Abstract
Background
Endometrial carcinoma (EC) remain a malignancy with incompletely understood risk factors. To address this knowledge gap, we employed mendelian randomization study to investigate potential protective and risk elements associated with endometrial cancer.
Methods
We conducted a two-sample Mendelian randomization (MR) study using genetic association data for overall EC and its subtypes from a large-scale genome-wide association study (GWAS). This GWAS encompassed 12,906 EC patients and 108,979 healthy controls. The EC cases were further categorized into 8758 endometrioid and 1230 non-endometrioid subtypes. To serve as instrumental variables, we identified independent genetic variants strongly associated with 5 lifestyle factors and 14 metabolic factors from relevant GWASs. Subsequently, we conducted univariable Mendelian randomization (MR) analyses.
Results
Our study revealed the relationship among EC with lifetime smoking index (OR: 1.43; 95% CI 1.05–1.96), frequency of alcohol consumption (OR:1.23; 95% CI 1.04–1.45), body mass index (BMI) (OR:1.82; 95% CI 1.64–2.01), type 2 diabetes mellitus (T2DM) (OR:1.06; 95% CI 1.00–1.12), and fasting insulin (OR:1.97; 95% CI 1.30–2.98). Conversely, inverse associations with EC were observed for education level (OR:0.72, 95% CI 0.62–0.83), moderate-level physical exercise (OR 0.35, 95% CI 0.15–0.84), and low-density lipoproteins (LDL) (OR 0.91, 95% CI 0.84–0.99).
Conclusions
Our findings underscore a causal association between genetically predicted lifetime smoking index, alcohol intake frequency, BMI, T2DM, and fasting insulin with EC risk. Furthermore, our study highlights the potential protective effects of a high education level, moderate-intensity physical exercise, and LDL reduction against EC risk. This MR analysis provided valuable insights into underlying EC risk mechanisms and paved new ways for EC prevention strategies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12672-024-01439-6.
Keywords: Lifestyle factors, Metabolic factor, Mendelian randomization, Endometrial Carcinoma
Introduction
Endometrial cancer (EC) ranks as the sixth most prevalent cancer among women, with 417,367 newly diagnosed cases worldwide in 2020 [1]. Its incidence demonstrates a steady rise, with projections indicating a 40–50% increase in the coming decade [2]. EC is broadly classified into two main subtypes, including endometrioid (Type I) and non-endometrioid (Type II), each with distinct risk factors, molecular profiles, and clinical outcomes [3]. Understanding the etiology of these subtypes is crucial for developing targeted prevention and treatment strategies.
Previous observational studies have delineated potential risk factors associated with EC, including lifestyle factors such as smoking [4, 5], alcohol consumption [6, 7], coffee intake [8, 9], educational attainment [10, 11], and physical activity levels [12, 13]. Additionally, metabolic factors such as blood pressure [14], blood glucose level [15], blood lipid profiles [16] and anthropometric indices [17] have been implicated. However, the inherent limitations of observational studies, including confounding variables, impede definitive causal inference. Moreover, most studies have not differentiated between EC subtypes, potentially obscuring subtype-specific risk factors.
The complex biological mechanisms underlying these associations, particularly in the context of EC subtypes. Understanding subtype-specific risk factors is essential for developing tailored prevention strategies, improving risk assessment, gaining insights into disease mechanisms, and informing personalized treatment approaches. These advancements could significantly improve patient outcomes and contribute to more effective management of endometrial cancer.
In recent years, Mendelian randomization (MR) analyses have offered a promising approach to overcome some limitations of observational studies by using genetic variants as proxies for modifiable risk factors. Existing MR studies have investigated individual risk factors for EC in [18–24], but they often focus on these factors in isolation and rarely address subtype-specific risks. In this study, we aim to expand upon existing analyses by comprehensively estimating the causal effects of 5 lifestyle and 14 potentially modifiable risk factors on EC risk through the MR approach. Importantly, we will differentiate between endometrioid and non-endometrioid EC subtypes, addressing a significant gap in current research. This approach seeks to provide a more nuanced understanding of EC etiology, potentially uncovering subtype-specific risk factors that could inform targeted prevention strategies and improve patient outcomes.
