time, alterations in reproductive behavior, and acceleration of puberty. It is imperative to understand these hidden ecological threats to aquatic organisms and mitigate them by introducing and enforcing strict regulations for elimination of BPA and its congeners in domestic and industrial effluents. This will protect watercourses, safeguarding not only the environment, but also sustainable agriculture and food production worldwide.
Funding
Funding was not received from public and private sector.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
Declaration of Competing Interest
The authors report no declarations of interest.
References
- Acconcia F, Pallottini V, Marino M, 2015. Molecular Mechanisms of Action of BPA. Dose-response : a publication of International Hormesis Society 13, 1559325815610582. 10.1177/1559325815610582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksakal E, Ceyhun SB, Erdoğan O, Ekinci D, 2010. Acute and long-term genotoxicity of deltamethrin to insulin-like growth factors and growth hormone in rainbow trout. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 152, 451–455. 10.1016/j.cbpc.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Algoblan A, Alalfi M, Khan M, 2014. Mechanism linking diabetes mellitus and obesity. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy 7, 587. 10.2147/DMSO.S67400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Morimoto S, Ripoll C, Fuentes E, Nadal A, 2006. The estrogenic effect of bisphenol A disrupts pancreatic beta-cell function in vivo and induces insulin resistance. Environmental health perspectives 114, 106–112. 10.1289/ehp.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Ropero AB, Soriano S, García-Aŕevalo M, Ripoll C, Fuentes E, Quesada I, Nadal Á, 2012. Bisphenol-A acts as a potent estrogen via non-classical estrogen triggered pathways. Molecular and Cellular Endocrinology 355, 201–207. 10.1016/j.mce.2011.12.012. [DOI] [PubMed] [Google Scholar]
- Alonso-Magdalena P, Quesada I, Nadal Á, 2015. Prenatal Exposure to BPA and Offspring Outcomes: The Diabesogenic Behavior of BPA. Dose-response : a publication of International Hormesis Society 13, 1559325815590395. 10.1177/1559325815590395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru N, Leatherland JF, Vijayan MM, 2010a. Bisphenol a in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS ONE 5, 1–10. 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluru N, Leatherland JF, Vijayan MM, 2010b. Bisphenol a in oocytes leads to growth suppression and altered stress performance in juvenile rainbow trout. PLoS ONE. 10.1371/journal.pone.0010741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle BM, Do RP, Ponzi D, Stahlhut RW, Drury BE, Nagel SC, Welshons WV, Besch-Williford CL, Palanza P, Parmigiani S, Vom Saal FS, Taylor JA,Edu T, 2013. Metabolic disruption in male mice due to fetal exposure to low but not high doses of bisphenol A (BPA): Evidence for effects on body weight, food intake, adipocytes, leptin, adiponectin, insulin and glucose regulation. Reprod Toxicol 42. 10.1016/j.reprotox.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankley GT, Gray LE, 2013. Cross-species conservation of endocrine pathways: A critical analysis of tier 1 fish and rat screening assays with 12 model chemicals. Environmental Toxicology and Chemistry 32, 1084–1087. 10.1002/etc.2151. [DOI] [PubMed] [Google Scholar]
- Arukwe A, 1998. Xenobiotic modulation of fish endocrine systems: Molecular and biochemical studies of the estrogen- and Ah-receptor pathways in Atlantic salmon (Salmo salar). PhD Dissertation, University of Bergen, Norway. [Google Scholar]
- Azevedo D, de A, Lacorte S, Viana P, Barceló D, 2001. Occurrence of nonylphenol and bisphenol-A in surface waters from Portugal. Journal of the Brazilian Chemical Society 12, 532–537. 10.1590/S0103-50532001000400015. [DOI] [Google Scholar]
- Balton ML, Specker JL, 2007. The hypothalamic-pituitary-thyroid (HPT) axis in fish and its role in fish development and reproduction. Critical Reviews in Toxicology 37 (1–2), 97–115. 10.1080/10408440601123529. [DOI] [PubMed] [Google Scholar]
- Belfroid A, van Velzen M, van der Horst B, Vethaak D, 2002. Occurrence of bisphenol A in surface water and uptake in fish: evaluation of field measurements. Chemosphere 49, 97–103. 10.1016/S0045-6535(02)00157-1. [DOI] [PubMed] [Google Scholar]
- Ben-Jonathan N, Steinmetz R, 1998. Xenoestrogens: The emerging story of Bisphenol A. Trends in Endocrinology and Metabolism 9, 124–128. [DOI] [PubMed] [Google Scholar]
- Berger J, Moller DE, 2002. The Mechanisms of Action of PPARs. Annual Review of Medicine 53, 409–435. 10.1146/annurev.med.53.082901.104018. [DOI] [PubMed] [Google Scholar]
- Bhandari RK, Deem SL, Holliday DK, Jandegian CM, Kassotis CD, Nagel SC, Tillitt DE, vom Saal FS, Rosenfeld CS, 2015a. Effects of the environmental estrogenic contaminants bisphenol A and 17α-ethinyl estradiol on sexual development and adult behaviors in aquatic wildlife species. General and Comparative Endocrinology 214, 195–219. 10.1016/j.ygcen.2014.09.014. [DOI] [PubMed] [Google Scholar]
- Bhandari RK, vom Saal FS, Tillitt DE, 2015b. Transgenerational effects from early developmental exposures to bisphenol A or 17α-ethinylestradiol in medaka, Oryzias latipes. Scientific Reports 5, 9303. 10.1038/srep09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann S, Tschudin P, Grob K, 2010. Transfer of bisphenol A from thermal printer paper to the skin. Analytical and Bioanalytical Chemistry 398, 571–576. 10.1007/s00216-010-3936-9. [DOI] [PubMed] [Google Scholar]
- Bindhumol V, Chitra KC, Mathur PP, 2003. Bisphenol A induces reactive oxygen species generation in the liver of male rats. Toxicology 188 (2–3), 117–124. 10.1016/s0300-483x(03)00056-8. [DOI] [PubMed] [Google Scholar]
- Birceanu O, Servos MR, Vijayan MM, 2015. Bisphenol A accumulation in eggs disrupts the endocrine regulation of growth in rainbow trout larvae. Aquatic Toxicology 161, 51–60. 10.1016/j.aquatox.2015.01.028. [DOI] [PubMed] [Google Scholar]
- Björnsson BT, Johansson V, Benedet S, Einarsdottir IE, Hildahl J, Agustsson T, Jönsson E, 2002. Growth Hormone Endocrinology of Salmonids: Regulatory Mechanisms and Mode of Action. Fish Physiology and Biochemistry 27, 227–242. 10.1023/B:FISH.0000032728.91152.10. [DOI] [Google Scholar]
- Blokhina O, Virolainen E, Fagerstedt KV, 2003. Antioxidants, oxidative damage and oxygen deprivation stress: a review. Annals of Botany (Spec No(2)), 179–194. 10.1093/aob/mcf118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent GA, Braverman LE, Zoeller T, 2007. Thyroid Health and the Environment. Thyroid 17 (9), 807–809. 10.1089/thy.2007.1514. [DOI] [PubMed] [Google Scholar]
- Bulayeva NN, Watson CS, 2004. Xenoestrogen-induced ERK-1 and ERK-2 activation via multiple membrane-initiated signaling pathways. Environmental health perspectives 112, 1481–1487. 10.1289/ehp.7175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canesi L, Lorusso LC, Ciacci C, Betti M, Zampini M, Gallo G, 2004. Environmental estrogens can affect the function of mussel hemocytes through rapid modulation of kinase pathways. General and Comparative Endocrinology 138, 58–69. 10.1016/j.ygcen.2004.05.004. [DOI] [PubMed] [Google Scholar]
