Abstract
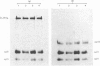
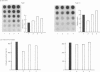
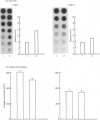
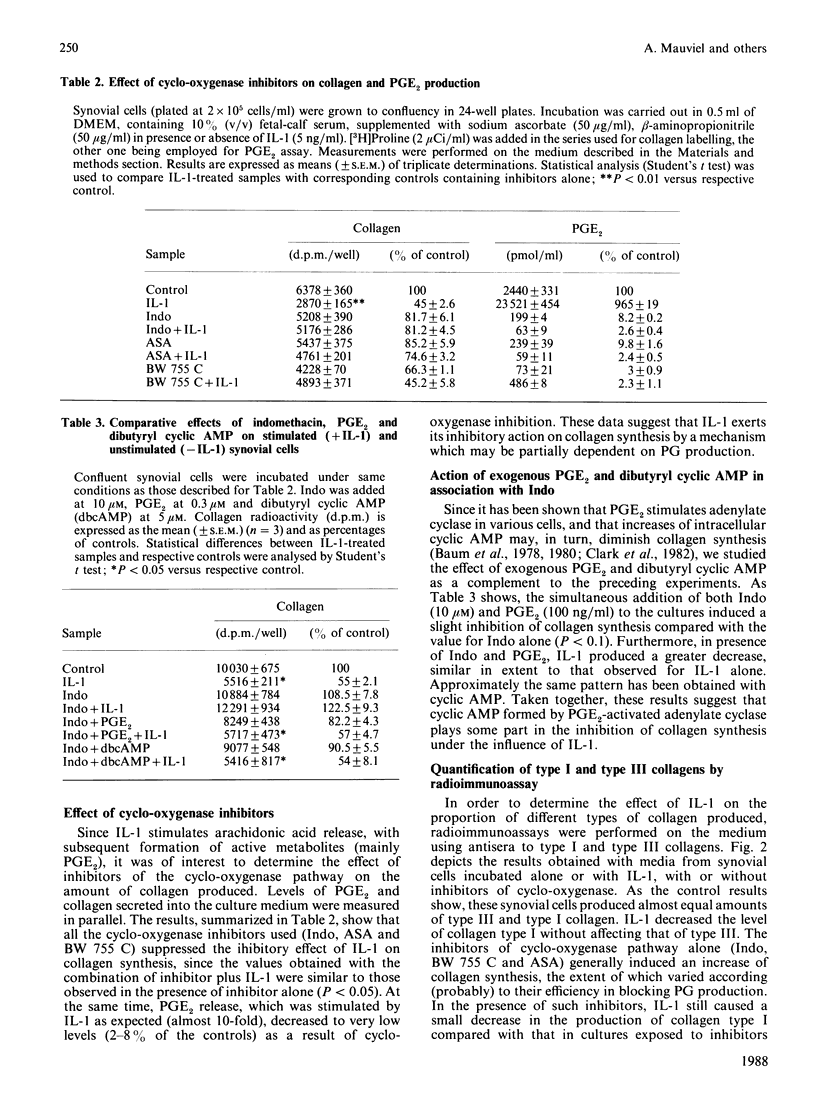
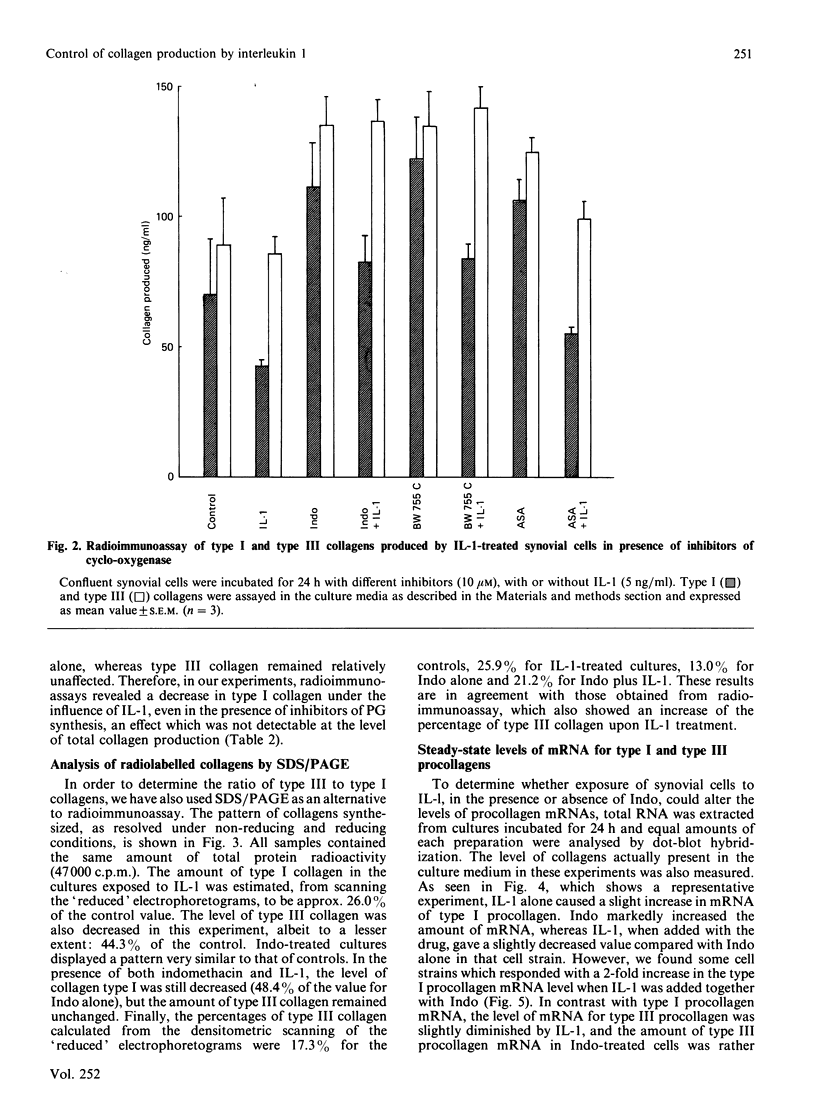
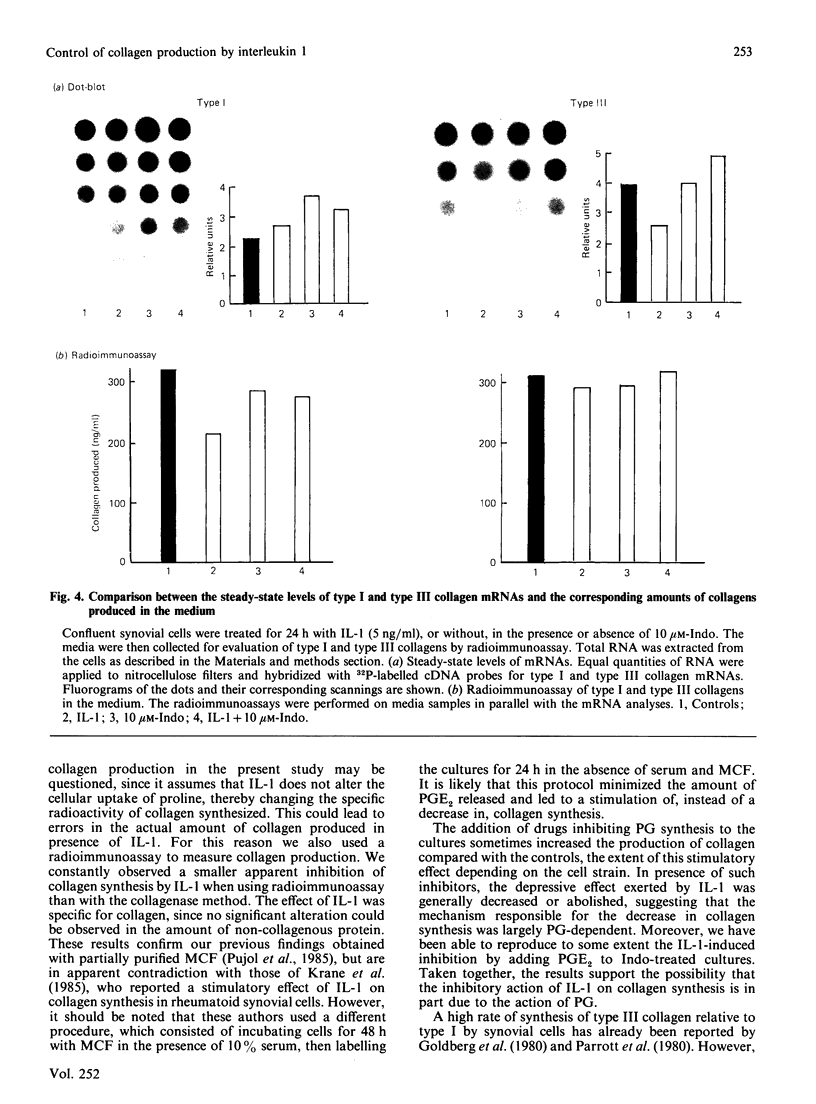
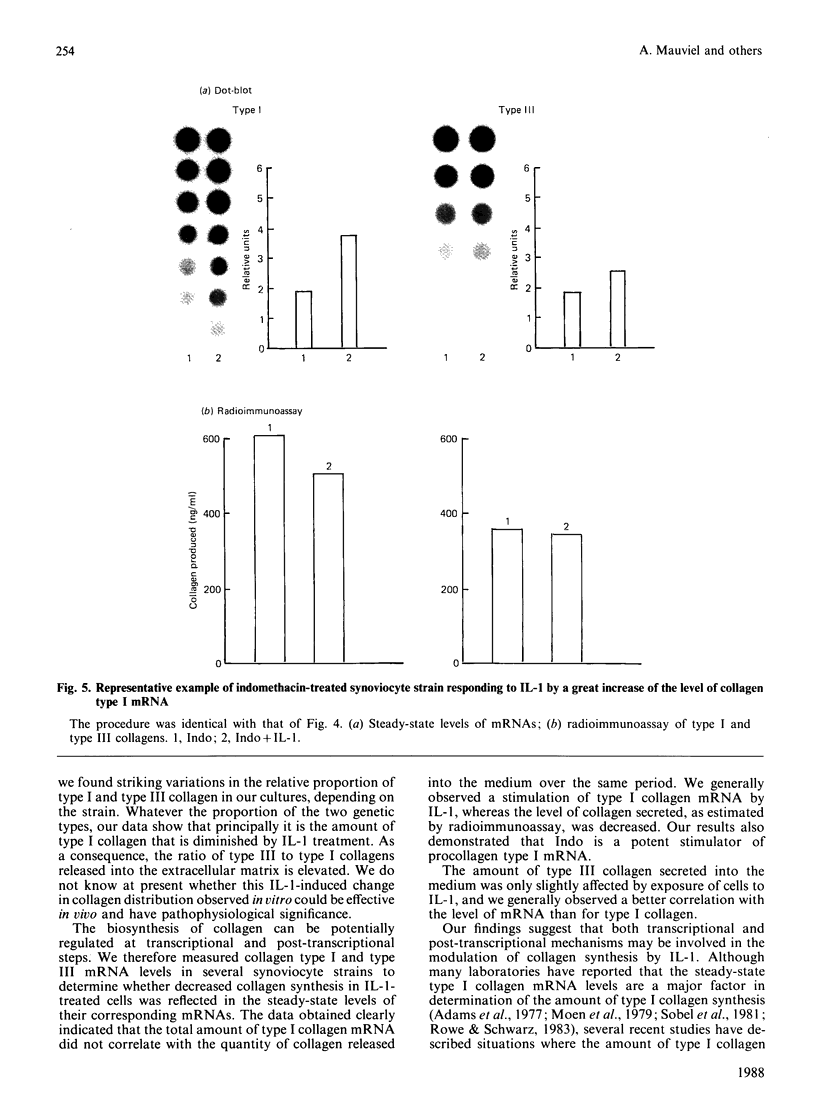
The effects of porcine interleukin-1 (IL-1) alpha on collagen production were studied in cultured human rheumatoid synovial cells. Addition of 0.05-5 ng of IL-1/ml into the cultures resulted in a dose-dependent decreased rate of collagen released into the medium over 24 h. To determine whether this inhibition was due to secondary action of prostaglandin E2 (PGE2) secreted in response to IL-1, cultures were incubated in presence of various inhibitors of arachidonate metabolism. Depending on the cell strains, these inhibitors were able to suppress or diminish the effect of IL-1, suggesting that PGE2 is involved in the mechanism. Depression of collagen production caused by IL-1 mainly affected type I collagen and therefore led to a change in the type I/type III collagen ratio in the extracellular medium. Steady-state levels of mRNA for types I and III procollagens were estimated by dot-blot hybridization and compared with the amounts of respective collagens produced in the same cultures. IL-1 generally increased procollagen type I mRNA, but to a variable extent, as did indomethacin (Indo). Depending on the cell strain, the combination of indo and IL-1 could elevate the mRNA level of type I procollagen compared with Indo alone. These results did not correlate with the production rate of collagen in the medium, which was diminished by exposure to IL-1. The level of mRNA for collagen type III was not greatly changed by incubation with IL-1, and a better correlation was generally observed with the amount of type III collagen found