Materials and methods
Study design
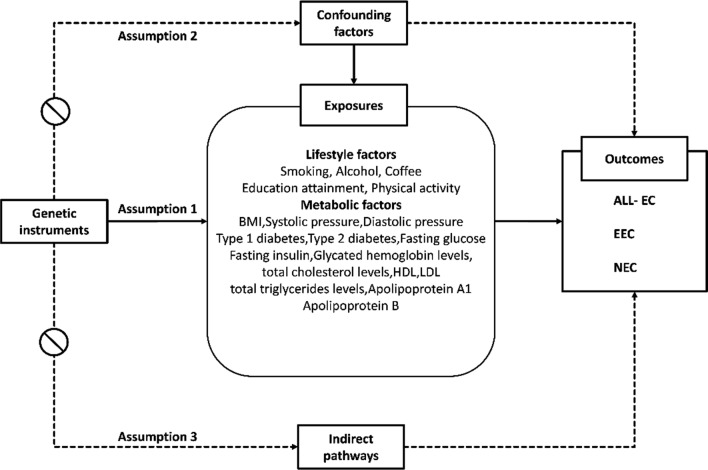
A two-sample Mendelian randomization (MR) analysis was employed to investigate the causal relationships between genetic variants and endometrial cancer risk in this study. The study protocol has not been registered elsewhere. The Mendelian randomization (MR) approach relies on the fulfillment of three fundamental assumptions (Fig. 1): (1) the chosen instrumental variables exhibit associations with the targeted lifestyle and metabolic factors; (2) the genetic variants remain unaffected by any unmeasured confounders influencing the exposure-outcome relationship; and (3) the genetic variants exclusively influence EC risk through the mediation of lifestyle and metabolic factors, without involvement in alternative pathways. For this investigation, we utilized publicly available summary-level statistics, which have previously obtained ethics approval from the original genome-wide association studies (GWASs). Consequently, no additional ethical clearance was necessary for the present MR analysis.
Fig. 1.
Fundamental assumptions of the Mendelian Randomization (MR) approach
Instrumental variables identification and data source
Single-nucleotide polymorphisms (SNPs) correlated with lifestyle and metabolic elements were obtained at the genome-wide significance level (p ≤ 5 × 10–8) from relevant genome-wide association studies (GWASs). The genetic correlation between these SNPs was assessed by calculating linkage disequilibrium, utilizing data from the European cohort of the 1000 Genomes Project as a reference population [24]. SNPs exhibiting substantial correlation which defined by a linkage disequilibrium threshold of r2 ≥ 0.01 were filtered out. From each correlated cluster, we retained only the variant demonstrating the highest statistical significance with lowest p-value in our genome-wide association analysis. We investigated the relationships between 5 lifestyle factors (tobacco consumption, alcohol drinking, coffee intake, education level, and physical activity) and 14 metabolic factors with the risk of EC, including its subtypes such as endometrioid and non-endometrioid EC. Detailed information regarding the GWASs of the studied exposures is provided in supplemetal Table 1.
Table 1.
Lifestyle factors F statistic and R2
| Lifestyle factors | Outcomes | Mean F (min,max) | R2 |
|---|---|---|---|
| Age of smoking initiation | ALL-EC | 39.17 (30.7–52.7) | 0.001 |
| Age of smoking initiation | EC | 32.35 (30.7–52.7) | 0.001 |
| Age of smoking initiation | NEC | 39.17 (30.7–52.7) | 0.001 |
| Smoking initiation | ALL-EC | 42.16 (29.8–145) | 0.006 |
| Smoking initiation | EC | 42.16 (29.8–145) | 0.006 |
| Smoking initiation | NEC | 67.63 (27–514.87) | 0.053 |
| Cigarettes per day | ALL-EC | 103.34 (30.9–961) | 0.009 |
| Cigarettes per day | EC | 103.34 (30.9–961) | 0.009 |
| Cigarettes per day | NEC | 106.73 (30.9–961) | 0.009 |
| Lifetime smoking index | ALL-EC | 44.41 (29.9–172.8) | 0.011 |
| Lifetime smoking index | EC | 44.41 (29.9–172.8) | 0.011 |
| Lifetime smoking index | NEC | 44.52 (29.9–172.8) | 0.011 |
| Alcohol intake frequency | ALL-EC | 53.44 (29.74–811.85) | 0.011 |
| Alcohol intake frequency | EC | 53.44 (29.74–811.85) | 0.011 |
| Alcohol intake frequency | NEC | 34.79 (29.74–269.71) | 0.005 |
| Alcoholic drinks per week | ALL-EC | 78.64 (29.8–926.99) | 0.005 |
| Alcoholic drinks per week | EC | 78.64 (29.8–926.99) | 0.005 |
| Alcoholic drinks per week | NEC | 52.73 (29.8–206) | 0.003 |
| Coffee intake | ALL-EC | 74.71 (30.1–646.73) | 0.007 |
| Coffee intake | EC | 39.72 (30.1–646.73) | 0.003 |
| Coffee intake | NEC | 74.71 (30.1–646.73) | 0.007 |
| Years of schooling | ALL-EC | 48.98 (29.69–240.25) | 0.020 |
| Years of schooling | EC | 48.98 (29.69–240.25) | 0.020 |
| Years of schooling | NEC | 49.03 (29.69–240.25) | 0.020 |
| Moderate to vigorous physical activity | ALL-EC | 34.39 (29.98–51.82) | 0.002 |
| Moderate to vigorous physical activity | EC | 34.39 (29.98–51.82) | 0.002 |
| Moderate to vigorous physical activity | NEC | 34.39 (29.98–51.82) | 0.002 |
| Vigorous physical activity | ALL-EC | 40.81 (32.13–55.26) | 0.001 |
| Vigorous physical activity | EC | 40.81 (32.13–55.26) | 0.001 |
| Vigorous physical activity | NEC | 40.81 (32.13–55.26) | 0.001 |
We extracted aggregated genetic association statistics for both overall EC susceptibility and subtype-specific risk from a large-scale genomic analysis. This comprehensive study incorporated data from 12,906 individuals diagnosed with EC, comprising 8758 cases of endometrioid subtype and 1230 cases of non-endometrioid variants. The analysis was further strengthened by the inclusion of up to 108,979 unaffected individuals, all of whom were of European descent. Comprehensive details regarding the GWASs conducted for the studied outcomes are delineated in supplemental Table S1.