- Canesi L, Betti M, Lorusso LC, Ciacci C, Gallo G, 2005. ‘In vivo’ effects of Bisphenol A in Mytilus hemocytes: modulation of kinase-mediated signalling pathways. Aquatic Toxicology 71, 73–84. 10.1016/j.aquatox.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Cantonwine DE, Hauser R, Meeker JD, 2013. Bisphenol A and Human Reproductive Health. Expert review of obstetrics & gynecology 8. 10.1586/17474108.2013.811939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Xiao Y, Gai Z, Li R, Zhu Z, Bai C, Tanguay RL, Xu X, Huang C, Dong Q, 2015. Reproductive toxicity of low level bisphenol A exposures in a two-generation zebrafish assay: Evidence of male-specific effects. Aquatic Toxicology 169, 204–214. 10.1016/j.aquatox.2015.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshenko K, Pakdel F, Segner H, Kah O, Eggen RIL, 2008. Interference of endocrine disrupting chemicals with aromatase CYP19 expression or activity, and consequences for reproduction of teleost fish. General and Comparative Endocrinology 155, 31–62. [DOI] [PubMed] [Google Scholar]
- Chung E, Genco MC, Megrelis L, Ruderman JV, 2011. Effects of bisphenol A and triclocarban on brain-specific expression of aromatase in early zebrafish embryos. Proceedings of the National Academy of Sciences of the United States of America 108, 17732–17737. 10.1073/pnas.1115187108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Circu ML, Aw TY, 2010. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radical Biology and Medicine 48 (6), 749–762. 10.1016/j.freeradbiomed.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA, Lee DP, 2004. Enzymes of triacylglycerol synthesis and their regulation. Progress in lipid research 43, 134–176. [DOI] [PubMed] [Google Scholar]
- Corrales J, Kristofco LA, Steele WB, Yates BS, Breed CS, Williams ES, Brooks BW, 2015. Global Assessment of Bisphenol A in the Environment. Dose-Response 13, 155932581559830. 10.1177/1559325815598308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crain DA, Eriksen M, Iguchi T, Jobling S, Laufer H, LeBlanc G. a., Guillette LJ, 2007. An ecological assessment of bisphenol-A: Evidence from comparative biology. Reproductive Toxicology 24, 225–239. 10.1016/j.reprotox.2007.05.008. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Brommage NR, Duston J, Eales JG, 1988. Seasonal patterns in plasma levels of thyroid hormones and sex steroids in relation to photoperiod-induced changes in spawning time in rainbow trout, Salmo gairdneri. General and Comparative Endocrinology 69, 217–225. [DOI] [PubMed] [Google Scholar]
- Cyr DG, Eales JG, 1996. Interrelationships between thyroidal and reproductive endocrine systems in fish. Reviews in Fish Biology and Fisheries 6, 165–200. [Google Scholar]
- Darras VM, Mol KA, Van der Geyten S, Kuhn ER, 1998. Control of peripheral thyroid hormone levels by activating and inactivating deiodinases. Trends in Comparative Endocrinology and Neurobiology 839, 80–86. [DOI] [PubMed] [Google Scholar]
- Den Broeder MJ, Kopylova VA, Kamminga LM, Legler J, 2015. Zebrafish as a Model to Study the Role of Peroxisome Proliferating-Activated Receptors in Adipogenesis and Obesity. PPAR Research 2015, 1–11. 10.1155/2015/358029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon J-P, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC, 2009. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocrine reviews 30, 293–342. 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diotel N, Do Rego J-L, Anglade I, Vaillant C, Pellegrini E, Vaudry H, Kah O, 2011. The Brain of Teleost Fish, a Source, and a Target of Sexual Steroids. Frontiers in Neuroscience 5, 137. 10.3389/fnins.2011.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong W, Willett KL, 2008. Local expression of CYP19A1 and CYP19A2 in developing and adult killifish (Fundulus heteroclitus). General and comparative endocrinology 155, 307–317. 10.1016/j.ygcen.2007.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan Z, Zhu Lin, Zhu Lingyan, Kun Y, Zhu X, 2008. Individual and joint toxic effects of pentachlorophenol and bisphenol A on the development of zebrafish (Danio rerio) embryo. Ecotoxicology and Environmental Safety 71, 774–780. 10.1016/j.ecoenv.2008.01.021. [DOI] [PubMed] [Google Scholar]
- Eales JG, 1979. Thyroid function in cyclostomes and fishes in Barrington. In: Barrington EJ (Ed.), Hormones and Evolution, Vol 1. Academic Press, New York, pp. 341–436. [Google Scholar]
- Eales JG, Brown SB, 1993. Measurement and regulation of thyroidal status in teleost fish. Reviews in Fish Biology and Fisheries 3, 299–347. 10.1007/BF00043383. [DOI] [Google Scholar]
- Eales JG, Brown SB, Cyr DG, Adams BA, Finnson KR, 1999. Deiodination as an index of chemical disruption of thyroid hormone homeostasis and thyroidal status in fish. United States: N. p. Web. [Google Scholar]
- Eckstrum KS, Weis KE, Baur NG, Yoshihara Y, Raetzman LT, 2016. Icam5 expression exhibits sex differences in the neonatal pituitary and is regulated by estradiol and bisphenol A. Endocrinology 157, 1408–1420. 10.1210/en.2015-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman DR, Hartig PC, Cardon M, Skelton DM, Teng Q, Durhan EJ, Jensen KM, Kahl MD, Villeneuve DL, Gray LE, Collette TW, Ankley GT, 2012. Metabolite Profiling and a Transcriptional Activation Assay Provide Direct Evidence of Androgen Receptor Antagonism by Bisphenol A in Fish. Environmental Science & Technology 46, 9673–9680. 10.1021/es3014634. [DOI] [PubMed] [Google Scholar]
- Eladak S, Grisin T, Moison D, Guerquin MJ, N’Tumba-Byn T, Pozzi-Gaudin S, Benachi A, Livera G, Rouiller-Fabre V, Habert R, 2015. A new chapter in the bisphenol a story: Bisphenol S and bisphenol F are not safe alternatives to this compound. Fertility and Sterility 103, 11–21. 10.1016/j.fertnstert.2014.11.005. [DOI] [PubMed] [Google Scholar]
- ElAmin M, Elobeid M, Daghestani M, Almarhoon Z, AlOlayan E, Virk P, Omer S, Hassan Z, 2013. Bisphenol A Detection in Various Brands of Drinking Bottled Water in Riyadh, Saudi Arabia Using Gas Chromatography/Mass Spectrometer. Tropical Journal of Pharmaceutical Research 11, 455–459. 10.4314/tjpr.v11i3.15. [DOI] [Google Scholar]
- El-Shenawy SM, 2010. Effects of insecticides fenitrothion, endosulfan and abamectin on antioxidant parameters of isolated rat hepatocytes. Toxicology in vitro 24 (4), 1148–1157. 10.1016/j.tiv.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Faheem M, Khaliq S, Ahmad HU, Lone KP, 2017a. Bisphenol-A (BPA) alters plasma thyroid hormones and sex steroids in female Pakistani major carp (Catla catla; Cyprinidae). Pakistan Veterinary Journal 37. [Google Scholar]
- Faheem M, Khaliq S, Lone KP, 2017b. Non-monotonic endocrine-disrupting effects of bisphenol-a on vitellogenin expression in juvenile freshwater cyprinid, Catla catla. Pakistan Journal of Zoology 49. 10.17582/journal.pjz/2017.49.4.sc9. [DOI] [Google Scholar]
- Faheem M, Khaliq S, Lone KP, 2017c. Disruption of the Reproductive Axis in Freshwater Fish, Catla catla, after Bisphenol-A Exposure. Zoological Science 34. 10.2108/zs170009. [DOI] [PubMed] [Google Scholar]
- Faheem M, Adeel M, Khaliq S, Lone KP, El-Din-H-Sayed Alaa, 2020. Bisphenol-A induced antioxidants imbalance and cytokines alteration leading to immune suppression during larval development of Labeo rohita. Environmental science and pollution research 27, 26800–26809. 10.1007/s11356-020-08959-y. [DOI] [PubMed] [Google Scholar]
- Faheem M, Jahan N, Khaliq S, Lone KP, 2019a. Modulation of brain kisspeptin expression after bisphenol-A exposure in a teleost fish, Catla catla. Fish Physiology and Biochemistry 45, 33–42. 10.1007/s10695-018-0532-y. [DOI] [PubMed] [Google Scholar]
