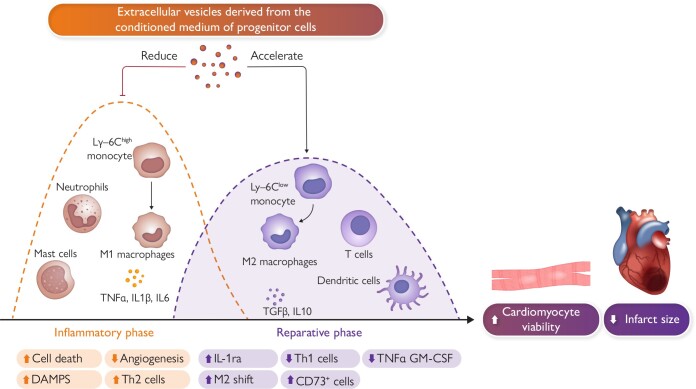
Graphical Abstract
Graphical Abstract.
Inflammation plays a significant role in acute myocardial infarction, affecting both the early phase following the clinical presentation of acute coronary syndrome and the subsequent chronic phase. During the acute phase, various inflammatory cell types, such as T-cells, granulocytes, monocytes, and B-cells, are attracted to the infarcted area to remove necrotic tissue and promote scar tissue formation. Initially, pro-inflammatory cells like polymorphonuclear neutrophils and M1 pro-inflammatory macrophages are predominant. Within 24–48 h, these M1 macrophages transition into an anti-inflammatory M2 state, contributing to the healing process. T-cells, including CD8+ and CD4+ types, are activated within lymph nodes connected to the heart and play a role in the healing process. Experiments conducted in small and large animals suggest a potential therapeutic role for exogenous extracellular vesicles (EVs) as an adjunctive therapy to percutaneous coronary intervention. EV-mediated modulation of the immune response facilitates a transition from an inflammatory state to a resolving phase after ischaemic damage. Abbreviations: DAMPS, damage-associated molecular pattern molecules; GM-CSF, granulocyte-macrophage colony stimulating factor; IL, Interleukin; IL-1ra, interleukin-1 receptor antagonist; Ly-6C, lymphocyte antigen; M1, inflammatory monocytes/macrophages; M2, reparative monocytes/macrophages; TGFB, transforming growth factor beta; Th2, T-helper cells; TNF-α, tumour necrosis factor alpha.
Keywords: Extracellular vesicles, Acute myocardial infarction, Exosomes, Large-animal studies
Abstract
Despite improvements in clinical outcomes following acute myocardial infarction, mortality remains high, especially in patients with severely reduced left ventricular ejection fraction (LVEF <30%), emphasizing the need for effective cardioprotective strategies adjunctive to recanalization. Traditional cell therapy has shown equivocal success, shifting the focus to innovative cardioactive biologicals and cell mimetic therapies, particularly extracellular vesicles (EVs). EVs, as carriers of non-coding RNAs and other essential biomolecules, influence neighbouring and remote cell function in a paracrine manner. Compared to cell therapy, EVs possess several clinically advantageous traits, including stability, ease of storage (enabling off-the-shelf clinical readiness), and decreased immunogenicity. Allogeneic EVs from mesenchymal and/or cardiac stromal progenitor cells demonstrate safety and potential efficacy in preclinical settings. This review delves into the translational potential of EV-based therapeutic approaches, specifically highlighting findings from large-animal studies, and offers a synopsis of ongoing early-stage clinical trials in this domain.
Introduction
With the implementation of early reperfusion therapy by percutaneous coronary intervention (PCI), clinical outcomes following acute myocardial infarction (AMI) have improved greatly.1 However, recent data reveal further room for improvement in mortality after acute coronary syndrome (ACS), both for ST (ST segment of ECG)-elevation myocardial infarction (STEMI) and non-STEMI patients. At 28 days of follow-up, non-STEMI patients have a lower probability of death than STEMI patients, but long-term (10-year follow-up) mortality is high and similar in the two groups (19.6% and 22.8% for STEMI and non-STEMI patients, respectively).2 Moreover, in patients with severely reduced left ventricular ejection fraction (LVEF <30%), in-hospital mortality remains >20%.3 Thus, an effective cardioprotective approach, adjunctive to early PCI, would be a welcome addition to the armamentarium. Cell therapy has proven to be rather disappointing,4,5 such that the focus has shifted to novel approaches involving cardioactive biologicals6 or cell mimetic therapies, such as therapeutic microparticles functionalized with biomimetic cardiac stem cell membranes and secretome,7 as well as native extracellular vesicles (EVs) secreted from multiple types of stem/progenitor cells.8–11 Although promising preclinical results have not yet translated into demonstrated clinical benefits, ongoing research on EVs in animal models of AMI highlights new opportunities to recruit cardioprotection after PCI (recognizing the difficulties in extrapolating preclinical data to clinical practice). EVs, including large, medium, and small cell-secreted vesicles, are carriers of non-coding RNAs including microRNAs (miRNA), as well as lipids, proteins, and (sometimes) DNA.12 The RNA fingerprints of EV cargo are quite specific to cell type of origin13 and sensitive to culture conditions.14 Through cell–cell transfer of these cargoes, EVs regulate neighbouring and remote cell function in a paracrine manner. Compared to cell therapy, EVs offer several appealing features for clinical applications as PCI-adjunctive therapy: they are acellular, non-replicating, stable during storage, and elicit a minimal immune response even when allogeneic (i.e. unmatched to the recipient).15 The hypoimmunogenic nature of allogeneic EVs facilitates clinical applications, as demonstrated in a recent pilot trial where allogeneic placenta mesenchymal stromal cell-derived EVs were safely injected into stroke patients.16
Evidence for the efficacy of EVs in small and large-animal models of AMI has mainly been demonstrated through intramyocardial (IM) delivery in open-chest models.8,9,11,17 The potential of intracoronary (IC) delivery in a translational closed-chest pig model of reperfused AMI has also been tested, with seemingly conflicting outcomes. In this review, we explore the translational potential of EV-based therapeutic approaches, with a specific focus on findings from large-animal studies (Table 1). Additionally, we provide a summary of the current landscape of ongoing clinical trials in this field.
Table 1.