Mendelian randomization analysis
The estimation of the causal effect of each lifestyle and metabolic factor on outcomes was primarily conducted using inverse-variance weighting under a multiplicative random-effect model, which synthesizes a combined causal estimate from each single-nucleotide polymorphism (SNP). The assumptions and advantages of the employed methodologies are succinctly presented in Tables 1, 2.
Table 2.
Metabolic factor F statistic and R2
| Metabolic factors | Outcomes | Mean F (min,max) | R2 |
|---|---|---|---|
| BMI | ALL-EC | 73.37 (28.62–1426.17) | 0.049 |
| BMI | EC | 73.37 (28.62–1426.17) | 0.049 |
| BMI | NEC | 73.37 (28.62–1426.17) | 0.049 |
| Systolic blood pressure | ALL-EC | 67.09 (27–514.87) | 0.052 |
| Systolic blood pressure | EC | 67.09 (27–514.87) | 0.052 |
| Systolic blood pressure | NEC | 67.63 (27–514.87) | 0.053 |
| Diastolic blood pressure | ALL-EC | 69.42 (26.67–496.7) | 0.052 |
| Diastolic blood pressure | EC | 69.33 (26.67–496.7) | 0.052 |
| Diastolic blood pressure | NEC | 71.12 (26.67–668.05) | 0.053 |
| Type 1 diabetes | ALL-EC | 201.72 (30.1–1402.98) | 0.007 |
| Type 1 diabetes | EC | 201.72 (30.1–1402.98) | 0.007 |
| Type 1 diabetes | NEC | 192.71 (30.1–1402.98) | 0.007 |
| Type 2 diabetes | ALL-EC | 78.92 (29.61–1066.63) | 0.028 |
| Type 2 diabetes | EC | 78.92 (29.61–1066.63) | 0.028 |
| Type 2 diabetes | NEC | 78.64 (29.61–1066.63) | 0.027 |
| Fasting glucose | ALL-EC | 136.48 (24.53–1650.91) | 0.043 |
| Fasting glucose | EC | 136.48 (24.53–1650.91) | 0.043 |
| Fasting glucose | NEC | 136.48 (24.53–1650.91) | 0.043 |
| Fasting insulin | ALL-EC | 51.8 (22.44–173.13) | 0.013 |
| Fasting insulin | EC | 51.8 (22.44–173.13) | 0.013 |
| Fasting insulin | NEC | 51.8 (22.44–173.13) | 0.013 |
| Glycated hemoglobin levels | ALL-EC | 106.74 (25–1391.6) | 0.051 |
| Glycated hemoglobin levels | EC | 106.74 (25–1391.6) | 0.051 |
| Glycated hemoglobin levels | NEC | 107.29 (25–1391.6) | 0.051 |
| Total cholesterol levels | ALL-EC | 148.98 (30.87–3063.03) | 0.076 |
| Total cholesterol levels | EC | 148.98 (30.87–3063.03) | 0.076 |
| Total cholesterol levels | NEC | 145.01 (30.87–3063.03) | 0.072 |
| HDL | ALL-EC | 137.54 (30.39–2806.33) | 0.097 |
| HDL | EC | 137.54 (30.39–2806.33) | 0.097 |
| HDL | NEC | 136.2 (30.39–2806.33) | 0.095 |
| LDL | ALL-EC | 209.52 (30.78–4531.38) | 0.082 |
| LDL | EC | 209.52 (30.78–4531.38) | 0.082 |
| LDL | NEC | 205.85 (30.78–4531.38) | 0.079 |
| Apolipoprotein A1 | ALL-EC | 131.74 (29.98–1859.31) | 0.080 |
| Apolipoprotein A1 | EC | 131.74 (29.98–1859.31) | 0.080 |
| Apolipoprotein A1 | NEC | 128.33 (29.98–1859.31) | 0.077 |
| Apolipoprotein B | ALL-EC | 176.86 (30.2–3528.44) | 0.082 |
| Apolipoprotein B | EC | 176.86 (30.2–3528.44) | 0.082 |
| Apolipoprotein B | NEC | 172.17 (30.2–3528.44) | 0.078 |