- Faheem M, Khaliq S, Lone KP, 2019b. Pakistan Veterinary Journal Effect of Bisphenol-A on Serum Biochemistry and Liver Function in the Freshwater Fish, Catla catla. pakistan veterinary journal 39, 71–75. 10.29261/pakvetj/2019.003. [DOI] [Google Scholar]
- Faheem M, Lone KP, 2017. Oxidative stress and histopathologic biomarkers of exposure to bisphenol-A in the freshwater fish, Ctenopharyngodon idella. Brazilian Journal of Pharmaceutical Sciences 53 (3), e17003. 10.1590/s2175-97902017000317003. [DOI] [Google Scholar]
- Felip A, Zanuy S, Pineda R, Pinilla L, Carrillo M, Tena-Sempere M, Gómez A, 2009. Evidence for two distinct KiSS genes in non-placental vertebrates that encode kisspeptins with different gonadotropin-releasing activities in fish and mammals. Molecular and Cellular Endocrinology 312, 61–71. 10.1016/j.mce.2008.11.017. [DOI] [PubMed] [Google Scholar]
- Fini Jean-Baptiste, Mével Sébastien Le, Turque Nathalie, Palmier Karima, Zalko Daniel, Cravedi Jean-Pierre, Demeneix Barbara A., 2007. An In Vivo Multiwell-Based Fluorescent Screen for Monitoring Vertebrate Thyroid Hormone Disruption. 10.1021/ES0704129. [DOI] [PubMed] [Google Scholar]
- Freitas J, Cano P, Craig-Veit C, Goodson ML, David Furlow J, Murk AJ, 2011. Detection of thyroid hormone receptor disruptors by a novel stable in vitro reporter gene assay. Toxicology in Vitro 25, 257–266. 10.1016/j.tiv.2010.08.013. [DOI] [PubMed] [Google Scholar]
- Froehner S, Machado KS, Falcão F, Monnich C, Bessa M, 2011. Inputs of Domestic and Industrial Sewage in Upper Iguassu, Brazil Identified by Emerging Compounds. Water, Air, & Soil Pollution 215, 251–259. 10.1007/s11270-010-0475-0. [DOI] [Google Scholar]
- Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A, 2002a. Occurrence of phthalates and bisphenol A and F in the environment. Water Research 36, 1429–1438. 10.1016/S0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Fromme H, Küchler T, Otto T, Pilz K, Müller J, Wenzel A, 2002b. Occurrence of phthalates and bisphenol A and F in the environment. Water Research 36, 1429–1438. 10.1016/S0043-1354(01)00367-0. [DOI] [PubMed] [Google Scholar]
- Goksøyr A, 2006. Endocrine Disruptors in the Marine Environment: Mechanisms of Toxicity and their Influence on Reproductive Processes in Fish. Journal of Toxicology and Environmental Health, Part A 69, 175–184. 10.1080/15287390500259483. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kitamura S, Kashiwagi K, Oofusa K, Tooi O, Yoshizato K, Sato J, Ohta S, Kashiwagi A, 2006. Suppression of Amphibian Metamorphosis by Bisphenol A and Related Chemical Substances. JOURNAL OF HEALTH SCIENCE 52, 160–168. 10.1248/jhs.52.160. [DOI] [Google Scholar]
- Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW, 1998. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Molecular and cellular endocrinology 142, 203–214. [DOI] [PubMed] [Google Scholar]
- Gu J, Zhang J, Chen Y, Wang H, Guo M, Wang L, Wang Z, Wu S, Shi L, Gu A, Ji G, 2019. Neurobehavioral effects of bisphenol S exposure in early life stages of zebrafish larvae (Danio rerio). Chemosphere 629–635. 10.1016/j.chemosphere.2018.10.218. [DOI] [PubMed] [Google Scholar]
- Gu Z, Jia R, He Q, Cao L, Du J, Jeney G, Xu P, Yin G, 2020. Oxidative stress, ion concentration change and immune response in gills of common carp (Cyprinus carpio) under long-term exposure to bisphenol A. Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology 230, 108711. 10.1016/j.cbpc.2020.108711. [DOI] [PubMed] [Google Scholar]
- Guan Y, Gao J, Zhang Y, Chen S, Yuan C, Wang Z, 2016. Effects of bisphenol A on lipid metabolism in rare minnow Gobiocypris rarus. Comparative Biochemistry and Physiology Part - C: Toxicology and Pharmacology 179, 144–149. 10.1016/j.cbpc.2015.10.006. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Korbut R, Adamek-Guzik T, 2003. Nitric oxide and superoxide in inflammation and immune regulation. Journal of physiology and pharmacology : an official journal of the Polish Physiological Society 54, 469–487. [PubMed] [Google Scholar]
- Hall JM, McDonnell DP, 2005. Coregulators in Nuclear Estrogen Receptor Action: From Concept to Therapeutic Targeting. Molecular Interventions 5, 343–357. 10.1124/mi.5.6.7. [DOI] [PubMed] [Google Scholar]
- Hanson AM, Kittilson JD, Martin LE, Sheridan MA, 2014. Environmental estrogens inhibit growth of rainbow trout (Oncorhynchus mykiss) by modulating the growth hormone-insulin-like growth factor system. General and Comparative Endocrinology 196, 130–138. 10.1016/j.ygcen.2013.11.013. [DOI] [PubMed] [Google Scholar]
- Harrison GS, Wierman ME, Nett TM, Glode LM, 2004. Gonadotropin-releasing hormone and its receptor in normal and malignant cells. Endocrine-Related Cancer 11, 725–748. 10.1677/erc.1.00777. [DOI] [PubMed] [Google Scholar]
- Hatef A, Alavi SMH, Abdulfatah A, Fontaine P, Rodina M, Linhart O, 2012. Adverse effects of bisphenol A on reproductive physiology in male goldfish at environmentally relevant concentrations. Ecotoxicology and Environmental Safety 76, 56–62. 10.1016/j.ecoenv.2011.09.021. [DOI] [PubMed] [Google Scholar]
- Haubruge E, Petit F, Gage MJG, 2000. Reduced sperm counts in guppies (Poecilia reticulata) following exposure to low levels of tributyltin and bisphenol A. 10.1098/rspb.2000.1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbison AE, 2009. Rapid actions of oestrogen on gonadotropin-releasing hormone neurons; from fantasy to physiology? The Journal of Physiology 587, 5025–5030. 10.1113/jphysiol.2009.179838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann AC, Kim CH, 2005. Effects of Arsenic on Zebrafish Innate Immune System. Marine Biotechnology 7, 494–505. 10.1007/s10126-004-4109-7. [DOI] [PubMed] [Google Scholar]
- Hiroi T, Okada K, Imaoka S, Osada M, Funae Y, 2006. Bisphenol A Binds to Protein Disulfide Isomerase and Inhibits Its Enzymatic and Hormone-Binding Activities. Endocrinology 147, 2773–2780. 10.1210/en.2005-1235. [DOI] [PubMed] [Google Scholar]
- Huang G-M, Tian X-f, Fang d X, Ji F-j, 2016. Waterborne exposure to bisphenol F causes thyroid endocrine disruption in zebrafish larvae. Chemosphere 147, 188–194. [DOI] [PubMed] [Google Scholar]
- Hohenblum P, Gans O, Moche W, Scharf S, Lorbeer G, 2004. Monitoring of selected estrogenic hormones and industrial chemicals in groundwaters and surface waters in Austria. Science of The Total Environment 333, 185–193. 10.1016/j.scitotenv.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Horie Y, Kanazwa N, Chiho T, Norihisa T, Taisen I, 2020. Bisphenol A induces a shift in sex differentiation gene expression with testis-ova or sex reversal in Japanese medaka (Oryzias latipes), pp. 1–11. 10.1002/jat.3945. [DOI] [PubMed] [Google Scholar]
- Hossein Rashidi B, Amanlou M, Behrouzi Lak T, Ghazizadeh M, Haghollahi F, Bagheri M, Eslami B, 2017. The Association Between Bisphenol A and Polycystic Ovarian Syndrome: A Case-Control Study. Acta medica Iranica 55, 759–764. [PubMed] [Google Scholar]
- Huang Q, Fang C, Chen Y, Wu X, Ye T, Lin Y, Dong S, 2012. Embryonic exposure to low concentration of bisphenol A affects the development of Oryzias melastigma larvae. Environmental Science and Pollution Research 19, 2506–2514. 10.1007/s11356-012-1034-6. [DOI] [PubMed] [Google Scholar]
- Hurst CH, Waxman DJ, 2003. Activation of PPAR and PPAR by Environmental Phthalate Monoesters. Toxicological Sciences 74, 297–308. 10.1093/toxsci/kfg145. [DOI] [PubMed] [Google Scholar]