in the medium. These data suggest that an additional control mechanism at translational or post-translational level must exist, counterbalancing the stimulatory effect of IL-1 on collagen mRNA transcription. It is likely that IL-1 could modulate the production of collagen in synovial cells by an interplay of different mechanisms, some of them limiting the effect of primary elevation of the steady-state mRNA level.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams S. L., Sobel M. E., Howard B. H., Olden K., Yamada K. M., de Crombrugghe B., Pastan I. Levels of translatable mRNAs for cell surface protein, collagen precursors, and two membrane proteins are altered in Rous sarcoma virus-transformed chick embryo fibroblasts. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3399–3403. doi: 10.1073/pnas.74.8.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Berg R. A., Crystal R. G. Effect of cyclic AMP on the intracellular degradation of newly synthesized collagen. J Biol Chem. 1980 Apr 10;255(7):2843–2847. [PubMed] [Google Scholar]
- Baum B. J., Moss J., Breul S. D., Crystal R. G. Association in normal human fibroblasts of elevated levels of adenosine 3':5'-monophosphate with a selective decrease in collagen production. J Biol Chem. 1978 May 25;253(10):3391–3394. [PubMed] [Google Scholar]
- Bhatnagar R., Penfornis H., Mauviel A., Loyau G., Saklatvala J., Pujol J. P. Interleukin-1 inhibits the synthesis of collagen by fibroblasts. Biochem Int. 1986 Oct;13(4):709–720. [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Cathala G., Savouret J. F., Mendez B., West B. L., Karin M., Martial J. A., Baxter J. D. A method for isolation of intact, translationally active ribonucleic acid. DNA. 1983;2(4):329–335. doi: 10.1089/dna.1983.2.329. [DOI] [PubMed] [Google Scholar]