Randomized large-animal studies
| Cell source | Method of purification of EV | Dose | Delivery method | Anti-platelet treatments | Readouts of EV-treated animals vs. control at final endpoint | |
|---|---|---|---|---|---|---|
| Emmert et al.18 [Yorkshire landrace pigs Ꝑ] | Human cardiac-progenitor cells (CPCs) | Tangential flow filtration GMP-grade | 22 mg protein equivalent of EV [≍20 × 1011 particles] | IC | Yes | CO[L/min]↑ LVEF[%]↑ IV [mL]↓ IS↓ Collagen↓ Vessel density↑ |
| Gallet et al.19 [Yucatan mini-pigs Ꝑ] | Human cardiosphere-derived cells (CDCs) | Polyethylene glycol (PEG) | 7.5 mg protein equivalent of EV [≍16.5 × 1011 particles] | IM | ? | CMs proliferation↑ CM hypertrophy↓ LVEF[%]↑ Transmurality↓ IS↓ Collagen↓ Vessel density↑ |
| Charles et al.20 [Yorkshire landrace pigs] | Human embryonic stem cell-derived MSC | Tangential flow filtration | 1 mg protein equivalent of EV [7 mg cumulative dose] | IV (twice daily bolus for seven days) | Yes | Mid-size wall thickness↑ LVEF[%] 7days↑ IS↓ |
| Gao et al.11 [Yorkshire landrace pigs] | Human iPSC-derived cardiac cells | Ultrafiltration + polyethylene glycol (PEG) | 7.5 mg protein equivalent of EV | IM | ? | LVEF[%]↑ LVEDV [mL]↓ Wall stress↓ IS↓ LVW/BW[g/kg]↓ Vessel density↑ CM hypertrophy↓ |
| Monguió-Tortajada et al.21 [Yorkshire landrace pigs 50% Ꝑ] | Pig cardiac adipose tissue-derived MSC (cATMSC) | Ultrafiltration + size exclusion chromatography (SEC) | EV from 2 × 107 cATMSCs | Human decellularised cardiac scaffold secured over the ischaemic myocardium | ? | %CCR2+ MØ↓ Collagen I↓ CD73+ MØ ↑ PBMC/µL blood 2 days↓ Lymphocytes/µL blood 2 days↓ |
| Li et al.22 [Cynomolgus monkeys] | Monkey bone marrow-derived MSC | Differential ultracentrifugation | 1 mg protein equivalent of EV | IM | ? | LVEF[%] ↑ LVEDV [mL]↓ LVESV↓ IS↓ MMP19↓ Vessel density↑ |
| López et al.23 [Large White pigs] | Pig cardiosphere-derived cells (CDCs) | Ultrafiltration | 9.16 protein equivalent of EV | Intrapericardially | Yes | WBC↑ (72 h) Neutrophils↑ (72 h) CD14 + CD16+↑ (72 h) Arg1+↑ (24 h) |
Summary of major large-animal studies utilizing a randomized approach to investigate the application of extracellular vesicles in the context of myocardial infarction. We have specifically chosen interventional studies and, for each study, have emphasized the cell source, method of EV purification, dosage, delivery methods, the incorporation of anti-platelet treatments, and the principal findings in EV-treated animals compared to the control group at the study endpoint. An upward-pointing arrow signifies improvement in the treated group compared to the control, while a downward-pointing arrow indicates decline in EV-treated animals vs. control.
Abbreviations: CPC, cardiac progenitor cells; CDC, cardiosphere-derived cells; CM, cardiomyocytes; CO, cardiac output; GMP, good manufacturing practice; IS, infarct size; LVEF, left ventricular ejection fraction; LVEDV, left ventricular end-diastolic volume; LVESV, left ventricular end-systolic volume; LVW, left ventricular weight BW, body weight; MSC, mesenchymal stem cells; PEG, polyethylene glycol; TNF, tumour necrosis factor; TGF-β, Transforming growth factor beta; IL, interleukin; Th2, T-helper cells; TFF, tangential flow filtration; WBC, white blood cell count.
The methodology employed in this review paper involves analysis of studies focused on EVs in the context of large-animal models. A systematic literature search was conducted across PubMed, encompassing articles that investigated the role of EVs in large-animal studies. The inclusion criteria prioritized studies involving pigs and primates to provide an understanding of the translational relevance of EV research. As for EV sources, we prioritized EVs isolated from a medium conditioned by cells cultured in vitro (Graphical Abstract). With regard to ongoing clinical trials, we searched ClinicalTrials.gov with a specific emphasis on interventional trials of EVs and/or exosomes for any cardiovascular indication (not limited to AMI). Searches were current as of 28 November 2023.
Extracellular vesicles
EVs are a diverse group of small membranous particles released by cells into the extracellular space, playing roles in intercellular communication. The lipid bilayer of EVs resembles the cell membrane, providing a protective envelope for their cargo. Proteins found on their surface or intercalated within the membrane, including various receptors and signaling molecules, contribute to EVs’ specific functions in cell-to-cell communication.9,24 Moreover, EVs carry a payload of nucleic acids, especially non-coding RNAs, which can be transferred to recipient cells, influencing gene expression and cellular processes. EVs can be categorized based on their distinct mechanisms of release and/or size. Exosomes (30–150 nm), originating within multivesicular bodies, encapsulate cytoplasmic cargo through inward budding before being released upon fusion with the cell membrane. Exosomes are viral-sized; for size reference, an human immunodeficiency virus virion has a diameter of ∼100 nm.25 Microvesicles, with a broader size range of 100–1000 nm, formed through outward budding of the plasma membrane, directly package cellular components, and are shed into the extracellular space. Apoptotic bodies, byproducts of programmed cell death, encapsulate cellular fragments and are released during apoptosis; their diameter is variable but generally >1000 nm, approximating the size of platelets.26 Given the varied usage and the inconsistencies across different literature sources, to maintain consistency throughout the manuscript and avoid potential confusion from varied terminology, we universally refer to EVs, encompassing both exosomes and microvesicles. While there is some interest in them as therapeutic candidates,27,28 apoptotic bodies are not considered here given the sparsity of their application to cardiovascular disease models.