- Ishido M, Masuo Y, Kunimoto M, Oka S, Morita M, 2004. Bisphenol A causes hyperactivity in the rat concomitantly with impairment of tyrosine hydroxylase immunoreactivity. Journal of Neuroscience Research 76, 423–433. 10.1002/jnr.20050. [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Sakakibara M, Terao M, Ozawa A, Kurobe C, Shigeura T, Kato M, Kikuyama S, 2003. Teratogenic and anti-metamorphic effects of bisphenol A on embryonic and larval Xenopus laevis. General and comparative endocrinology 133, 189–198. [DOI] [PubMed] [Google Scholar]
- Iwamuro S, Yamada M, Kato M, Kikuyama S, 2006. Effects of bisphenol A on thyroid hormone-dependent up-regulation of thyroid hormone receptor α and β and down-regulation of retinoid X receptor γ in Xenopus tail culture. Life Sciences 79, 2165–2171. 10.1016/j.lfs.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Janz DM, 2000. Endocrine system. (Chapter 25). In: Ostrander GK (Ed.), The Laboratory Fish. Academic Press, San Diego, CA. [Google Scholar]
- Ji K, Hong S, Kho Y, Choi K, 2013. Effects of bisphenol S exposure on endocrine functions and reproduction of zebrafish. Environmental Science and Technology 47, 8793–8800. 10.1021/es400329t. [DOI] [PubMed] [Google Scholar]
- Jin H, Zhu L, 2016. Occurrence and partitioning of bisphenol analogues in water and sediment from Liaohe River Basin and Taihu Lake. China. Water Research 103, 343–351. 10.1016/j.watres.2016.07.059. [DOI] [PubMed] [Google Scholar]
- Jones BA, Shimell JJ, Watson NV, 2011. Pre- and postnatal bisphenol A treatment results in persistent deficits in the sexual behavior of male rats, but not female rats, in adulthood. Hormones and Behavior 59, 246–251. 10.1016/j.yhbeh.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Jung KK, Kim SY, Kim TG, Kang JH, Kang SY, Cho JY, Kim SH, 2007. Differential regulation of thyroid hormone receptor-mediated function by endocrine disruptors. Archives of pharmacal research 30, 616–623. [DOI] [PubMed] [Google Scholar]
- Kandaraki E, Chatzigeorgiou A, Livadas S, Palioura E, Economou F, Koutsilieris M, Palimeri S, Panidis D, Diamanti-Kandarakis E, 2011. Endocrine Disruptors and Polycystic Ovary Syndrome (PCOS): Elevated Serum Levels of Bisphenol A in Women with PCOS. The Journal of Clinical Endocrinology & Metabolism 96, E480–E484. 10.1210/jc.2010-1658. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Okada R, Yamamoto K, Nakamura M, Mosconi G, Polzonetti-Magni AM, Kikuyama S, 2008. Bisphenol A acts differently from and independently of thyroid hormone in suppressing thyrotropin release from the bullfrog pituitary. General and Comparative Endocrinology 155 (3), 574–580. 10.1016/j.ygcen.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Kang IJ, Yokota H, Oshima Y, Tsuruda Y, Oe T, Imada N, Tadokoro H, Honjo T, 2002. Effects of bisphenol a on the reproduction of Japanese medaka (Oryzias latipes). Environmental toxicology and chemistry 21, 2394–2400. [PubMed] [Google Scholar]
- Keiter S, Baumann L, Färber H, Holbech H, Skutlarek D, Engwall M, Braunbeck T, 2012. Long-term effects of a binary mixture of perfluorooctane sulfonate (PFOS) and bisphenol A (BPA) in zebrafish (Danio rerio). Aquatic Toxicology 118–119, 116–129. 10.1016/j.aquatox.2012.04.003. [DOI] [PubMed] [Google Scholar]
- Kim SS, Hwang KS, Yang JY, Chae JS, Kim GR, Kan H, Jung MH, Lee HY, Song JS, Ahn S, Shin DS, Lee KR, Kim SK, Bae MA, 2020. Neurochemical and behavioral analysis by acute exposure to bisphenol A in zebrafish larvae model. Chemosphere 239, 124751. 10.1016/j.chemosphere.2019.124751. [DOI] [PubMed] [Google Scholar]
- Kime D, Nash J, Scott A, 1999. Vitellogenesis as a biomarker of reproductive disruption by xenobiotics. Aquaculture 177, 345–352. 10.1016/S0044-8486(99)00097-6. [DOI] [Google Scholar]
- Kinch CD, Ibhazehiebo K, Jeong J-H, Habibi HR, Kurrasch DM, 2015. Low-dose exposure to bisphenol A and replacement bisphenol S induces precocious hypothalamic neurogenesis in embryonic zebrafish. Proceedings of the National Academy of Sciences 112, 1475–1480. 10.1073/pnas.1417731112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishida M, McLellan M, Miranda J. a., Callard GV, 2001. Estrogen and xenoestrogens upregulate the brain aromatase isoform (P450aromB) and perturb markers of early development in zebrafish (Danio rerio). Comparative Biochemistry and Physiology - B Biochemistry and Molecular Biology 129, 261–268. 10.1016/S1096-4959(01)00319-0. [DOI] [PubMed] [Google Scholar]
- Kitahashi T, Ogawa S, Parhar IS, 2009. Cloning and Expression of kiss2 in the Zebrafish and Medaka. Endocrinology 150, 821–831. 10.1210/en.2008-0940. [DOI] [PubMed] [Google Scholar]
- Kitamura S, Suzuki T, Sanoh S, Kohta R, Jinno N, Sugihara K, Yoshihara S, Fujimoto N, Watanabe H, Ohta S, 2005. Comparative Study of the Endocrine-Disrupting Activity of Bisphenol A and 19 Related Compounds. Toxicological Sciences 84, 249–259. 10.1093/toxsci/kfi074. [DOI] [PubMed] [Google Scholar]
- Klinge CM, 2001. Estrogen receptor interaction with estrogen response elements. Nucleic Acids Research 29, 2905–2919. 10.1093/nar/29.14.2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloas W, Lutz I, 2006. Amphibians as model to study endocrine disrupters. Journal of Chromatography A 1130 (1), 16–27. 10.1016/j.chroma.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Kong HJ, Kim JL, Moon JY, Kim W-J, Kim HS, Park JY, Cho HK, An CM, 2014. Characterization, expression profile, and promoter analysis of the Rhodeus uyekii vitellogenin Ao1 gene. International journal of molecular sciences 15, 18804–18818. 10.3390/ijms151018804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo Y, Yamauchi K, 2005. In Vitro and in Vivo Analysis of the Thyroid Disrupting Activities of Phenolic and Phenol Compounds in Xenopus laevis. Toxicological Sciences 84, 29–37. 10.1093/toxsci/kfi049. [DOI] [PubMed] [Google Scholar]
- Kuiper GGJM, Carlsson B, Grandien K, Enmark E, Häggblad J, Nilsson S, Gustafsson J-Å, 1997. Comparison of the Ligand Binding Specificity and Transcript Tissue Distribution of Estrogen Receptors α and β. Endocrinology 138, 863–870. 10.1210/endo.138.3.4979. [DOI] [PubMed] [Google Scholar]
- Kwak HI, Bae MO, Lee MH, Lee YS, Lee BJ, Kang KS, Chae CH, Sung HJ, Shin JS, Kim JH, Mar WC, Sheen YY, Cho MH, 2001. Effects of nonylphenol, bisphenol A, and their mixture on the viviparous swordtail fish (Xiphophorus helleri). Environmental toxicology and chemistry / SETAC 20, 787–795. [PubMed] [Google Scholar]
- Labadie P, Budzinski H, 2006. Alteration of steroid hormone balance in juvenile turbot (Psetta maxima) exposed to nonylphenol, bisphenol A, tetrabromodiphenyl ether 47, diallylphthalate, oil, and oil spiked with alkylphenols. Archives of Environmental Contamination and Toxicology 50, 552–561. 10.1007/s00244-005-1043-2. [DOI] [PubMed] [Google Scholar]
- Lahnsteiner F, Berger B, Kletzl M, Weismann T, 2005. Effect of bisphenol A on maturation and quality of semen and eggs in the brown trout, Salmo trutta f. fario. Aquatic Toxicology 75, 213–224. 10.1016/j.aquatox.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Le Fol V, Aït-Aïssa S, Sonavane M, Porcher JM, Balaguer P, Cravedi JP, Zalko D, Brion F, 2017. In vitro and in vivo estrogenic activity of BPA, BPF and BPS in zebrafish-specific assays. Ecotoxicology and Environmental Safety 142, 150–156. 10.1016/j.ecoenv.2017.04.009. [DOI] [PubMed] [Google Scholar]
