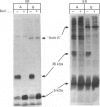
Abstract
Phospholamban of isolated sarcoplasmic reticulum of cardiac and smooth muscle is phosphorylated by cyclic GMP-dependent protein kinase (G-kinase). Concomitantly, the affinity of the Ca2+ pump for Ca2+ is increased. These effects are very similar to those seen with cyclic AMP-dependent protein kinase (A-kinase). The phosphate incorporation into phospholamban and the stimulatory effects of both kinases on the Ca2+ pump are not additive, suggesting that G-kinase phosphorylates the same serine residue as A-kinase. A possible physiological role for phosphorylation of phospholamban by G-kinase is discussed.
Full text
PDF
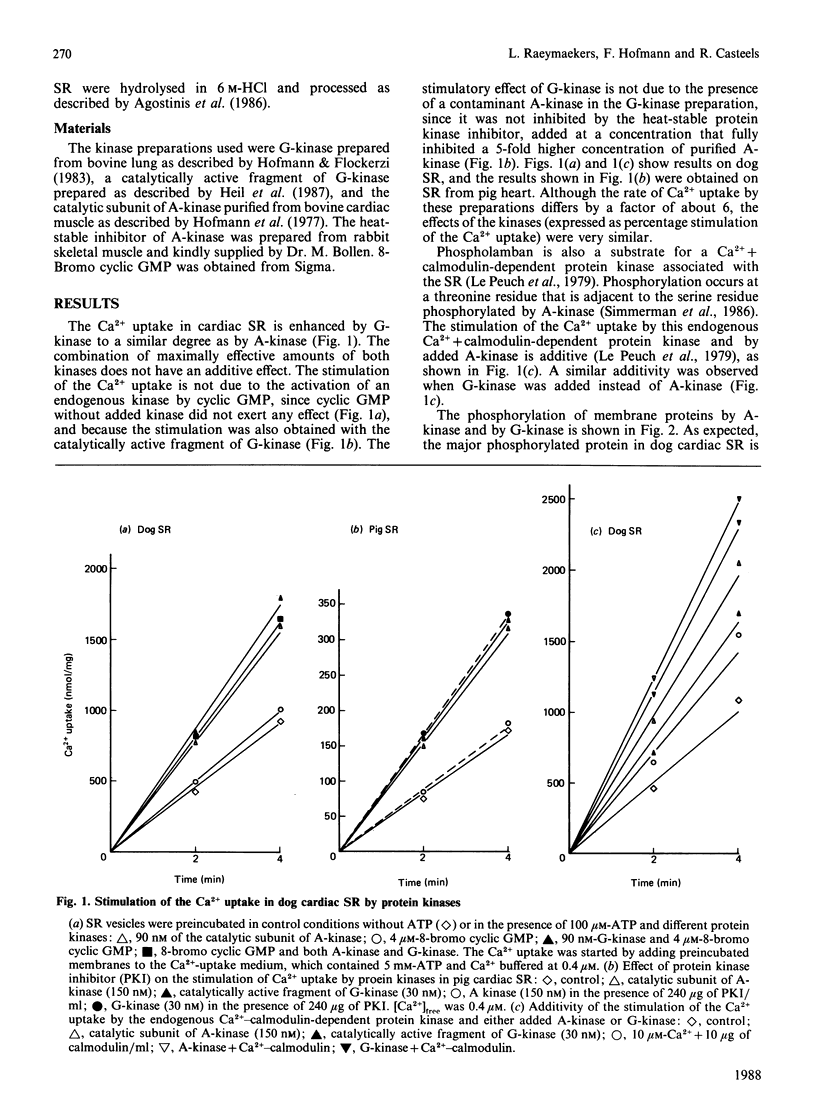
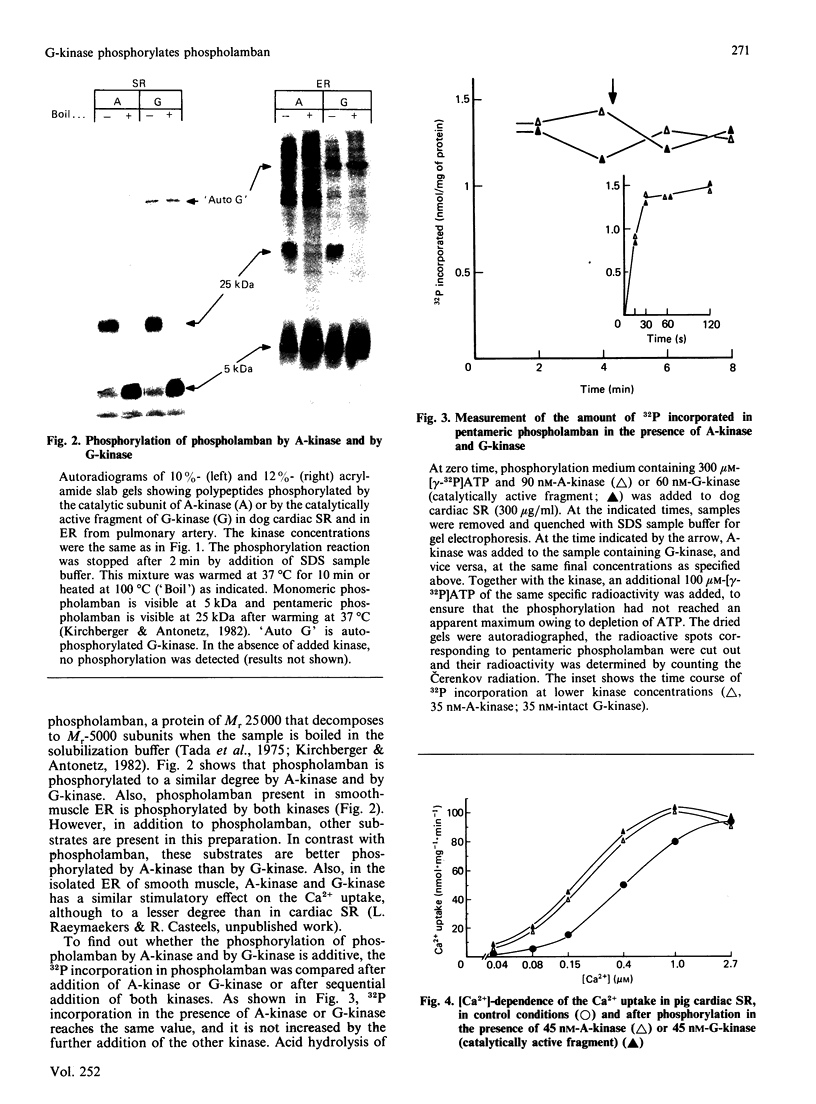


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostinis P., Goris J., Vandenheede J. R., Waelkens E., Pinna L. A., Merlevede W. Phosphorylation of the modulator protein of the ATP, Mg-dependent protein phosphatase by casein kinase TS. Reversal by PCS phosphatases and control by distinct phosphorylation site(s). FEBS Lett. 1986 Oct 20;207(1):167–172. doi: 10.1016/0014-5793(86)80033-3. [DOI] [PubMed] [Google Scholar]
- Eggermont J. A., Vrolix M., Raeymaekers L., Wuytack F., Casteels R. Ca2+-transport ATPases of vascular smooth muscle. Circ Res. 1988 Feb;62(2):266–278. doi: 10.1161/01.res.62.2.266. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Nakamura H. Cyclic GMP regulation of the plasma membrane (Ca2+-Mg2+)ATPase in vascular smooth muscle. J Biochem. 1987 Jan;101(1):287–290. doi: 10.1093/oxfordjournals.jbchem.a121904. [DOI] [PubMed] [Google Scholar]
- Hassid A. Atriopeptin II decreases cytosolic free Ca in cultured vascular smooth muscle cells. Am J Physiol. 1986 Nov;251(5 Pt 1):C681–C686. doi: 10.1152/ajpcell.1986.251.5.C681. [DOI] [PubMed] [Google Scholar]