Extracellular vesicles in acute cardioprotection
In AMI patients, urgent PCI is the main objective, such that cardioprotection would most realistically be implemented around the time of reperfusion or afterwards.5 The goal is to increase the number of surviving cardiomyocytes (CM) and reduce infarct size, limiting long-term remodelling and adverse events.29 However, therapies adjunctive to PCI have yielded either neutral results or demonstrated only marginal benefits in patients. The only therapy that appears to show significant effectiveness when combined with PCI is the current ‘standard-of-care’ with anti-platelet therapy; potent P2Y purinoceptor inhibitors30,31 reduce the risk of ischaemic events after ACS.31 Tailoring the duration and intensity of anti-platelet therapy to balance the risks of ischaemia and bleeding for individual patients may significantly reduce mortality after an acute ischaemic event. Interestingly, the protective effects of such agents seem to go beyond their antithrombotic properties. Anti-platelet agents confer direct postconditioning-like protection through mediators such as protein kinase C (PKC), mitochondrial adenosine triphosphate-sensitive potassium channels, and survival kinases.32 Not yet translated to humans is the phenomenon of cellular postconditioning, in which EVs from cardiac stromal/progenitor cells (cardiosphere-derived cells, CDCs) recapitulate the cardioprotective effects of cell therapy. When such heart-derived EVs are injected after reperfusion in small animal AMI models,9,33,34 they activate pro-survival pathways including Akt and ERK1/2, decrease caspase activation, and reduce cardiomyocyte apoptosis.9,34 Furthermore, the vesicular transfer of miR-181b into activated monocytes reduces PKCδ expression and enhances the macrophage-mediated cardioprotective effects.35 These findings suggest a potential therapeutic role for EVs as an adjunct to PCI, even if delayed until reflow has been established.
Recently, a mesenchymal-like cell population that is derived ex vivo from cardiac biopsies, either shedding from cardiac tissue (cardiac progenitor cells, CPCs) or obtained by plating self-assembling spherical aggregates of biopsy outgrowth in culture (CDCs), has emerged as a potential alternative cellular source for cardioprotection. The cytoprotective benefits of these cells in vivo are mediated primarily by their release of EVs, rather than by direct differentiation into new CM.8,34,36,37 In a recent large-animal preclinical trial, Emmert et al. evaluated the feasibility, safety, and effectiveness of EVs derived from human CPCs for the treatment of AMI in a pig model.18 The study compared IM and IC delivery. Two to three days post-treatment, pigs receiving IM EVs experienced decreased cardiac output, potentially due to needle-related injury. However, by 1-month post-treatment, both IM and IC groups showed a trend towards an increase in LVEF and a significant rise in left ventricular stroke volume (LVSV), indicating improved cardiac function. Additionally, infarct size decreased in the IC-delivered EV group compared to the IM-delivered EV group and the control group. The study included a second randomized protocol exploiting IC delivery as an elective approach with a longer follow-up. EV-treated animals showed increased LVEF and reduced infarct size compared to the control group. Furthermore, IC-delivered EVs were associated with reduced myocardial fibrosis (scar tissue) and increased blood vessel density in the heart. Notably, this improvement was observed over a 3-month period.18 The study found IC delivery of EVs to be safe, with no adverse events related to the EV therapy during follow-up. In an earlier study by Gallet et al.,19 pigs received human CDC-EVs or a control solution 30 min after reperfusion, with the EVs delivered either IC or IM. After 48 h, micro-vascular obstruction (MVO) was reduced by both IM and IC EVs, but the scar was reduced only by IM-delivered EVs.19 The two studies concur in showing clear benefits of heart-derived EVs administered after reflow but differ in the relative merits of IC vs. IM delivery. Such differences may simply reflect the duration of follow-up, insofar as Gallet’s study examined left ventricular function and infarct size only 48 h after treatment, whereas Emmert et al. extended follow-up to 3 months. In any case, the authors concur that IC delivery aligns well with the routine clinical practices of PCI.
Another alternative may be intravenous (IV) delivery of EVs. Charles et al. showed the effectiveness of EVs from clonal immortalized human embryonic mesenchymal stem cells in a porcine model of AMI.20 After being administered through IV bolus injection starting 60 min after ligation followed by twice daily boluses for seven days, EVs led to a significant reduction (30%–40%) in infarct size. This reduction was observed both 7 and 28 days after the AMI event, even though the levels of high-sensitivity troponin T were similar in the treated and control groups. Although the overall LVEF was not different from control, treatment with EVs reduced transmurality and lessened wall thinning in the infarct zone. The study concluded that systemic administration of EVs led to a notable reduction in infarct size while maintaining relatively better cardiac function compared to the control groups.20
Recently, an increasing body of research has demonstrated that induced pluripotent stem cells (iPSC) and their derived heart cells exhibit therapeutic effects through a paracrine mechanism, and specifically via EVs, in addition to their capacity for differentiation into heart cells. Gao et al. studied the effects of EVs secreted from a combination of CM, endothelial cells, and smooth muscle cells derived from human induced pluripotent stem cells in a pig model of AMI, or a blend of homogenized cell fragments.11 The cells, fragments, and EVs were directly injected into five sites in the border zone of the injured myocardium at reperfusion, 60 min after left anterior descending artery occlusion. Four weeks later, the EV-treated group exhibited superior outcomes in comparison to the control group (which received saline injections), as manifested by improved LVEF, wall stress, myocardial bioenergetics, cardiac hypertrophy, scar size, apoptosis, and angiogenesis within the infarcted region. Remarkably, the EV-treated group was not different from the groups that received intact cell injections, indicating that EV-based therapy alone can replicate the benefits of cell therapy.