- Lee YM, Seo JS, Kim IC, Yoon YD, Lee JS, 2006. Endocrine disrupting chemicals (bisphenol A, 4-nonylphenol, 4-tert-octylphenol) modulate expression of two distinct cytochrome P450 aromatase genes differently in gender types of the hermaphroditic fish Rivulus marmoratus. Biochemical and Biophysical Research Communications 345, 894–903. 10.1016/j.bbrc.2006.04.137. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, Sanderson JT, Bokkers A, Giesy JP, van den Berg M, 2005. Effects of bisphenol A-related diphenylalkanes on vitellogenin production in male carp (Cyprinus carpio) hepatocytes and aromatase (CYP19) activity in human H295R adrenocortical carcinoma cells. Toxicology and applied pharmacology 209, 95–104. 10.1016/j.taap.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Li Y, Burns KA, Arao Y, Luh CJ, Korach KS, 2012. Bisphenol AF, and Zearalenone through Estrogen Receptor α and β in Vitro, vol. 120, pp. 1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Chen Q, Cao J, Chen H, Li L, Cedergreen N, Xie H, Xie L, 2016. The chronic effects of lignin-derived bisphenol and bisphenol A in Japanese medaka Oryzias latipes. Aquatic Toxicology 170, 199–207. 10.1016/j.aquatox.2015.11.024. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2013. Concentrations and profiles of bisphenol a and other bisphenol analogues in foodstuffs from the united states and their implications for human exposure. Journal of Agricultural and Food Chemistry 61, 4655–4662. 10.1021/jf400445n. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2014a. A survey of alkylphenols, bisphenols, and triclosan in personal care products from China and the United States. Archives of Environmental Contamination and Toxicology 67, 50–59. 10.1007/s00244-014-0016-8. [DOI] [PubMed] [Google Scholar]
- Liao C, Kannan K, 2014b. A survey of bisphenol A and other bisphenol analogues in foodstuffs from nine cities in China. Food Additives and Contaminants - Part A Chemistry, Analysis. Control, Exposure and Risk Assessment 31, 319–329. 10.1080/19440049.2013.868611. [DOI] [PubMed] [Google Scholar]
- Liao C, Liu F, Guo Y, Moon H-B, Nakata H, Wu Q, Kannan K, 2012. Occurrence of Eight Bisphenol Analogues in Indoor Dust from the United States and Several Asian Countries: Implications for Human Exposure. Environmental Science & Technology 46, 9138–9145. 10.1021/es302004w. [DOI] [PubMed] [Google Scholar]
- Liu S, Qin F, Wang H, Wu T, Zhang Y, Zheng Y, Li M, Wang Z, 2012. Effects of 17α-ethinylestradiol and bisphenol A on steroidogenic messenger ribonucleic acid levels in the rare minnow gonads. Aquatic Toxicology 122–123, 19–27. 10.1016/j.aquatox.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Liu W, Zhang X, Wei P, Tian H, Wang W, Ru S, 2017. Long#xp#term exposure to bisphenol S damages the visual system and reduces the tracking capability of male zebrafish (Danio rerio). Journal of Applied Toxicology s1. 10.1002/jat.3519. [DOI] [PubMed] [Google Scholar]
- Liu Y, Wang L, Zhu L, Ran B, Wang Z, 2020. Bisphenol A disturbs transcription of steroidogenic genes in ovary of rare minnow Gobiocypris rarus via the abnormal DNA and histone methylation. Chemosphere 240, 124935. 10.1016/j.chemosphere.2019.124935. [DOI] [PubMed] [Google Scholar]
- Mandich A, Bottero S, Benfenati E, Cevasco A, Erratico C, Maggioni S, Massari A, Pedemonte F, Viganó L, 2007. In vivo exposure of carp to graded concentrations of bisphenol A. General and Comparative Endocrinology 153, 15–24. 10.1016/j.ygcen.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Maradonna F, Nozzi V, Valle DL, Traversi I, Gioacchini G, Benato F, Colletti E, Gallo P, Pisciottano ID, Mita DG, Hardiman G, Mandich A, Carnevali O, 2014. A developmental hepatotoxicity study of dietary bisphenol A in Sparus aurata juveniles. Comperative Biochemistry & Physiology C 166, 1–13. [DOI] [PubMed] [Google Scholar]
- Mathieu-Denoncourt J, Wallace SJ, de Solla SR, Langlois VS, 2015. Plasticizer endocrine disruption: Highlighting developmental and reproductive effects in mammals and non-mammalian aquatic species. General and Comparative Endocrinology 219, 74–88. 10.1016/j.ygcen.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Matsushima A, Kakuta Y, Teramoto T, Koshiba T, Liu X, Okada H, Tokunaga T, Kawabata S. -i., Kimura M, Shimohigashi Y, 2007. Structural Evidence for Endocrine Disruptor Bisphenol A Binding to Human Nuclear Receptor ERR. Journal of Biochemistry 142, 517–524. 10.1093/jb/mvm158. [DOI] [PubMed] [Google Scholar]
- Mendum T, Stoler E, VanBenschoten H, Warner JC, 2011. Concentration of bisphenol A in thermal paper. Green Chemistry Letters and Reviews 4, 81–86. 10.1080/17518253.2010.502908. [DOI] [Google Scholar]
- Migliaccio M, Chioccarelli T, Ambrosino C, Suglia A, Manfrevola F, Carnevali O, Fasano S, Pierantoni R, Cobellis G, 2018. Characterization of follicular atresia responsive to BPA in zebrafish by morphometric analysis of follicular stage progression. International Journal of Endocrinology 2018. 10.1155/2018/4298195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar RP, Lu Z-L, Pawson AJ, Flanagan CA, Morgan K, Maudsley SR, 2004. Gonadotropin-Releasing Hormone Receptors. Endocrine Reviews 25, 235–275. 10.1210/er.2003-0002. [DOI] [PubMed] [Google Scholar]
- Mol KA, Van der Geyten S, Burel C, Kühn ER, Boujard T, Darras VM, 1998. Comparative study of iodothyronine outer ring and inner ring deiodinase activities in five teleostean fishes. Fish Physiology and Biochemistry 18, 253–266. [Google Scholar]
- Moreira M, Aquino S, Coutrim M, Silva J, Afonso R, 2011. Determination of endocrine-disrupting compounds in waters from Rio das Velhas, Brazil, by liquid chromatography/high resolution mass spectrometry (ESI-LC-IT-TOF/MS). Environmental Technology 32, 1409–1417. 10.1080/09593330.2010.537829. [DOI] [PubMed] [Google Scholar]
- Moreman J, Lee O, Trznadel M, David A, Kudoh T, Tyler CR, 2017. Acute Toxicity, Teratogenic, and Estrogenic Effects of Bisphenol A and Its Alternative Replacements Bisphenol S, Bisphenol F, and Bisphenol AF in Zebrafish Embryo-Larvae. Environmental Science and Technology 51, 12796–12805. 10.1021/acs.est.7b03283. [DOI] [PubMed] [Google Scholar]
- Moriyama K, Tagami T, Akamizu T, Usui T, Saijo M, Kanamoto N, Hataya Y, Shimatsu A, Kuzuya H, Nakao K, 2002. Thyroid Hormone Action Is Disrupted by Bisphenol A as an Antagonist. The Journal of Clinical Endocrinology & Metabolism 87, 5185–5190. 10.1210/jc.2002-020209. [DOI] [PubMed] [Google Scholar]
- Naderi M, Wong MYL, Gholami F, 2014. Developmental exposure of zebrafish (Danio rerio) to bisphenol-S impairs subsequent reproduction potential and hormonal balance in adults. Aquatic Toxicology 148, 195–203. 10.1016/j.aquatox.2014.01.009. [DOI] [PubMed] [Google Scholar]
- Negishi T, Kawasaki K, Suzaki S, Maeda H, Ishii Y, Kyuwa S, Kuroda Y, Yoshikawa Y, 2004. Behavioral Alterations in Response to Fear-Provoking Stimuli and Tranylcypromine Induced by Perinatal Exposure to Bisphenol A and Nonylphenol in Male Rats. Environmental Health Perspectives 112, 1159–1164. 10.1289/ehp.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orozco A, Valverde RC, 2005. Thyroid hormone deiodination in fish Thyroid, 15, pp. 799–813. [DOI] [PubMed] [Google Scholar]
- Paige LA, Christensen DJ, Gron H, Norris JD, Gottlin EB, Padilla KM, Chang C. -y., Ballas LM, Hamilton PT, McDonnell DP, Fowlkes DM, 1999. Estrogen receptor (ER) modulators each induce distinct conformational changes in ER and ER. Proceedings of the National Academy of Sciences 96, 3999–4004. 10.1073/pnas.96.7.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palanza P, Gioiosa L, vom Saal FS, Parmigiani S, 2008. Effects of developmental exposure to bisphenol A on brain and behavior in mice. Environmental Research 108, 150–157. 10.1016/j.envres.2008.07.023. [DOI] [PubMed] [Google Scholar]