| Griglycerides | ALL-EC | 135.98 (30.32–1393.58) | 0.076 |
| Griglycerides | EC | 135.98 (30.32–1393.58) | 0.076 |
| Griglycerides | NEC | 137.55 (30.32–1393.58) | 0.076 |
We calculated the weighted median, penalized weighted median, MR pleiotropy residual sum and outlier approaches by sensitivity analyses, were performed (Table 3). Additionally, we applied the Egger regression intercept test to examine potential directional pleiotropy. An intercept not significantly different from zero (p > 0.05) in the MR-Egger analysis was interpreted as evidence against substantial pleiotropic bias. Additionally, we implemented the Cochran Q test to assess potential instrument heterogeneity to evaluate the presence of multifunctional genetic effects. Single SNP analysis utilizing the wald ratio approach and leave-one-out sensitivity test were conducted to ascertain whether associations between genetic variants and EC were influenced by individual SNPs. The F-statistic was computed to assess the association advantages of genetic variants for each exposure. "TwoSampleMR" package in R 4.0.3 was used in the analyses (Tables 1–3).
Table 3.
Heterogeneity and pleiotropy assessment
| Exposure | Outcome | SNP | Cochran’s Q | Intercept | P for intercept | Outliers |
|---|---|---|---|---|---|---|
| Age of smoking initiation | ALL-EC | 13 | 3.96 | 0.00 | 0.83 | 0 |
| Age of smoking initiation | EC | 13 | 2.12 | 0.00 | 0.89 | 0 |
| Age of smoking initiation | NEC | 13 | 9.79 | − 0.05 | 0.42 | 0 |
| Smoking initiation | ALL-EC | 85 | 99.56 | 0.01 | 0.38 | 0 |
| Smoking initiation | EC | 85 | 82.84 | 0.01 | 0.58 | 0 |
| Smoking initiation | NEC | 85 | 89.69 | − 0.01 | 0.78 | 0 |
| Cigarettes per day | ALL-EC | 21 | 26.37 | − 0.01 | 0.56 | 1 |
| Cigarettes per day | EC | 22 | 28.13 | − 0.02 | 0.11 | 0 |
| Cigarettes per day | NEC | 21 | 23.25 | 0.02 | 0.25 | 0 |
| Lifetime smoking index | ALL-EC | 108 | 116.43 | 0.02 | 0.02 | 0 |
| Lifetime smoking index | EC | 108 | 110.77 | 0.01 | 0.19 | 0 |
| Lifetime smoking index | NEC | 106 | 112.78 | 0.02 | 0.32 | 0 |
| Alcohol intake frequency | ALL-EC | 93 | 122.92 | 0.00 | 0.83 | 2 |
| Alcohol intake frequency | EC | 93 | 118.32 | 0.00 | 0.55 | 2 |
| Alcohol intake frequency | NEC | 95 | 90.32 | − 0.03 | 0.02 | 0 |
| Alcoholic drinks per week | ALL-EC | 33 | 54.90 | 0.00 | 0.81 | 0 |
| Alcoholic drinks per week | EC | 33 | 38.77 | − 0.01 | 0.41 | 0 |
| Alcoholic drinks per week | NEC | 32 | 43.81 | 0.04 | 0.01 | 0 |
| Coffee intake | ALL-EC | 37 | 50.62 | 0.00 | 0.62 | 1 |
| Coffee intake | EC | 38 | 51.57 | 0.00 | 0.77 | 0 |
| Coffee intake | NEC | 38 | 28.38 | 0.03 | 0.08 | 0 |
| Years of schooling | ALL-EC | 306 | 329.71 | 0.00 | 0.42 | 0 |
| Years of schooling | EC | 306 | 323.81 | 0.01 | 0.09 | 0 |