To mitigate non-specific and unintended impacts of EV delivery, targeted and localized administration via polymeric scaffolds presents a potential solution. The integration of EVs within scaffolds can enhance tissue recovery, wound healing, bone regeneration, immunomodulation, and vascular functionality.38 Consequently, the approach of delivering EV through biopolymeric scaffolds is gaining substantial traction in the realm of tissue engineering. Monguió-Tortajada et al. employed a tissue engineering approach to locally deliver EVs derived from cardiac adipose tissue mesenchymal stromal cells (cATMSC-EV) in an AMI pig model with a human decellularized pericardial scaffold.21,39 After inducing AMI by permanent coronary artery ligation via thoracotomy, the treated group received a decellularized pericardial scaffold filled with a peptide hydrogel and cATMSC-EV, while the control group received a scaffold exposed only to buffer. Thirty days after placing these scaffolds over the infarcted region, cardiac magnetic resonance imaging (MRI) demonstrated structural and functional improvements in EV-treated animals, including a higher right ventricular ejection fraction (increased by 20.8% compared to untreated) and reduced ventricular dilation, indicative of diminished adverse remodelling. Scar size was smaller in the EV-treated group, with less fibrosis observed in the distal myocardium (reduced by 42%).21 Notably, animals treated with EVs displayed distinct changes in monocyte/macrophage interactions, with reduced inflammatory macrophages (CCR2+), and more macrophages expressing the ‘anti-inflammatory’ marker CD73+, in the infarct zone. Meanwhile, fewer peripheral blood mononuclear cells (PBMCs) were circulating 2 days after AMI, contributing to lower levels of tumour necrosis factor (TNF)α and granulocyte-macrophage colony stimulating factor (GM-CSF), indicating that the treatment had positive effects on systemic inflammation.21 While conceptually innovative, the clinical utility of this approach is limited by its highly-invasive surgical nature. Independently, miR-486-5p-overexpressing EVs were tested in a relevant non-human primate model of myocardial infarction (MI). These EVs, sourced from primate MSCs, were administered IM 30 min after AMI induction.22 MRI conducted one month later demonstrated enhanced LVEF, reduced end-systolic volume (ESV), and decreased infarct size. Immunofluorescence analysis of heart sections revealed that animals treated with miR-486-5p-overexpressing EVs exhibited higher vascular density in both the border zone and the infarcted zone compared to controls. Cardiac coronary artery computed tomography (CT) angiography also suggested better vascularization in the infarcted region of the miR-486-5p-overexpressing EV group.22 It is worth noting that animals in the aforementioned studies received daily doses of anti-platelet agents (i.e. clopidogrel or clopidogrel plus aspirin), such that the benefits observed were additive to those of anti-platelet therapy. Nevertheless, enthusiasm once again must be tempered by the fact most of the studies involved open-chest induction of AMI and injection of test agents, making extrapolation to clinical practice tenuous, if not implausible.
Extracellular vesicles in late cardioprotection
AMI produces the abrupt demise of cardiac tissue due to ischaemia, but also leads to prolonged detrimental alterations in the affected myocardium, causing disruptions in metabolism and ionic balance at the cellular level. These disruptions ultimately lead to cellular death late after the acute event, through both necrosis and apoptosis. Within the infarct border zone, CM uncoupled from the surviving myocardium are subjected to mechanical stress that results in apoptosis. Meanwhile, other cell types become senescent in response to injury, releasing a pro-inflammatory secretome.40,41 In a murine model, 1 week post-AMI, there is an increase in markers of apoptosis (e.g. TUNEL and cleaved caspase-3-positive nuclei).42 This delayed onset of apoptosis persists during the chronic phase of ischaemia, peaking two weeks after left coronary artery ligation.43 Post-AMI apoptosis is predominantly localized in the border zone and the infarct area itself. It may also occur following reperfusion in regions experiencing global hypoxia. These experimental observations lay the foundation for investigating a ‘late-cardioprotective approach’ using EVs as a biotherapeutic strategy. This concept was explored by Gallet et al. in their second preclinical randomized trial. Closed-chest AMI was induced in female Yucatan mini-pigs.19 After 4 weeks, 12 animals were randomly divided into two groups: one receiving vehicle and the other receiving closed-chest IM injections of CDC-EVs. Relative to baseline (before treatment), MRIs obtained 1 month post-MI revealed LV end-systolic volume to be increased in controls, but not in EV-treated pigs, indicating reduced adverse ventricular remodelling. EV-treated pigs had higher LVEF at endpoint despite similar baseline values. Circumferential strain measurements also reflected improved systolic function in EV-treated pigs. By late-gadolinium enhanced MRI, scar mass and scar size decreased in the EV group but not in the CDC group, resulting in a smaller scar at endpoint. Taken together, the studies show that EVs may, potentially, be cardioprotective even when administered 1–4 weeks post-AMI. An alternative strategy that could be explored to achieve the goal of delayed cardioprotection is the intrapericardial administration of EVs.23,44 In large-animal studies, allogeneic pig-derived CDC-EVs (equivalent to 9.16 mg total proteins) were intrapericardially injected via a mini-thoracotomy.23 The injection of EVs resulted in a peripheral and local (pericardial fluid) increase in pro-angiogenic and immunomodulatory subsets of M2 macrophages, aligning with findings from previous studies that employed open-chest IM injections. Although this approach was tested no more than 72 h post-MI and requires a minimally invasive surgical procedure, Zhu et al. showed that a similar approach can be used to deliver lncRNA therapy to prevent cardiac fibrosis and promote cardiac repair.44
Safety assessments
Although EVs from MSCs of different tissue origins have been shown to exert immunomodulatory effects, some questions remain unanswered particularly if repeat dosing is to be considered. Immunomodulatory effects of syngeneic EVs have been demonstrated in both short-term (6 days)39 and long-term (30 days)21 porcine models of AMI, showing reductions in macrophage and lymphocyte infiltration. Monguió-Tortajada et al. reported both local and systemic effects of scaffold-embedded EVs when administered acutely post-AMI. On a systemic level, they observed a twofold rise in circulating interleukin (IL)-1ra a natural competitive antagonist of the IL-1 receptor which blocks IL-1α/β inflammatory effect, as well as a reduction in post-AMI PBMC rush on the second day. The levels of TNFα and GM-CSF were decreased 30 days post-AMI. Furthermore, they observed recruitment within myocardium of an anti-inflammatory cell subset, namely CD73+ monocytes.21 These findings suggest that EV-mediated modulation of the immune response facilitates a transition from an inflammatory state to a resolving phase after ischaemic damage, consistent with previous observations with CDC-EVs.35,45
Transplanting human EVs into non-immunosuppressed pigs in a xenogeneic experimental design has been shown to recapitulate the entire benefit profile of auto- or allogeneic parental cells without apparent adverse effects.18,19,46 Histological grade 1 rejection was observed in pigs from both EV- and vehicle-injected groups, suggesting that this inflammatory reaction is more likely to be related to factors other than the EVs themselves (e.g. needle track injury).19 Pro-arrhythmic complications present a significant safety concern in cardiac biotherapeutics, given the ventricular tachycardias seen with embrionic stem cells (ESC)-derived cardiomyocyte transplantation in large-animals.47–49 Only a limited number of studies have systematically addressed the arrhythmogenic potential of EV transplantation, but no complications in cardiac rhythm have been observed. Gao et al. found that animals injected with secreted EVs by iPSC-derived cardiac cells did not exhibit an increased propensity to ventricular tachycardia and/or ventricular fibrillation, induced by programmed electrical stimulation.11 In non-human primates, IM injection of EVs did not increase the occurrence of paroxysmal supraventricular tachycardia, premature ventricular contractions, or non-sustained ventricular tachycardia in the initial 48-hour period following AMI. Furthermore, no changes were detected in the effective refractory period or ventricular fibrillation threshold (in treated vs. control groups) 28 days post-AMI.22 Dawkins et al. demonstrated that CDC-EVs, when injected into the heart, inhibited the inducibility of ventricular arrhythmias by programmed electrical stimulation,46 providing further reassurance that EVs may not share the arrhythmogenic potential of cell transplantation. The pro-arrhythmic effects of iPSC- and ESC-derived cell transplantation are likely due to engraftment and coupling of surviving cells, which introduce repolarization inhomogeneities and abnormal automaticity into the post-AMI heart. EVs,50 on the other hand, favour scar healing and improve endogenous tissue recovery without introducing exogenous cells that produce electrophysiological complications,46 rationalizing their superior performance in terms of arrhythmias.