- Heil W. G., Landgraf W., Hofmann F. A catalytically active fragment of cGMP-dependent protein kinase. Occupation of its cGMP-binding sites does not affect its phosphotransferase activity. Eur J Biochem. 1987 Oct 1;168(1):117–121. doi: 10.1111/j.1432-1033.1987.tb13395.x. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Hofmann F., Flockerzi V. Characterization of phosphorylated and native cGMP-dependent protein kinase. Eur J Biochem. 1983 Feb 15;130(3):599–603. doi: 10.1111/j.1432-1033.1983.tb07191.x. [DOI] [PubMed] [Google Scholar]
- Ignarro L. J., Kadowitz P. J. The pharmacological and physiological role of cyclic GMP in vascular smooth muscle relaxation. Annu Rev Pharmacol Toxicol. 1985;25:171–191. doi: 10.1146/annurev.pa.25.040185.001131. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Casnellie J. E., Greengard P., Jamieson J. D. Subcellular localization of cyclic GMP-dependent protein kinase and its substrates in vascular smooth muscle. J Biol Chem. 1980 Apr 25;255(8):3777–3785. [PubMed] [Google Scholar]
- Iwasa Y., Hosey M. M. Phosphorylation of cardiac sarcolemma proteins by the calcium-activated phospholipid-dependent protein kinase. J Biol Chem. 1984 Jan 10;259(1):534–540. [PubMed] [Google Scholar]
- Jorgensen A. O., Jones L. R. Localization of phospholamban in slow but not fast canine skeletal muscle fibers. An immunocytochemical and biochemical study. J Biol Chem. 1986 Mar 15;261(8):3775–3781. [PubMed] [Google Scholar]
- Kai H., Kanaide H., Matsumoto T., Nakamura M. 8-Bromoguanosine 3':5'-cyclic monophosphate decreases intracellular free calcium concentrations in cultured vascular smooth muscle cells from rat aorta. FEBS Lett. 1987 Sep 14;221(2):284–288. doi: 10.1016/0014-5793(87)80941-9. [DOI] [PubMed] [Google Scholar]
- Kirchberger M. A., Antonetz T. Phospholamban: dissociation of the 22,000 molecular weight protein of cardiac sarcoplasmic reticulum into 11,000 and 5,500 molecular weight forms. Biochem Biophys Res Commun. 1982 Mar 15;105(1):152–156. doi: 10.1016/s0006-291x(82)80024-7. [DOI] [PubMed] [Google Scholar]
- Kirchberger M. A., Tada M. Effects of adenosine 3':5'-monophosphate-dependent protein kinase on sarcoplasmic reticulum isolated from cardiac and slow and fast contracting skeletal muscles. J Biol Chem. 1976 Feb 10;251(3):725–729. [PubMed] [Google Scholar]