| Years of schooling | NEC | 305 | 280.96 | − 0.02 | 0.11 | 0 |
| Moderate to vigorous physical activity | ALL-EC | 18 | 31.25 | 0.01 | 0.69 | 1 |
| Moderate to vigorous physical activity | EC | 18 | 29.99 | 0.02 | 0.70 | 1 |
| Moderate to vigorous physical activity | NEC | 19 | 19.91 | 0.06 | 0.25 | 0 |
| Vigorous physical activity | ALL-EC | 7 | 5.47 | 0.05 | 0.42 | 0 |
| Vigorous physical activity | EC | 7 | 6.69 | 0.04 | 0.62 | 0 |
| Vigorous physical activity | NEC | 7 | 1.95 | − 0.06 | 0.69 | 0 |
| BMI | ALL-EC | 489 | 547.52 | 0.00 | 0.20 | 3 |
| BMI | EC | 490 | 598.67 | 0.00 | 0.45 | 2 |
| BMI | NEC | 492 | 474.35 | − 0.01 | 0.17 | 0 |
| Systolic blood pressure | ALL-EC | 323 | 369.06 | 0.00 | 0.24 | 6 |
| Systolic blood pressure | EC | 326 | 381.33 | 0.00 | 0.64 | 3 |
| Systolic blood pressure | NEC | 329 | 370.02 | 0.01 | 0.28 | 0 |
| Diastolic blood pressure | ALL-EC | 314 | 379.57 | 0.00 | 0.22 | 3 |
| Diastolic blood pressure | EC | 315 | 369.15 | 0.01 | 0.16 | 3 |
| Diastolic blood pressure | NEC | 316 | 403.20 | 0.01 | 0.37 | 1 |
| Type 1 diabetes | ALL-EC | 14 | 23.44 | − 0.01 | 0.61 | 1 |
| Type 1 diabetes | EC | 14 | 23.97 | 0.00 | 0.96 | 1 |
| Type 1 diabetes | NEC | 16 | 12.52 | 0.00 | 0.84 | 0 |
| Type 2 diabetes | ALL-EC | 169 | 242.65 | 0.00 | 0.37 | 3 |
| Type 2 diabetes | EC | 171 | 245.06 | 0.01 | 0.00 | 1 |
| Type 2 diabetes | NEC | 169 | 199.59 | 0.00 | 0.88 | 1 |
| Fasting glucose | ALL-EC | 62 | 88.38 | − 0.01 | 0.02 | 2 |
| Fasting glucose | EC | 63 | 104.72 | − 0.01 | 0.07 | 1 |
| Fasting glucose | NEC | 64 | 49.62 | − 0.02 | 0.06 | 0 |
| Fasting insulin | ALL-EC | 37 | 49.90 | − 0.02 | 0.16 | 1 |
| Fasting insulin | EC | 36 | 38.53 | 0.00 | 0.77 | 2 |
| Fasting insulin | NEC | 38 | 40.56 | − 0.01 | 0.74 | 0 |
| Glycated hemoglobin levels | ALL-EC | 71 | 120.23 | 0.00 | 0.82 | 0 |
| Glycated hemoglobin levels | EC | 71 | 111.29 | 0.00 | 0.49 | 0 |
| Glycated hemoglobin levels | NEC | 70 | 56.97 | 0.01 | 0.63 | 0 |
| Total cholesterol levels | ALL-EC | 59 | 74.74 | 0.00 | 0.45 | 0 |
| Total cholesterol levels | EC | 59 | 63.52 | 0.00 | 0.85 | 0 |
| Total cholesterol levels | NEC | 58 | 77.24 | 0.02 | 0.10 | 0 |
| HDL | ALL-EC | 81 | 110.74 | 0.00 | 0.34 | 1 |
| HDL | EC | 81 | 107.75 | 0.00 | 0.82 | 1 |
| HDL | NEC | 81 | 101.39 | − 0.01 | 0.27 | 0 |
| LDL | ALL-EC | 44 | 51.40 | 0.00 | 0.89 | 2 |
| LDL | EC | 46 | 56.48 | 0.00 | 0.77 | 0 |
| LDL | NEC | 45 | 56.67 | 0.01 | 0.61 | 0 |
| Apolipoprotein A1 | ALL-EC | 70 | 109.13 | 0.00 | 0.72 | 0 |
| Apolipoprotein A1 | EC | 69 | 97.88 | 0.00 | 0.74 | 1 |
| Apolipoprotein A1 | NEC | 68 | 89.08 | − 0.02 | 0.13 | 1 |
| Apolipoprotein B | ALL-EC | 54 | 75.51 | 0.01 | 0.16 | 0 |
| Apolipoprotein B | EC | 54 | 53.34 | 0.01 | 0.21 | 0 |
| Apolipoprotein B | NEC | 53 | 68.37 | 0.02 | 0.09 | 0 |