Extracellular vesicles in clinical trials
Clinical trials of EVs for cardiac indications are in their infancy. While we review the available information here, the self-declared nature of study information, combined with limited verification, can result in inconsistent updates, potentially excluding recent data from both completed trials and published studies. While several Phase I-II clinical trials are underway to test the potential of EVs derived from autologous dendritic cells (DCs) to enhance immunoreaction immunotherapy against cancer antigens,51–53 limited evidence currently exists regarding the clinical application of EVs in patients with AMI. The ongoing EV-AMI study (Safety Evaluation of Intracoronary Infusion of Extracellular Vesicles in Patients With AMI, NCT04327635), a single-centre study in the USA, aims to evaluate the safety of administering up to three doses of purified exosome product (PEP) to 18 patients with AMI, within 12 h of symptom onset to PCI. According to the ClinicalTrials.gov description, PEP is derived from banked human blood. Infusion takes place within 20 min of stent placement or post-dilation and is delivered distal to the stent over ∼5 min. The primary objective of this open-label study is to assess dose-limiting toxicities and the maximum tolerated dose of PEP with escalating concentrations of EVs. Secondary outcomes include infarct size and LV function, evaluated using cardiac MRI, along with monitoring for alloimmune responses.
The Co-transplantation of Mesenchymal Stem Cell-Derived Exosomes and Autologous Mitochondria for Patients Candidates for Coronary Artery Bypass Grafting (CABG) Surgery (NCT05669144), a single-centre study in Iran, involves multiple intervention groups: IC and IM injection of mesenchymal stromal cells-derived EVs, IC and IM injection of mitochondria, combined IC and IM injection of EVs and mitochondria, and placebo (five patients). Primary outcome measures include LVEF and monitoring for adverse reactions like angioedema, hypotension, and acute allergic reactions.
A distinct study design has been devised for the Treatment of Non-ischemic Cardiomyopathies by Intravenous Extracellular Vesicles of Cardiovascular Progenitor Cells (SECRET-HF) (NCT05774509), a single-centre study in France. This clinical trial seeks to evaluate the safety and efficacy of three IV injections of EV-enriched secretome derived from CPCs in severely symptomatic patients with drug-refractory LV dysfunction due to non-ischaemic dilated cardiomyopathy.
These clinical trials may begin to address several critical questions, such as the safety profile of EV after cardiac injection. However, many key areas of concern remain unaddressed, such as the safety and tolerability of repeated injections.
Conclusions
The emerging field of EV-based therapeutics holds promise in reshaping our approach to cardiac health and recovery.54 EVs possess the unique ability to address multiple pathways simultaneously, encapsulating a variety of active biomolecules.55,56 This distinctive attribute becomes especially advantageous in the aftermath of AMI. Studies in pigs have consistently revealed positive outcomes in infarcted hearts following EV administration. Among these benefits, EVs have demonstrated the capacity to promote scar vascularization, mitigate fibrosis, and regulate the infiltration of macrophages and lymphocytes into the infarct zone. The remarkable immunomodulatory effects observed in rodent and porcine models of AMI underscore the potential of EVs to orchestrate a transition from inflammatory to resolving phases after ischaemic injury. The findings suggest that EV-mediated immune response modulation triggers the recruitment of anti-inflammatory cell subsets, contributing to post-AMI recovery. Notably, xenogeneic experiments introducing human EVs into non-immunosuppressed pigs have showcased the benefits of xenogeneic EV-based therapies without apparent adverse effects, at least with single doses.11,18,19
The concern surrounding pro-arrhythmogenic complications of EV-based therapy still needs systematic assessment; however, emerging evidence indicates that EV transplantation may not significantly increase arrhythmia susceptibility. Indeed, studies to date point towards a potential suppression of ventricular arrhythmias, hinting at favourable cardiac electrophysiological dynamics after EV implantation.11,46
In describing the creation of the EU-CARDIOPROTECTION COST Action programme, Hausenloy and Heusch stated: ‘the translation of adjunct cardioprotection to clinical practice has been largely disappointing so far’.57 In navigating the future of EV-based interventions, it is important to emphasize the need for further investigations into dosing strategies, long-term effects, and routes of administration. A significant challenge will be to construct a representative disease model for AMI trying to accurately model MI-induced heart failure in humans. Human AMI arises from the convergence of numerous factors evolving over time, often compounded by the presence of coexisting medical conditions and concurrent medication regimens.58 A wide spectrum of risk factors, such as smoking, alcohol abuse, cancer, and diabetes have a substantial impact on the severity of AMI outcomes. Furthermore, the progression of AMI is influenced by age and gender, displaying biases that result in higher occurrences among older males compared to younger individuals and women. However, the animal models employed in current laboratory investigations fail to recapitulate the diversity of comorbidities and other relevant variables in patients.59 These animal models tend to exhibit homogeneity, possessing youth, and good health, without genetic predisposition or underlying medical disorders.
The potential to harness these ‘tiny’ vesicles for targeted cardiac protection and regeneration may also reside in identifying specific subsets of AMI patients most likely to benefit from adjunctive EV therapy.55 In this regard, a recent pooled analysis of four randomized controlled trials using bone marrow-derived mononuclear cells (BM-MNC) after AMI indicated that the presence of MVO, as detected by MRI, identifies a subgroup of patients who may benefit from IC stem cell therapy.60,61 Notably, Gallet et al. showed that MVO decreased in EV-injected pigs regardless of the routes of administration (IC and/or IM).19 Even in cases where AMI patients undergo rapid PCI, up to 60% of them may experience MVO, characterized by impaired blood flow within the infarct zone potentially resulting in larger infarct size, unfavourable cardiac remodelling, and decreased LV function.60–62 Therefore, patients with large AMI and MVO experiencing higher risk of unfavourable outcomes62 may represent a targeted patient cohort most likely to benefit from EV treatment.