- Pennie W, Aldridge T, Brooks A, 1998. Differential activation by xenoestrogens of ER alpha and ER beta when linked to different response elements. Journal of Endocrinology 158, R11–R14. 10.1677/joe.0.158r011. [DOI] [PubMed] [Google Scholar]
- Peteffi GP, Fleck JD, Kael IM, Rosa DC, Antunes MV, Linden R, 2019. Brazilian Journal of Biology Ecotoxicological risk assessment due to the presence of bisphenol A and caffeine in surface waters in the Sinos River Basin-Rio Grande do Sul-Brazil. Braz. J. Biol 79, 712–721. 10.1590/1519-6984.189752. [DOI] [PubMed] [Google Scholar]
- Qin F, Wang L, Wang X, Liu S, Xu P, Wang H, Wu T, Zhang Y, Zheng Y, Li M, Zhang X, Yuan C, Hu G, Wang Z, 2013. Bisphenol A affects gene expression of gonadotropin-releasing hormones and type I GnRH receptors in brains of adult rare minnow Gobiocypris rarus. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP 157, 192–202. 10.1016/j.cbpc.2012.11.002. [DOI] [PubMed] [Google Scholar]
- Qiu W, Zhao Y, Yang M, Farajzadeh M, Pan C, Wayne NL, 2016a. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology 157, 636–647. 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- Qiu W, Zhao Y, Yang M, Farajzadeh M, Pan C, Wayne NL, 2016b. Actions of bisphenol A and bisphenol S on the reproductive neuroendocrine system during early development in zebrafish. Endocrinology 157, 636–647. 10.1210/en.2015-1785. [DOI] [PubMed] [Google Scholar]
- Qiu W, Shao H, Lei P, Zheng C, Qiu C, Yang M, Zheng Y, 2018. Immunotoxicity of bisphenol S and F are similar to that of bisphenol A during zebrafish early development. Chemosphere 194, 1–8. 10.1016/j.chemosphere.2017.11.125. [DOI] [PubMed] [Google Scholar]
- Qiu W, Fang M, Liu J, Fu C, Zheng C, Chen B, Wang KJ, 2019. In vivo actions of Bisphenol F on the reproductive neuroendocrine system after long-term exposure in zebrafish. Science of the Total Environment 665, 995–1002. 10.1016/j.scitotenv.2019.02.154. [DOI] [PubMed] [Google Scholar]
- Quesada I, Fuentes E, Viso-León MC, Soria B, Ripoll C, Nadal A, 2002. Low doses of the endocrine disruptor bisphenol-A and the native hormone 17β-estradiol rapidly activate transcription factor CREB. The FASEB Journal 16, 1671–1673. 10.1096/fj.02-0313fje. [DOI] [PubMed] [Google Scholar]
- Rahman MS, Kwon W-S, Lee J-S, Yoon S-J, Ryu B-Y, Pang M-G, 2015. Bisphenol-A Affects Male Fertility via Fertility-related Proteins in Spermatozoa. Scientific Reports 5, 9169. 10.1038/srep09169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmani S, Pour Khalili N, Khan F, Hassani S, Ghafour-Boroujerdi E, Abdollahi M, 2018. Bisphenol A: What lies beneath its induced diabetes and the epigenetic modulation? Life Sciences 214, 136–144. 10.1016/J.LFS.2018.10.044. [DOI] [PubMed] [Google Scholar]
- Raine JC, Takemura A, Leatherland JF, 2001. Assessment of thyroid function in adult medaka (Oryzias latipes) and juvenile rainbow trout (Oncorhynchus mykiss) using immunostaining methods. Journal of Experimental Zoology 290 (4), 366–378. [DOI] [PubMed] [Google Scholar]
- Raldúa D, Babin PJ, 2009. Simple, Rapid Zebrafish Larva Bioassay for Assessing the Potential of Chemical Pollutants and Drugs to Disrupt Thyroid Gland Function. Environmental Science & Technology 43 (17), 6844–6850. 10.1021/es9012454. [DOI] [PubMed] [Google Scholar]
- Rather MA, Bhat IA, Gireesh-Babu P, Chaudhari A, Sundaray JK, Sharma R, 2016. Molecular characterization of kisspeptin gene and effect of nano–encapsulted kisspeptin-10 on reproductive maturation in Catla catla. Domestic Animal Endocrinology 56, 36–47. 10.1016/j.domaniend.2016.01.005. [DOI] [PubMed] [Google Scholar]
- Reif DM, Martin MT, Tan SW, Houck KA, Judson RS, Richard AM, Knudsen TB, Dix DJ, Kavlock RJ, 2010. Endocrine Profiling and Prioritization of Environmental Chemicals Using ToxCast Data. Environmental Health Perspectives 118, 1714–1720. 10.1289/ehp.1002180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke M, 2010. Influences of the environment on the endocrine and paracrine fish growth hormone-insulin-like growth factor-I system. Journal of Fish Biology 76, 1233–1254. 10.1111/j.1095-8649.2010.02605.x. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Kim BM, Lee CJ, Yoon YD, Lee YM, Lee JS, 2011. Bisphenol A modulates expression of sex differentiation genes in the self-fertilizing fish, Kryptolebias marmoratus. Aquatic Toxicology 104, 218–229. 10.1016/j.aquatox.2011.04.020. [DOI] [PubMed] [Google Scholar]
- Riu A, McCollum CW, Pinto CL, Grimaldi M, Hillenweck A, Perdu E, Zalko D, Bernard L, Laudet V, Balaguer P, Bondesson M, Gustafsson J-A, 2014. Halogenated Bisphenol-A Analogs Act as Obesogens in Zebrafish Larvae (Danio rerio). Toxicological Sciences 139, 48–58. 10.1093/toxsci/kfu036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers JA, Mirza RS, 2013. The Effects of Bisphenol-A on the Immune System of Wild Yellow Perch, Perca flavescens. Water, Air, & Soil Pollution 224, 1728. 10.1007/s11270-013-1728-5. [DOI] [Google Scholar]
- Routledge EJ, White R, Parker MG, Sumpter JP, 2000. Differential Effects of Xenoestrogens on Coactivator Recruitment by Estrogen Receptor (ER) α and ERβ. Journal of Biological Chemistry 275, 35986–35993. 10.1074/jbc.M006777200. [DOI] [PubMed] [Google Scholar]
- Saili KS, Corvi MM, Weber DN, Patel AU, Das SR, Przybyla J, Anderson KA, Tanguay RL, 2012. Neurodevelopmental low-dose bisphenol A exposure leads to early life-stage hyperactivity and learning deficits in adult zebrafish. Toxicology 291, 83–92. 10.1016/j.tox.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez-Avila J, Bonet J, Velasco G, Lacorte S, 2009. Determination and occurrence of phthalates, alkylphenols, bisphenol A, PBDEs, PCBs and PAHs in an industrial sewage grid discharging to a Municipal Wastewater Treatment Plant. Science of The Total Environment 407, 4157–4167. 10.1016/j.scitotenv.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Santangeli S, Notarstefano V, Maradonna F, Giorgini E, Gioacchini G, Forner-Piquer I, Habibi HR, Carnevali O, 2018. Effects of diethylene glycol dibenzoate and Bisphenol A on the lipid metabolism of Danio rerio. Science of The Total Environment 636, 641–655. 10.1016/j.scitotenv.2018.04.291. [DOI] [PubMed] [Google Scholar]
- Santhi VA, Sakai N, Ahmad ED, Mustafa AM, 2012. Occurrence of bisphenol A in surface water, drinking water and plasma from Malaysia with exposure assessment from consumption of drinking water. Science of The Total Environment 427–428, 332–338. 10.1016/J.SCITOTENV.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Schmutzler C, Hamann I, Hofmann PJ, Kovacs G, Stemmler L, Mentrup B, Schomburg L, Ambrugger P, Grüters A, Seidlova-Wuttke D, Jarry H, Wuttke W, Köhrle J, 2004. Endocrine active compounds affect thyrotropin and thyroid hormone levels in serum as well as endpoints of thyroid hormone action in liver, heart and kidney. Toxicology 205, 95–102. 10.1016/j.tox.2004.06.041. [DOI] [PubMed] [Google Scholar]
- Segner H, Navas JM, Schäfers C, Wenzel A, 2003. Potencies of estrogenic compounds in in vitro screening assays and in life cycle tests with zebrafish in vivo. Ecotoxicology and Environmental Safety 54, 315–322. 10.1016/S0147-6513(02)00040-4. [DOI] [PubMed] [Google Scholar]
- Segner H, Wenger M, Möller AM, Köllner B, Casanova-Nakayama A, 2012. Immunotoxic effects of environmental toxicants in fish — how to assess them? Environmental Science and Pollution Research 19, 2465–2476. 10.1007/s11356-012-0978-x. [DOI] [PubMed] [Google Scholar]
- Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O’Rahilly S, Carlton MBL, Crowley WF, Aparicio SAJR, Colledge WH, 2003. The GPR54 Gene as a Regulator of Puberty. New England Journal of Medicine 349, 1614–1627. 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- Shahjahan M, Motohashi E, Doi H, Ando H, 2010. Elevation of Kiss2 and its receptor gene expression in the brain and pituitary of grass puffer during the spawning season. General and Comparative Endocrinology 169, 48–57. 10.1016/j.ygcen.2010.07.008. [DOI] [PubMed] [Google Scholar]
- Sheng ZG, Zhu BZ, 2011. Low concentrations of Bisphenol A induce mouse spermatogonial cell proliferation by G protein-coupled receptor 30 and estrogen receptor-?? Environmental Health Perspectives 119, 1775–1780. 10.1289/ehp.1103781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd BS, Johnson JK, Silverstein JT, Parhar IS, Vijayan MM, McGuire A, Weber GM, 2007. Endocrine and orexigenic actions of growth hormone secretagogues in rainbow trout (Oncorhynchus mykiss). Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 146, 390–399. 10.1016/j.cbpa.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Shibata N, Yoshikuni M, Nagahama Y, 1993. Vitellogenin Incorporation into Oocytes of Rainbow Trout, Oncorhynchus mykiss, in Vitro. Effect of Hormones on Denuded Oocytes. 10.1111/j.1440-169X.1993.00115.x. [DOI] [PubMed] [Google Scholar]
- Sogorb MA, Estévez J, Vilanova E, 2019. Case study: Is bisphenol S safer than bisphenol A in thermal papers? Archives of Toxicology. 10.1007/s00204-019-02474-x. [DOI] [PubMed] [Google Scholar]
- Sohoni P, Tyler CR, Hurd K, Caunter J, Hetheridge M, Williams T, Woods C, Evans M, Toy R, Gargas M, Sumpter JP, 2001. Reproductive effects of long-term exposure to Bisphenol A in the fathead minnow (Pimephales promelas). Environmental science & technology 35, 2917–2925. 10.1021/es000198n. [DOI] [PubMed] [Google Scholar]
- Song S, Song M, Zeng L, Wang T, Liu R, Ruan T, Jiang G, 2014. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environmental Pollution 186, 14–19. 10.1016/j.envpol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- Staples C. a, Dom PB, Klecka GM, Sandra TO, Harris LR, 1998. A review of the environmental fate, effects, and exposures of Bisphenol A. Chemosphere 36, 2149–2173. 10.1016/S0045-6535(97)10133-3. [DOI] [PubMed] [Google Scholar]
- Sun H, Shen O-X, Wang X-R, Zhou L, Zhen S, Chen X, 2009. Anti-thyroid hormone activity of bisphenol A, tetrabromobisphenol A and tetrachlorobisphenol A in an improved reporter gene assay. Toxicology in Vitro 23, 950–954. 10.1016/j.tiv.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Sun S-X, Zhang Y-N, Lu D-L, Wang W-L, Limbu SM, Chen L-Q, Zhang M-L, Du Z-Y, 2019. Concentration-dependent effects of 17β-estradiol and bisphenol A on lipid deposition, inflammation and antioxidant response in male zebrafish (Danio rerio). Chemosphere 237, 124422. 10.1016/j.chemosphere.2019.124422. [DOI] [PubMed] [Google Scholar]
- Takayanagi S, Tokunaga T, Liu X, Okada H, Matsushima A, Shimohigashi Y, 2006. Endocrine disruptor bisphenol A strongly binds to human estrogen-related receptor γ (ERRγ) with high constitutive activity. Toxicology Letters 167, 95–105. 10.1016/j.toxlet.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Tang Tianle, Yang Yang, Chen Yawen, Tang Wenhao, Wang Fuqiang, Diao Xiaoping, 2015. Thyroid Disruption in Zebrafish Larvae by Short-Term Exposure to Bisphenol AF. International Journal of Environmental Research Public Health 12 (10), 13069–13084. 10.3390/ijerph121013069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testai E, Hartemann P, Rodríguez-Farre E, Rastogi SC, Bustos J, Gundert-Remy U, Hensten A, Kopperud HM, Olea N, Piersma A, De Jong W, 2016. The safety of the use of bisphenol A in medical devices. Regulatory Toxicology and Pharmacology 79, 106–107. 10.1016/j.yrtph.2016.01.014. [DOI] [PubMed] [Google Scholar]
- Thomas P, Dong J, 2006. Binding and activation of the seven-transmembrane estrogen receptor GPR30 by environmental estrogens: A potential novel mechanism of endocrine disruption. The Journal of Steroid Biochemistry and Molecular Biology 102, 175–179. 10.1016/j.jsbmb.2006.09.017. [DOI] [PubMed] [Google Scholar]
- Tohmé M, Prud’homme SM, Boulahtouf A, Samarut E, Brunet F, Bernard L, Bourguet W, Gibert Y, Balaguer P, Laudet V, 2014. Estrogen-related receptor γ is an in vivo receptor of bisphenol A. The FASEB Journal 28, 3124–3133. 10.1096/fj.13-240465. [DOI] [PubMed] [Google Scholar]
- Tzatzarakis MN, Karzi V, Vakonaki E, Goumenou M, Kavvalakis M, Stivaktakis P, Tsitsimpikou C, Tsakiris I, Rizos AK, Tsatsakis AM, 2017. Bisphenol A in soft drinks and canned foods and data evaluation. Food Additives & Contaminants: Part B 10, 85–90. 10.1080/19393210.2016.1266522. [DOI] [PubMed] [Google Scholar]
- Valavanidis A, Vlahogianni T, Dassenakis M, Scoullos M, 2006. Molecular biomarkers of oxidative stress in aquatic organisms in relation to toxic environmental pollutants. Ecotoxicology and Environmental Safety 64 (2), 178–189. 10.1016/j.ecoenv.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Van der Geyten S, Byamungu N, Reyns GE, Kuhn ER, Darras VM, 2005. Iodothyronine deiodinases and the control of plasma and tissue thyroid hormone levels in hyperthyroid tilapia (Oreochromis niloticus). Journal of Endocrinology. 184, 467–479. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Hauser R, Marcus M, Olea N, Welshons WV, 2007. Human exposure to bisphenol A (BPA). Reproductive Toxicology 24, 139–177. 10.1016/j.reprotox.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Colborn T, Hayes TB, Heindel JJ, Jacobs DR, Lee DH, Shioda T, Soto AM, vom Saal FS, Welshons WV, Zoeller RT, Myers JP, 2012. Hormones and endocrine-disrupting chemicals: Low-dose effects and nonmonotonic dose responses. Endocrine Reviews 33, 378–455. 10.1210/er.2011-1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vom Saal FS, Welshons WV, 2006. Large effects from small exposures. II. The importance of positive controls in low-dose research on bisphenol A. Environmental Research 100, 50–76. 10.1016/j.envres.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Wabuke-Bunoti MAN, Firling CE, 1983. The prehatching development of the thyroid-gland of the fathead minnow, Pimephales-promelas (Rafinesque). General and Comparative Endocrinology 49 (2), 320–331. [DOI] [PubMed] [Google Scholar]
- Wanderlaar Bonga SE, 1993. Endocrinology. In: Evans DH (Ed.), The Physiology of Fishes. Boca Raton, FL, CRC Press, pp. 469–502. [Google Scholar]
- Wang J, Liu X, Wang H, Wu T, Hu X, Qin F, Wang Z, 2010. Expression of two cytochrome P450 aromatase genes is regulated by endocrine disrupting chemicals in rare minnow Gobiocypris rarus juveniles. Comparative Biochemistry and Physiology - C Toxicology and Pharmacology 152, 313–320. 10.1016/j.cbpc.2010.05.007. [DOI] [PubMed] [Google Scholar]
- Wang L, Wang Z, Liu J, Ji G, Shi L, Xu J, Yang J, 2018a. Deriving the freshwater quality criteria of BPA, BPF and BPAF for protecting aquatic life. Ecotoxicology and Environmental Safety 164, 713–721. 10.1016/j.ecoenv.2018.08.073. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Wang Z, Qin J, Wang Wei, Tian H, Ru S, 2018b. Bisphenol S induces obesogenic effects through deregulating lipid metabolism in zebrafish (Danio rerio) larvae. Chemosphere 199, 286–296. 10.1016/j.chemosphere.2018.01.163. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhang X, Qin J, Wei P, Jia Y, Wang J, Ru S, 2019. Long-term bisphenol S exposure induces fat accumulation in liver of adult male zebrafish (Danio rerio) and slows yolk lipid consumption in F1 offspring. Chemosphere 221, 500–510. 10.1016/j.chemosphere.2019.01.020. [DOI] [PubMed] [Google Scholar]