- Clark J. G., Kostal K. M., Marino B. A. Modulation of collagen production following bleomycin-induced pulmonary fibrosis in hamsters. Presence of a factor in lung that increases fibroblast prostaglandin E2 and cAMP and suppresses fibroblast proliferation and collagen production. J Biol Chem. 1982 Jul 25;257(14):8098–8105. [PubMed] [Google Scholar]
- Dalgleish R., Trapnell B. C., Crystal R. G., Tolstoshev P. Copy number of a human type I alpha 2 collagen gene. J Biol Chem. 1982 Nov 25;257(22):13816–13822. [PubMed] [Google Scholar]
- Dayer J. M., Krane S. M., Russell R. G., Robinson D. R. Production of collagenase and prostaglandins by isolated adherent rheumatoid synovial cells. Proc Natl Acad Sci U S A. 1976 Mar;73(3):945–949. doi: 10.1073/pnas.73.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray F., Charbonnel B., Maclouf J. Radioimmunoassay of prostaglandins Falpha, E1 and E2 in human plasma. Eur J Clin Invest. 1975 Jul 29;5(4):311–318. doi: 10.1111/j.1365-2362.1975.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Goldberg R. L., Parrott D. P., Kaplan S. R., Fuller G. C. Effect of gold sodium thiomalate on proliferation of human rheumatoid synovial cells and on collagen synthesis in tissue culture. Biochem Pharmacol. 1980 Mar 15;29(6):869–876. doi: 10.1016/0006-2952(80)90216-6. [DOI] [PubMed] [Google Scholar]
- Gowen M., Wood D. D., Ihrie E. J., Meats J. E., Russell R. G. Stimulation by human interleukin 1 of cartilage breakdown and production of collagenase and proteoglycanase by human chondrocytes but not by human osteoblasts in vitro. Biochim Biophys Acta. 1984 Feb 14;797(2):186–193. doi: 10.1016/0304-4165(84)90121-1. [DOI] [PubMed] [Google Scholar]
- Hamerman D., Wood D. D. Interleukin 1 enhances synovial cell hyaluronate synthesis. Proc Soc Exp Biol Med. 1984 Oct;177(1):205–210. doi: 10.3181/00379727-177-1-rc1. [DOI] [PubMed] [Google Scholar]