| Griglycerides | ALL-EC | 65 | 85.00 | 0.01 | 0.30 | 0 |
| Griglycerides | EC | 65 | 80.54 | 0.00 | 0.77 | 0 |
| Griglycerides | NEC | 64 | 77.02 | 0.02 | 0.16 | 0 |
Results
Lifestyle factors and endometrial cancer
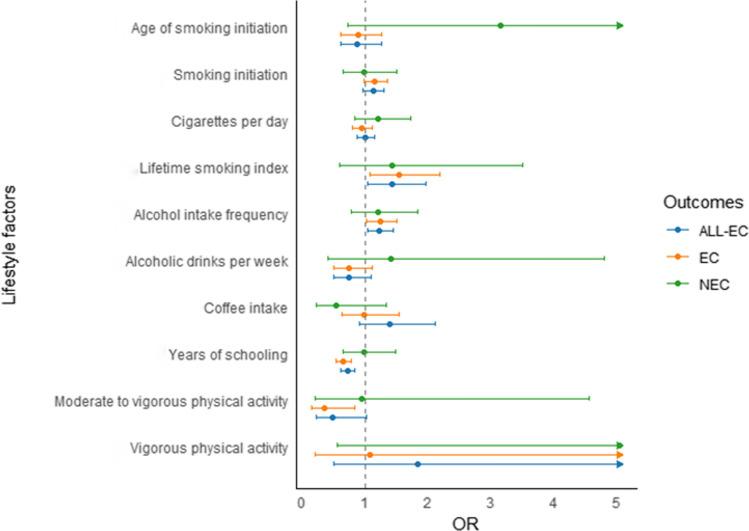
In the primary inverse-variance weighting (IVW) analysis, several lifestyles were examined in relation to ALL-EC risk. The results revealed that a higher lifetime smoking index was significantly associated with an increased risk of ALL-EC (OR: 1.43, 95% CI 1.05–1.96), suggesting a cumulative effect of smoking on cancer development (Fig. 2 and Supplemental Table S2). When examining alcohol intake, frequency of alcohol consumption was associated with a modest but significant increase in ALL-EC risk (OR: 1.23, 95% CI 1.04–1.45), although overall alcohol intake itself was not significantly linked. Additionally, coffee consumption did not significantly impact ALL-EC risk (OR: 1.39, 95% CI 0.91–2.12). Interestingly, higher education levels of education associated with a significantly decreased risk of ALL-EC (OR: 0.72, 95% CI 0.62–0.83). However, both moderate-intensity and intense physical activity did not show significant effects on ALL-EC.
Fig. 2.
Associations of lifestyle factors and genetic predisposition with endometrial cancer risk by histological subtypes
In secondary analyses, we examined the associations between the same factors with risks of specific histological subtypes of ALL-EC, including EC and NEC. For EC, the associations were largely consistent with those observed for ALL-EC. A genetic predisposition to a lifetime smoking index (OR: 1.54; 95% CI 1.08–2.20) and alcohol intake frequency (OR: 1.24; 95% CI 1.02–1.50) was associated with increased risks of endometrioid EC. However, age at smoking initiation, smoking status, cigarette amounts per day and overall alcohol intake were not significantly associated. Additionally, Higher education level continued to be protective (OR: 0.66, 95% CI 0.55–0.78), while moderate-intensity physical activity showed a significant reduction in risk (OR: 0.35, 95% CI 0.15–0.84).
The results revealed that higher education attainment (OR: 0.66; 95% CI 0.55–0.78) and engagement in moderate to vigorous physical activity (OR:0.35; 95% CI 0.15–0.84) were observed to correlate with decreased risks of endometrioid EC. Nevertheless, intense physical activity did not show a significant effect on EC.
For NEC, the associations with life styles were less pronounced. No associations were observed between lifestyle factors and non-endometrioid EC. These findings highlight that NEC may have a different etiological profile compared to EC, with fewer lifestyle factors exerting a significant influence. However, the underlying mechanisms need to explore in future studies.