Finally, it is important to note that the various studies presented in this review employed different EV purification protocols. EVs contain a diverse array of molecules, including proteins, nucleic acids, and lipids, which can vary depending on the source and method of isolation. This heterogeneity poses challenges in standardizing EV-based therapies, making it difficult to precisely define the therapeutic cargo and dosage. Additionally, the lack of standardized characterization methods and quality control measures for EVs may complicate their development as therapeutic agents. Addressing these issues is crucial for advancing the translational potential of EVs and establishing them as reliable and effective therapeutic products. The development of an EV-based therapeutic product requires the establishment of reproducible and scalable purification protocols for clinical-grade EVs.54 The large-scale manufacturing of Good Manufacturing Practice (GMP)-grade EVs should include a versatile protocol that can be easily applied to different cell sources, overcoming limitations such as yield efficiency, EV purity, and, most importantly, batch-to-batch variability. Commonly used bench-scale isolation methods, including ultracentrifugation, density gradient centrifugation, size exclusion chromatography, and polymer-based precipitation, can be challenging to adapt to GMP-compatible methods for the scalable production, concentration, and isolation of EV. Recently, a strategy involving tangential flow filtration has been implemented with promising results;63 however, further investigations geared at optimizing GMP-grade EV purification are warranted.
Acknowledgements
L.B. is supported by a research grant from the Swiss National Foundation and Swiss Heart Foundation. Work in EM’s laboratory is supported by grants from the National Heart, Lung and Blood Institute of the USA, and by the California Institute for Regenerative Medicine. E.M. holds the Mark Siegel Family Foundation Distinguished Chair of the Cedars-Sinai Medical Center.
Contributor Information
Lucio Barile, Cardiovascular Theranostics, Istituto Cardiocentro Ticino, Laboratories for Translational Research, Ente Ospedaliero Cantonale, CH-6500, Bellinzona, Switzerland; Euler Institute, Faculty of Biomedical Sciences, Università della Svizzera italiana, CH-6900 Lugano, Switzerland.
Eduardo Marbán, Cedars-Sinai Medical Center, Smidt Heart Institute, Los Angeles, CA, USA.
Supplementary data
Supplementary data are not available at European Heart Journal online.
Declarations
Disclosure of Interest
E.M. holds founder’s equity in Capricor Therapeutics.
Data Availability
No data were generated or analysed for or in support of this paper.
Funding
Nothing to declare.
References
- 1. Christensen DM, Schjerning A-M, Smedegaard L, Charlot MG, Ravn PB, Ruwald AC, et al. Long-term mortality, cardiovascular events, and bleeding in stable patients 1 year after myocardial infarction: a Danish nationwide study. Eur Heart J 2023;44:488–98. 10.1093/eurheartj/ehac667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bouisset F, Ruidavets J-B, Dallongeville J, Moitry M, Montaye M, Biasch K, et al. Comparison of short- and long-term prognosis between ST-elevation and non-ST-elevation myocardial infarction. J Clin Med 2021;10:180. 10.3390/jcm10020180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberto M, Hoepli A, Cattaneo M, Radovanovic D, Rickli H, Erne P, et al. Patients with AMI and severely reduced LVEF, a well-defined, still extremely vulnerable population (insights from AMIS plus registry). Am J Cardiol 2023;200:190–201. 10.1016/j.amjcard.2023.05.027 [DOI] [PubMed] [Google Scholar]
- 4. Mathur A, Fernández-Avilés F, Bartunek J, Belmans A, Crea F, Dowlut S, et al. The effect of intracoronary infusion of bone marrow-derived mononuclear cells on all-cause mortality in acute myocardial infarction: the BAMI trial. Eur Heart J 2020;41:3702–10. 10.1093/eurheartj/ehaa651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lefer DJ, Marbán E. Is cardioprotection dead? Circulation 2017;136:98–109. 10.1161/CIRCULATIONAHA.116.027039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ruozi G, Bortolotti F, Mura A, Tomczyk M, Falcione A, Martinelli V, et al. Cardioprotective factors against myocardial infarction selected in vivo from an AAV secretome library. Sci Transl Med 2022;14:eabo0699. 10.1126/scitranslmed.abo0699 [DOI] [PubMed] [Google Scholar]
- 7. Tang T-T, Zhu Y-C, Dong N-G, Zhang S, Cai J, Zhang L-X, et al. Pathologic T-cell response in ischaemic failing hearts elucidated by T-cell receptor sequencing and phenotypic characterization. Eur Heart J 2019;40:3924–33. 10.1093/eurheartj/ehz516 [DOI] [PubMed] [Google Scholar]
- 8. Ibrahim AG-E, Cheng K, Marbán E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2014;2:606–19. 10.1016/j.stemcr.2014.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barile L, Cervio E, Lionetti V, Milano G, Ciullo A, Biemmi V, et al. Cardioprotection by cardiac progenitor cell-secreted exosomes: role of pregnancy-associated plasma protein-A. Cardiovasc Res 2018;114:992–1005. 10.1093/cvr/cvy055 [DOI] [PubMed] [Google Scholar]
- 10. Balbi C, Lodder K, Costa A, Moimas S, Moccia F, van Herwaarden T, et al. Reactivating endogenous mechanisms of cardiac regeneration via paracrine boosting using the human amniotic fluid stem cell secretome. Int J Cardiol 2019;287:87–95. 10.1016/j.ijcard.2019.04.011 [DOI] [PubMed] [Google Scholar]
- 11. Gao L, Wang L, Wei Y, Krishnamurthy P, Walcott GP, Menasché P, et al. Exosomes secreted by hiPSC-derived cardiac cells improve recovery from myocardial infarction in swine. Sci Transl Med 2020;12:eaay1318. 10.1126/scitranslmed.aay1318 [DOI] [PubMed] [Google Scholar]
- 12. Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda) 2019;34:169–77. 10.1152/physiol.00045.2018 [DOI] [PubMed] [Google Scholar]