- Heino J., Viander M., Peltonen J., Kouri T. Adherent cells from rheumatoid synovia: identity of HLA-DR positive stellate cells. Ann Rheum Dis. 1987 Feb;46(2):114–120. doi: 10.1136/ard.46.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krane S. M., Dayer J. M., Simon L. S., Byrne M. S. Mononuclear cell-conditioned medium containing mononuclear cell factor (MCF), homologous with interleukin 1, stimulates collagen and fibronectin synthesis by adherent rheumatoid synovial cells: effects of prostaglandin E2 and indomethacin. Coll Relat Res. 1985 Mar;5(2):99–117. doi: 10.1016/s0174-173x(85)80033-9. [DOI] [PubMed] [Google Scholar]
- Kähäri V. M., Heino J., Vuorio E. Interleukin-1 increases collagen production and mRNA levels in cultured skin fibroblasts. Biochim Biophys Acta. 1987 Jul 6;929(2):142–147. doi: 10.1016/0167-4889(87)90169-8. [DOI] [PubMed] [Google Scholar]
- Magloire H., Callé A., Hartmann D. J., Joffre A., Serre B., Grimaud J. A., Schué F. Type-I collagen production by human odontoblast-like cells in explants cultured on cyanoacrylate films. Electron-immunolocalization of fibronectin at cell/film interface. Cell Tissue Res. 1986;244(1):133–140. doi: 10.1007/BF00218390. [DOI] [PubMed] [Google Scholar]
- Mizel S. B., Dayer J. M., Krane S. M., Mergenhagen S. E. Stimulation of rheumatoid synovial cell collagenase and prostaglandin production by partially purified lymphocyte-activating factor (interleukin 1). Proc Natl Acad Sci U S A. 1981 Apr;78(4):2474–2477. doi: 10.1073/pnas.78.4.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moen R. C., Rowe D. W., Palmiter R. D. Regulation of procollagen synthesis during the development of chick embryo calvaria. Correlation with procollagen mRNA content. J Biol Chem. 1979 May 10;254(9):3526–3530. [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Parrott