- Kobayashi S., Kanaide H., Nakamura M. Cytosolic-free calcium transients in cultured vascular smooth muscle cells: microfluorometric measurements. Science. 1985 Aug 9;229(4713):553–556. doi: 10.1126/science.3927484. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Peuch C. J., Haiech J., Demaille J. G. Concerted regulation of cardiac sarcoplasmic reticulum calcium transport by cyclic adenosine monophosphate dependent and calcium--calmodulin-dependent phosphorylations. Biochemistry. 1979 Nov 13;18(23):5150–5157. doi: 10.1021/bi00590a019. [DOI] [PubMed] [Google Scholar]
- Lindemann J. P., Watanabe A. M. Muscarinic cholinergic inhibition of beta-adrenergic stimulation of phospholamban phosphorylation and Ca2+ transport in guinea pig ventricles. J Biol Chem. 1985 Oct 25;260(24):13122–13129. [PubMed] [Google Scholar]
- Movsesian M. A., Nishikawa M., Adelstein R. S. Phosphorylation of phospholamban by calcium-activated, phospholipid-dependent protein kinase. Stimulation of cardiac sarcoplasmic reticulum calcium uptake. J Biol Chem. 1984 Jul 10;259(13):8029–8032. [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Parker I., Ito Y., Kuriyama H., Miledi R. Beta-adrenergic agonists and cyclic AMP decrease intracellular resting free-calcium concentration in ileum smooth muscle. Proc R Soc Lond B Biol Sci. 1987 Mar 23;230(1259):207–214. doi: 10.1098/rspb.1987.0016. [DOI] [PubMed] [Google Scholar]
- Popescu L. M., Panoiu C., Hinescu M., Nutu O. The mechanism of cGMP-induced relaxation in vascular smooth muscle. Eur J Pharmacol. 1985 Jan 8;107(3):393–394. doi: 10.1016/0014-2999(85)90269-9. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Jones L. R. Evidence for the presence of phospholamban in the endoplasmic reticulum of smooth muscle. Biochim Biophys Acta. 1986 Jun 19;882(2):258–265. doi: 10.1016/0304-4165(86)90163-7. [DOI] [PubMed] [Google Scholar]
- Raeymaekers L., Wuytack F., Casteels R. Subcellular fractionation of pig stomach smooth muscle. A study of the distribution of the (Ca2+ + Mg2+)-ATPase activity in plasmalemma and endoplasmic reticulum. Biochim Biophys Acta. 1985 May 28;815(3):441–454. doi: 10.1016/0005-2736(85)90372-4. [DOI] [PubMed] [Google Scholar]