- 13. Cambier L, de Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, et al. Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 2017;9:337–52. 10.15252/emmm.201606924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Jong OG, Verhaar MC, Chen Y, Vader P, Gremmels H, Posthuma G, et al. Cellular stress conditions are reflected in the protein and RNA content of endothelial cell-derived exosomes. J Extracell Vesicles 2012;16:1. 10.3402/jev.v1i0.18396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Marbán E. A mechanistic roadmap for the clinical application of cardiac cell therapies. Nat Biomed Eng 2018;2:353–61. 10.1038/s41551-018-0216-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barzegar M, Wang Y, Eshaq RS, Yun JW, Boyer CJ, Cananzi SG, et al. Human placental mesenchymal stem cells improve stroke outcomes via extracellular vesicles-mediated preservation of cerebral blood flow. EBioMedicine 2020;63:103161. 10.1016/j.ebiom.2020.103161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lai RC, Chen TS, Lim SK. Mesenchymal stem cell exosome: a novel stem cell-based therapy for cardiovascular disease. Regen Med 2011;6:481–92. 10.2217/rme.11.35 [DOI] [PubMed] [Google Scholar]
- 18. Emmert MY, Burrello J, Wolint P, Hilbe M, Andriolo G, Balbi C, et al. Intracoronary delivery of extracellular vesicles from human cardiac progenitor cells reduces infarct size in porcine acute myocardial infarction. Eur Heart J 2024;45:728–32. 10.1093/eurheartj/ehad636 [DOI] [PubMed] [Google Scholar]
- 19. Gallet R, Dawkins J, Valle J, Simsolo E, de Couto G, Middleton R, et al. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201–11. 10.1093/eurheartj/ehw240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charles CJ, Li RR, Yeung T, Mazlan SMI, Lai RC, de Kleijn DPV, et al. Systemic mesenchymal stem cell-derived exosomes reduce myocardial infarct size: characterization with MRI in a porcine model. Front Cardiovasc Med 2020;7:601990. 10.3389/fcvm.2020.601990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Monguió-Tortajada M, Prat-Vidal C, Martínez-Falguera D, Teis A, Soler-Botija C, Courageux Y, et al. Acellular cardiac scaffolds enriched with MSC-derived extracellular vesicles limit ventricular remodelling and exert local and systemic immunomodulation in a myocardial infarction porcine model. Theranostics 2022;12:4656–70. 10.7150/thno.72289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Q, Xu Y, Lv K, Wang Y, Zhong Z, Xiao C, et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci Transl Med 2021;13:eabb0202. 10.1126/scitranslmed.abb0202 [DOI] [PubMed] [Google Scholar]
- 23. López E, Blázquez R, Marinaro F, Álvarez V, Blanco V, Báez C, et al. The intrapericardial delivery of extracellular vesicles from cardiosphere-derived cells stimulates M2 polarization during the acute phase of porcine myocardial infarction. Stem Cell Rev and Rep 2020;16:612–25. 10.1007/s12015-019-09926-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Salomon C, Das S, Erdbrügger U, Kalluri R, Kiang Lim S, Olefsky JM, et al. Extracellular vesicles and their emerging roles as cellular messengers in endocrinology: an endocrine society scientific statement. Endocr Rev 2022;43:441–68. 10.1210/endrev/bnac009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ganser-Pornillos BK, Yeager M, Pornillos O. Assembly and architecture of HIV. Adv Exp Med Biol 2012;726:441–65. 10.1007/978-1-4614-0980-9_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Théry C, Witwer KW, Aikawa E, Alcaraz MJ, Anderson JD, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the international society for extracellular vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles 2018;7:1535750. 10.1080/20013078.2018.1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dou G, Tian R, Liu X, Yuan P, Ye Q, Liu J, et al. Chimeric apoptotic bodies functionalized with natural membrane and modular delivery system for inflammation modulation. Sci Adv 2020;6:eaba2987. 10.1126/sciadv.aba2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barile L, Guzik TJ. The swan song of dying cells. Cardiovasc Res 2020;116:e90–2. 10.1093/cvr/cvaa152 [DOI] [PubMed] [Google Scholar]
- 29. Heusch G, Andreadou I, Bell R, Bertero E, Botker H-E, Davidson SM, et al. Health position paper and redox perspectives on reactive oxygen species as signals and targets of cardioprotection. Redox Biol 2023;67:102894. 10.1016/j.redox.2023.102894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gorog DA, Ferreiro JL, Ahrens I, Ako J, Geisler T, Halvorsen S, et al. De-escalation or abbreviation of dual antiplatelet therapy in acute coronary syndromes and percutaneous coronary intervention: a consensus statement from an international expert panel on coronary thrombosis. Nat Rev Cardiol 2023;20:830–44. 10.1038/s41569-023-00901-2 [DOI] [PubMed] [Google Scholar]
- 31. Gragnano F, Cao D, Pirondini L, Franzone A, Kim H-S, von Scheidt M, et al. P2y12 inhibitor or aspirin monotherapy for secondary prevention of coronary events. J Am Coll Cardiol 2023;82:89–105. 10.1016/j.jacc.2023.04.051 [DOI] [PubMed] [Google Scholar]
- 32. Yang X-M, Liu Y, Cui L, Yang X, Liu Y, Tandon N, et al. Platelet P2Y12 blockers confer direct postconditioning-like protection in reperfused rabbit hearts. J Cardiovasc Pharmacol Ther 2013;18:251–62. 10.1177/1074248412467692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Correa BL, Harane NE, Desgres M, Perotto M, Alayrac P, Guillas C, et al. Extracellular vesicles fail to trigger the generation of new cardiomyocytes in chronically infarcted hearts. Theranostics 2021;11:10114–24. 10.7150/thno.62304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Roefs MT, Bauzá-Martinez J, van de Wakker SI, Qin J, Olijve WT, Tuinte R, et al. Cardiac progenitor cell-derived extracellular vesicles promote angiogenesis through both associated- and co-isolated proteins. Commun Biol 2023;6:800. 10.1038/s42003-023-05165-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. de Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, et al. Exosomal microRNA transfer into macrophages mediates cellular postconditioning. Circulation 2017;136:200–14. 10.1161/CIRCULATIONAHA.116.024590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chimenti I, Smith RR, Li T-S, Gerstenblith G, Messina E, Giacomello A, et al. Relative roles of direct regeneration versus paracrine effects of human cardiosphere-derived cells transplanted into infarcted mice. Circ Res 2010;106:971–80. 10.1161/CIRCRESAHA.109.210682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Barile L, Lionetti V, Cervio E, Matteucci M, Gherghiceanu M, Popescu LM, et al. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc Res 2014;103:530–41. 10.1093/cvr/cvu167 [DOI] [PubMed] [Google Scholar]