D. P., Kuttan R., Goldberg R. L., Kaplan S. R., Fuller G. C. Synthesis of type I and type III collagen by synovial cells in tissue culture derived from patients with rheumatoid arthritis, osteoarthritis, and normal individuals. Proc Soc Exp Biol Med. 1980 Nov;165(2):335–344. doi: 10.3181/00379727-165-40982. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Pujol J. P., Brisset M., Jourdan C., Bocquet J., Jouis V., Béliard R., Loyau G. Effect of a monocyte cell factor (MCF) on collagen production in cultured articular chondrocytes: role of prostaglandin E2. Biochem Biophys Res Commun. 1984 Mar 15;119(2):499–508. doi: 10.1016/s0006-291x(84)80276-4. [DOI] [PubMed] [Google Scholar]
- Pujol J. P., Penfornis H., Arenzana-Seisdedos F., Bocquet J., Farjanel J., Rattner A., Brisset M., Virelizier J. L., Beliard R., Loyau G. Mononuclear cell-mediated modulation of synovial cell metabolism. I. Collagen synthesis. Exp Cell Res. 1985 May;158(1):63–74. doi: 10.1016/0014-4827(85)90431-8. [DOI] [PubMed] [Google Scholar]
- Rowe L. B., Schwarz R. I. Role of procollagen mRNA levels in controlling the rate of procollagen synthesis. Mol Cell Biol. 1983 Feb;3(2):241–249. doi: 10.1128/mcb.3.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Pilsworth L. M., Sarsfield S. J., Gavrilovic J., Heath J. K. Pig catabolin is a form of interleukin 1. Cartilage and bone resorb, fibroblasts make prostaglandin and collagenase, and thymocyte proliferation is augmented in response to one protein. Biochem J. 1984 Dec 1;224(2):461–466. doi: 10.1042/bj2240461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saklatvala J., Sarsfield S. J., Townsend Y. Pig interleukin 1. Purification of two immunologically different leukocyte proteins that cause cartilage resorption, lymphocyte activation, and fever. J Exp Med. 1985 Oct 1;162(4):1208–1222. doi: 10.1084/jem.162.4.1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandberg M., Vuorio E. Localization of types I, II, and III collagen mRNAs in developing human skeletal tissues by in situ hybridization. J Cell Biol. 1987 Apr;104(4):1077–1084. doi: 10.1083/jcb.104.