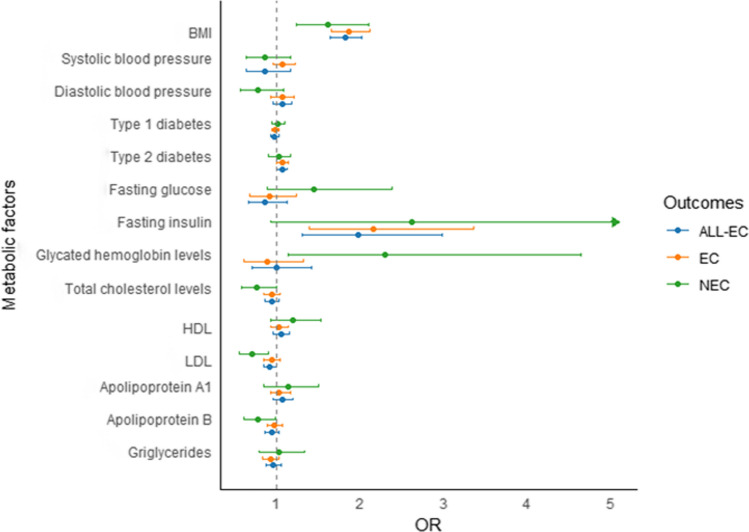
Metabolic factors and endometrial cancer
In the primary inverse-variance weighting (IVW) analyses, genetic predisposition to elevated body mass index (BMI) (OR: 1.82; 95% CI 1.64–2.01), type 2 diabetes (OR: 1.06; 95% CI 1.00–1.12), and fasting insulin levels (OR:1.97; 95% CI 1.30–2.98) were associated with an increased risk of EC (Fig. 3 and supplemental Table S2). However, systolic, diastolic blood pressure, Type 1 diabetes, fasting blood glucose and glycosylated hemoglobin were not significantly associated with ALL-EC risk. In terms of lipid profiles, higher levels of LDL (OR: 0.91; 95% CI 0.84–0.99) were linked to a decreased risk of EC, while systolic, diastolic blood pressure, Type 1 diabetes, fasting blood glucose and glycosylated hemoglobin were not significantly associated with ALL-EC risk.
Fig. 3.
Associations of metabolic factors and genetic predisposition with endometrial cancer risk by histological subtypes
In secondary analyses, the associations between metabolism factors and EC histological subtypes were observed. For EC, the results were consistent with ALL-EC. BMI remained a strong risk factor (OR: 1.86, 95% CI 1.65–2.11), and fasting insulin was again associated with increased risk (OR: 2.16, 95% CI 1.39–3.36). Additionally, higher levels of apolipoprotein B (OR: 0.77; 95% CI 0.60–0.98) and LDL (OR: 0.71; 95% CI 0.55–0.90) were associated with a reduced risk of endometrioid EC. For NEC, BMI was again associated with increased risk though less strongly (OR: 1.61, 95% CI 1.23–2.10) and increased glycated hemoglobin levels (OR: 2.30; 95% CI 1.14–4.64) were linked to an augmented risk. These results highlights a potential distinct metabolic pathway involved in EC and NEC.
Discussion
Our MR analyses revealed significant associations between EC risk and various lifestyle and metabolic factors based on large-scale GWAS summary statistics. We found compelling genetic evidence suggesting that higher lifetime smoking index, alcohol intake frequency, BMI, T2DM, and fasting insulin are associated with an increased risk of EC, particularly for endometrioid EC. Conversely, higher education attainment, engagement in moderate to high intensity exercise, and lower levels of triglycerides may reduce the risk of EC.
Our findings regarding the association between smoking and EC risk align with existing literature. Smoking, characterized by its carcinogenic properties, has been linked to cancer incidence and poorer long-term outcome [25, 26]. Nicotine, a prominent component of tobacco, acts as a cancer promoter, facilitating cancer cell division epithelial-mesenchymal transformation, and angiogenesis [27–30], thereby potentially contributing to a more aggressive phenotype conducive to metastasis. However, it is noteworthy that some studies [31], such as those by NIKI Dimiou, have reported inverse associations between lifetime smoking and EC risk [18], suggesting the necessity for further confirmation through larger Mendelian randomization studies.
Regarding alcohol intake frequency, our analysis revealed suggestive evidence of its association with EC, including both endometrioid and non-endometrioid subtypes. However, the precise biological mechanisms underlying this relationship remain unclear. Nonetheless, alcohol intake may lead to elevated cumulative estrogen burden, thereby promoting epithelial cell genotoxicity and mitosis [16], which could contribute to EC progression. Notably, our findings did not establish a significant association between weekly alcohol consumption and EC risk, consistent with previous cohort studies.
Additionally, we did not reveal a statistically significant relationship between coffee intake and the risk of developing EC, irrespective of coffee type. Nonetheless, prior research has indicated that caffeine consumption among premenopausal women may be associated with increased EC risk, while such an association is negligible among postmenopausal women [9, 32].