- Rashatwar S. S., Cornwell T. L., Lincoln T. M. Effects of 8-bromo-cGMP on Ca2+ levels in vascular smooth muscle cells: possible regulation of Ca2+-ATPase by cGMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5685–5689. doi: 10.1073/pnas.84.16.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmerman H. K., Collins J. H., Theibert J. L., Wegener A. D., Jones L. R. Sequence analysis of phospholamban. Identification of phosphorylation sites and two major structural domains. J Biol Chem. 1986 Oct 5;261(28):13333–13341. [PubMed] [Google Scholar]
- Suematsu E., Hirata M., Kuriyama H. Effects of cAMP- and cGMP-dependent protein kinases, and calmodulin on Ca2+ uptake by highly purified sarcolemmal vesicles of vascular smooth muscle. Biochim Biophys Acta. 1984 Jun 13;773(1):83–90. doi: 10.1016/0005-2736(84)90552-2. [DOI] [PubMed] [Google Scholar]
- Tada M., Kirchberger M. A., Katz A. M. Phosphorylation of a 22,000-dalton component of the cardiac sarcoplasmic reticulum by adenosine 3':5'-monophosphate-dependent protein kinase. J Biol Chem. 1975 Apr 10;250(7):2640–2647. [PubMed] [Google Scholar]
- Walter U. Cyclic-GMP-regulated enzymes and their possible physiological functions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1984;17:249–258. [PubMed] [Google Scholar]
- Watanabe A. M., Lindemann J. P., Fleming J. W. Mechanisms of muscarinic modulation of protein phosphorylation in intact ventricles. Fed Proc. 1984 Aug;43(11):2618–2623. [PubMed] [Google Scholar]
- Wegener A. D., Simmerman H. K., Liepnieks J., Jones L. R. Proteolytic cleavage of phospholamban purified from canine cardiac sarcoplasmic reticulum vesicles. Generation of a low resolution model of phospholamban structure. J Biol Chem. 1986 Apr 15;261(11):5154–5159. [PubMed] [Google Scholar]
- Winquist R. J. Possible mechanisms underlying the vasorelaxant response to atrial natriuretic factor. Fed Proc. 1986 Aug;45(9):2371–2375. [PubMed] [Google Scholar]