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J. A., Mizel S. B., Cohen D., Green I. Interleukin 1, a potential regulator of fibroblast proliferation. J Immunol. 1982 May;128(5):2177–2182. [PubMed] [Google Scholar]
- Sobel M. R., Yamamoto T., de Crombrugghe B., Pastan I. Regulation or procollagen messenger ribonucleic acid levels in Rous sarcoma virus transformed chick embryo fibroblasts. Biochemistry. 1981 Apr 28;20(9):2678–2684. doi: 10.1021/bi00512a049. [DOI] [PubMed] [Google Scholar]
- Sykes B., Puddle B., Francis M., Smith R. The estimation of two collagens from human dermis by interrupted gel electrophoresis. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1472–1480. doi: 10.1016/s0006-291x(76)80180-5. [DOI] [PubMed] [Google Scholar]
- Tolstoshev P., Berg R. A., Rennard S. I., Bradley K. H., Trapnell B. C., Crystal R. G. Procollagen production and procollagen messenger RNA levels and activity in human lung fibroblasts during periods of rapid and stationary growth. J Biol Chem. 1981 Mar 25;256(6):3135–3140. [PubMed] [Google Scholar]
- Tolstoshev P., Haber R., Trapnell B. C., Crystal R. G. Procollagen messenger RNA levels and activity and collagen synthesis during the fetal development of sheep lung, tendon, and skin. J Biol Chem. 1981 Sep 25;256(18):9672–9679. [PubMed] [Google Scholar]
- Whiteside T. L., Buckingham R. B., Prince R. K., Rodnan G. P. Products of activated mononuclear cells modulate accumulation of collagen by normal dermal and scleroderma fibroblasts in culture. J Lab Clin Med. 1984 Sep;104(3):355–369. [PubMed] [Google Scholar]