- 38. Sahoo S, Kariya T, Ishikawa K. Targeted delivery of therapeutic agents to the heart. Nat Rev Cardiol 2021;18:389–99. 10.1038/s41569-020-00499-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Monguió-Tortajada M, Prat-Vidal C, Moron-Font M, Clos-Sansalvador M, Calle A, Gastelurrutia P, et al. Local administration of porcine immunomodulatory, chemotactic and angiogenic extracellular vesicles using engineered cardiac scaffolds for myocardial infarction. Bioact Mater 2021;6:3314–27. 10.1016/j.bioactmat.2021.02.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Redgrave RE, Dookun E, Booth LK, Camacho Encina M, Folaranmi O, Tual-Chalot S, et al. Senescent cardiomyocytes contribute to cardiac dysfunction following myocardial infarction. NPJ Aging 2023;9:15. 10.1038/s41514-023-00113-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lazzarini E, Lodrini AM, Arici M, Bolis S, Vagni S, Panella S, et al. Stress-induced premature senescence is associated with a prolonged QT interval and recapitulates features of cardiac aging. Theranostics 2022;12:5237–57. 10.7150/thno.70884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang X, Guo Z, Ding Z, Mehta JL. Inflammation, autophagy, and apoptosis after myocardial infarction. J Am Heart Assoc 2018;7:e008024. 10.1161/JAHA.117.008024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lodrini AM, Goumans M-J. Cardiomyocytes cellular phenotypes after myocardial infarction. Front Cardiovasc Med 2021;8:750510. 10.3389/fcvm.2021.750510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu D, Liu S, Huang K, Li J, Mei X, Li Z, et al. Intrapericardial long non-coding RNA–Tcf21 antisense RNA inducing demethylation administration promotes cardiac repair. Eur Heart J 2023;44:1748–60. 10.1093/eurheartj/ehad114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. de Couto G, Jaghatspanyan E, DeBerge M, Liu W, Luther K, Wang Y, et al. Mechanism of enhanced MerTK-dependent macrophage efferocytosis by extracellular vesicles. Arterioscler Thromb Vasc Biol 2019;39:2082–96. 10.1161/ATVBAHA.119.313115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dawkins JF, Ehdaie A, Rogers R, Soetkamp D, Valle J, Holm K, et al. Biological substrate modification suppresses ventricular arrhythmias in a porcine model of chronic ischaemic cardiomyopathy. Eur Heart J 2022;43:2139–56. 10.1093/eurheartj/ehac042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shiba Y, Gomibuchi T, Seto T, Wada Y, Ichimura H, Tanaka Y, et al. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–91. 10.1038/nature19815 [DOI] [PubMed] [Google Scholar]
- 48. Gabisonia K, Prosdocimo G, Aquaro GD, Carlucci L, Zentilin L, Secco I, et al. MicroRNA therapy stimulates uncontrolled cardiac repair after myocardial infarction in pigs. Nature 2019;569:418–22. 10.1038/s41586-019-1191-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Chong JJH, Yang X, Don CW, Minami E, Liu Y-W, Weyers JJ, et al. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–7. 10.1038/nature13233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Li Q, Wang J, Wu Q, Cao N, Yang H-T. Perspective on human pluripotent stem cell-derived cardiomyocytes in heart disease modeling and repair. Stem Cells Transl Med 2020;9:1121–8. 10.1002/sctm.19-0340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Besse B, Charrier M, Lapierre V, Dansin E, Lantz O, Planchard D, et al. Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. Oncoimmunology 2016;5:e1071008. 10.1080/2162402X.2015.1071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J Transl Med 2005;3:9. 10.1186/1479-5876-3-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pitt JM, André F, Amigorena S, Soria J-C, Eggermont A, Kroemer G, et al. Dendritic cell-derived exosomes for cancer therapy. J Clin Invest 2016;126:1224–32. 10.1172/JCI81137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Davidson SM, Boulanger CM, Aikawa E, Badimon L, Barile L, Binder CJ, et al. Methods for the identification and characterization of extracellular vesicles in cardiovascular studies—from exosomes to microvesicles. Cardiovasc Res 2023;119:45–63. 10.1093/cvr/cvac031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Marbán E. The secret life of exosomes: what bees can teach US about next-generation therapeutics. J Am Coll Cardiol 2018;71:193–200. 10.1016/j.jacc.2017.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Lionetti V, Barile L. Fndc5/irisin-enriched extracellular vesicles: a new hormonal relay in the regular race against vascular ageing. Eur Heart J 2022;43:4596–8. 10.1093/eurheartj/ehac517 [DOI] [PubMed] [Google Scholar]
- 57. Hausenloy DJ, Heusch G. Translating cardioprotection for patient benefit: the EU-CARDIOPROTECTION COST action. J Am Coll Cardiol 2019;73:2001–3. 10.1016/j.jacc.2019.03.020 [DOI] [PubMed] [Google Scholar]
- 58. Pound P, Ebrahim S, Sandercock P, Bracken MB, Roberts I. Reviewing animal trials systematically (RATS) group. Where is the evidence that animal research benefits humans? BMJ 2004;328:514–7. 10.1136/bmj.328.7438.514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Pound P, Ritskes-Hoitinga M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J Transl Med 2018;16:304. 10.1186/s12967-018-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Niccoli G, Scalone G, Lerman A, Crea F. Coronary microvascular obstruction in acute myocardial infarction. Eur Heart J 2016;37:1024–33. 10.1093/eurheartj/ehv484 [DOI] [PubMed] [Google Scholar]
- 61. Davidson SJ, Roncalli J, Surder D, Corti R, Chugh AR, Yang PC, et al. Microvascular obstruction identifies a subgroup of patients who benefit from stem cell therapy following ST-elevation myocardial infarction. Am Heart J 2023;259:79–86. 10.1016/j.ahj.2023.02.004 [DOI] [PubMed] [Google Scholar]
- 62. Niccoli G, Montone RA, Ibanez B, Thiele H, Crea F, Heusch G, et al. Optimized treatment of ST-elevation myocardial infarction. Circ Res 2019;125:245–58. 10.1161/CIRCRESAHA.119.315344 [DOI] [PubMed] [Google Scholar]
- 63. Andriolo G, Provasi E, Brambilla A, Panella S, Soncin S, Cicero VL, et al. Methodologies for scalable production of high-quality purified small extracellular vesicles from conditioned Medium. Methods Mol Biol 2023;2668:69–98. 10.1007/978-1-0716-3203-1_7 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data were generated or analysed for or in support of this paper.