- Watson CS, Bulayeva NN, Wozniak AL, Alyea RA, 2007. Xenoestrogens are potent activators of nongenomic estrogenic responses. Steroids 72, 124–134. 10.1016/j.steroids.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiler K, Ramakrishnan S, 2019. Bisphenol F causes disruption of gonadotropin-releasing hormone neural development in zebrafish via an estrogenic mechanism. NeuroToxicology 71, 31–38. 10.1016/j.neuro.2018.12.001. [DOI] [PubMed] [Google Scholar]
- Wetherill YB, Akingbemi BT, Kanno J, McLachlan JA, Nadal A, Sonnenschein C, Watson CS, Zoeller RT, Belcher SM, 2007. In vitro molecular mechanisms of bisphenol A action. Reproductive Toxicology 24, 178–198. 10.1016/j.reprotox.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Wong J, Zsarnovszky A, Belcher S, 2003. Estrogens and ICI182,780 (Faslodex) modulate mitosis and cell death in immature cerebellar neurons via rapid activation of p44/p42 mitogen-activated protein kinase. The Journal of Neuroscience 23, 4984–4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood AW, Duan C, Bern HA, 2005. Insulin-Like Growth Factor Signaling in Fish. International Review of Cytology, pp. 215–285. 10.1016/S0074-7696(05)43004-1. [DOI] [PubMed] [Google Scholar]
- Wu M, Xu H, Shen Y, Qiu W, Yand M, 2011. Oxidative stress in zebrafish embryos induced by short-term exposure to bisphenol A, nonylphenol, and their mixture. Environmental toxicology and Chemistry 2335–2341. 10.1002/etc.634. [DOI] [PubMed] [Google Scholar]
- Xiong Q, Liu X, Shen Y, Yu P, Chen S, Hu J, Yu J, Li J, Wang H-S, Cheng X, Hong K, 2015. Elevated Serum Bisphenol A Level in Patients with Dilated Cardiomyopathy. International Journal of Environmental Research and Public Health 12, 5329–5337. 10.3390/ijerph120505329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Zhang J, Wang Y, Ye Y, Luo Q, 2010. Perinatal exposure to bisphenol-A impairs learning-memory by concomitant down-regulation of N-methyl-d-aspartate receptors of hippocampus in male offspring mice. Hormones and Behavior 58, 326–333. 10.1016/j.yhbeh.2010.02.012. [DOI] [PubMed] [Google Scholar]
- Xu H, Yang M, Qiu W, Pan C, Wu M, 2013. The impact of endocrine-disrupting chemicals on oxidative stress and innate immune response in zebrafish embryos. Environmental Toxicology and Chemistry 32, 1793–1799. 10.1002/etc.2245. [DOI] [PubMed] [Google Scholar]
- Yamazaki E, Yamashita N, Taniyasu S, Lam J, Lam PKS, Moon H-B, Jeong Y, Kannan P, Achyuthan H, Munuswamy N, Kannan K, 2015. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicology and Environmental Safety 122, 565–572. 10.1016/j.ecoenv.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Yang B, Jiang Q, Chan T, Ko WKW, Wong AOL, 2010. Goldfish kisspeptin: Molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. General and Comparative Endocrinology 165, 60–71. 10.1016/j.ygcen.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Yang Yunjia, Lu L, Zhang J, Yang Yi, Wu Y, Shao B, 2014. Simultaneous determination of seven bisphenols in environmental water and solid samples by liquid chromatography–electrospray tandem mass spectrometry. Journal of Chromatography A 1328, 26–34. 10.1016/j.chroma.2013.12.074. [DOI] [PubMed] [Google Scholar]
- Yang M, Qiu W, Chen B, Chen J, Liu S, Wu M, Wang K-J, 2015. The In Vitro Immune Modulatory Effect of Bisphenol A on Fish Macrophages via Estrogen Receptor α and Nuclear Factor-κB Signaling. Environmental Science & Technology 49, 1888–1895. 10.1021/es505163v. [DOI] [PubMed] [Google Scholar]
- Yang Q, Yang X, Liu J, Ren W, Chen Y, Shen S, 2017. Effects of BPF on steroid hormone homeostasis and gene expression in the hypothalamic–pituitary–gonadal axis of zebrafish. Environmental Science and Pollution Research 24, 21311–21322. 10.1007/s11356-017-9773-z. [DOI] [PubMed] [Google Scholar]
- Yin DQ, Hu SQ, Gu Y, Wei L, Liu SS, Zhang AQ, 2007. Immunotoxicity of bisphenol A to Carassius auratus lymphocytes and macrophages following in vitro exposure. J Environ Sci (China) 19, 232–237. [DOI] [PubMed] [Google Scholar]
- Yokota H, Tsuruda Y, Maeda M, Oshima Y, Tadokoro H, Nakazono A, Honjo T, Kobayashi K, 2000. Effect of bisphenol a on the early life stage in Japanese medaka (Oryzias latipes). Environmental Toxicology and Chemistry 19, 1925–1930. 10.1002/etc.5620190730. [DOI] [Google Scholar]
- Yoshiura Y, Sohn Y, Munakata A, 1999. et al. Molecular cloning of the cDNA encoding the β subunit of thyrotropin and regulation of its gene expression by thyroid hormones in the goldfish, Carassius auratus. Fish Physiology and Biochemistry 21, 201–210. 10.1023/A:1007884527397. [DOI] [Google Scholar]
- Yu BP, 1994. Cellular defenses against damage from reactive oxygen species. Physiological Reviews 74, 139–162. [DOI] [PubMed] [Google Scholar]
- Zelikoff JT, Carlson E, Li Y, Raymond A, Duffy J, Beaman JR, Anderson M, 2002. Immunotoxicity Biomarkers in Fish: Development, Validation and Application for Field Studies and Risk Assessment. Human and Ecological Risk Assessment: An International Journal 8, 253–263. 10.1080/20028091056890. [DOI] [Google Scholar]
- Zhang Y, Gao J, Xu P, Yuan C, Qin F, Liu S, Zheng Y, Yang Y, Wang Z, 2014. Low-dose bisphenol A disrupts gonad development and steroidogenic genes expression in adult female rare minnow Gobiocypris rarus. Chemosphere 112, 435–442. 10.1016/j.chemosphere.2014.04.089. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Tao S, Yuan C, Liu Y, Wang Z, 2016. Non-monotonic dose–response effect of bisphenol A on rare minnow Gobiocypris rarus ovarian development. Chemosphere 144, 304–311. [DOI] [PubMed] [Google Scholar]
- Zhang D-H, Zhou E-X, Yang Z-L, 2017. Waterborne exposure to BPS causes thyroid endocrine disruption in zebrafish larvae. PLoS One 12 (5), e0176927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang YF, Ren XM, Li YY, Yao XF, Li CH, Qin ZF, Guo LH, 2018. Bisphenol A alternatives bisphenol S and bisphenol F interfere with thyroid hormone signaling pathway in vitro and in vivo. Environmental Pollution 237, 1072–1079. 10.1016/j.envpol.2017.11.027. [DOI] [PubMed] [Google Scholar]
- Zhao F, Jiang G, Wei P, Wang H, Ru S, 2018. Bisphenol S exposure impairs glucose homeostasis in male zebrafish (Danio rerio). Ecotoxicology and Environmental Safety 147, 794–802. 10.1016/j.ecoenv.2017.09.048. [DOI] [PubMed] [Google Scholar]
- Zoeller RT, Bansal R, Parris C, 2005. Bisphenol-A, an Environmental Contaminant that Acts as a Thyroid Hormone Receptor Antagonist in Vitro, Increases Serum Thyroxine, and Alters RC3/Neurogranin Expression in the Developing Rat Brain. Endocrinology 146, 607–612. 10.1210/en.2004-1018. [DOI] [PubMed] [Google Scholar]
- Zohar Y, Muñoz-Cueto JA, Elizur A, Kah O, 2010. Neuroendocrinology of reproduction in teleost fish. General and Comparative Endocrinology 165, 438–455. 10.1016/j.ygcen.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Zwart W, de Leeuw R, Rondaij M, Neefjes J, Mancini MA, Michalides R, 2010. The hinge region of the human estrogen receptor determines functional synergy between AF-1 and AF-2 in the quantitative response to estradiol and tamoxifen. Journal of cell science 123, 1253–1261. 10.1242/jcs.061135. [DOI] [PubMed] [Google Scholar]