Educational attainment emerged as a noteworthy factor associated with EC risk reduction in our study, particularly for endometrioid EC. This finding is consistent with previous cohort studies, including one by Qi Xia Wang, which suggested that longer educational attainment could predict a significant reduction in EC risk [33]. The exact mechanisms mediating this association remain elusive, although various intermediate phenotypes may play mediating roles.
In terms of physical activity, our study revealed an association between moderate physical activity and reduced risk of endometrioid EC, whereas no significant relationship was observed with vigorous physical activity. Promoting physical activity and reducing sedentary behaviors are recognized as effective strategies for cancer prevention, independently of body fat [34], through various mechanisms.
Moreover, our Mendelian randomization study provided genetic support for the causal relationship between BMI, fasting insulin, type 2 diabetes, LDL cholesterol, and EC risk, particularly for endometrioid EC, with associations reported by observational and MR analyses [2, 35–37].However, no statistically significant associations were found between hypertension, type 1 diabetes, fasting blood glucose, glycated hemoglobin, HDL cholesterol, total cholesterol, and EC risk.
In conclusion, our comprehensive analysis sheds light on the complex interplay between lifestyle, metabolic factors, and EC risk. While certain factors demonstrate clear associations, further research is warranted to elucidate underlying mechanisms and confirm observed relationships, particularly those with conflicting findings across different studies.
Several strengths characterize our Mendelian randomization (MR) study. Foremost among them is the MR design, which effectively mitigates confounding and reverse causality biases to a significant extent [38, 39]. Through the application of Mendelian randomization, we comprehensively investigated the associations between 5 lifestyle factors, 14 metabolic factors, and EC and its subtypes. Furthermore, our study specifically targeted individuals of European descent, minimizing potential biases arising from population structure.
Limitation
Certain limitations warrant consideration when interpreting our findings. Firstly, our MR analysis predominantly focused on individuals of European ancestry, potentially limiting the generalizability of our results to other populations. This underscores the importance of conducting genomic studies encompassing diverse ancestral groups to capture broader insights into EC etiology. Secondly, the utilization of summarized data restricted the scope of analyses that could be performed, precluding non-linear MR investigations. Lastly, sample overlap was observed between several exposure genome-wide association studies (GWASs) and the outcome dataset in our MR analysis. While sample overlap is a common limitation in two-sample MR studies employing large genetic consortia, it may introduce weak instrument bias. Nevertheless, our stringent selection criteria for single-nucleotide polymorphisms (SNPs) at the genome-wide threshold, along with consistently high estimated F statistics (ranging from 33.45 to 209.52, all exceeding 10), suggest that significant weak instrument bias is unlikely, despite the consortia overlap.
Conclusions
Our study yields compelling evidence for a favorable causal association between genetically factors such as lifetime smoking index, alcohol intake frequency, BMI, T2DM, fasting insulin levels, and the risk of EC. Conversely, our findings suggest that higher education levels, engagement in moderate-intensity physical exercise, and lower levels of LDL cholesterol may reduce the risk of EC.
The comprehensive MR analysis conducted in this study offers valuable insights into potential causal mechanisms underpinning the relationship between lifestyle and metabolic factors and EC risk. Furthermore, the findings revealed a basis for developing potential strategies aimed at the prevention of EC.
Supplementary Information
Author contributions
X.Z., C.P., and J.W. conceptualized and designed the study; X.Z. and C.P. analyzed the data; X.Z., C.P., L.W., X.L., H.L., S.W., and L.W. interpreted the data; X.Z. and C.P. wrote the original draft; All authors commented on the manuscript; X.L., and J.W. reviewed and improved the manuscript; J.W. supervised all research work. All authors have read and agreed to the published version of the manuscript.
Data availability
Data is provided within the manuscript or supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xu Zhang and Caiyu Pu have contributed equally to this work.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Crosbie EJ, Kitson SJ, McAlpine JN, Mukhopadhyay A, Powell ME, Singh N. Endometrial cancer. Lancet. 2022;399(10333):1412–28. [DOI] [PubMed] [Google Scholar]
- 3.Morice P, Leary A, Creutzberg C, Abu-Rustum N, Darai E. Endometrial cancer. Lancet. 2016;387(10023):1094–108. [DOI] [PubMed] [Google Scholar]
- 4.Huang Z, Sun S, Lee M, Maslov AY, Shi M, Waldman S, Marsh A, Siddiqui T, Dong X, Peter Y, Sadoughi A, Shah C, Ye K, Spivack SD, Vijg J. Single-cell analysis of somatic mutations in human bronchial epithelial cells in relation to aging and smoking. Nat Genet. 2022;54(4):492–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dimou N, Omiyale W, Biessy C, Viallon V, Kaaks R, O’Mara TA, Aglago EK, Ardanaz E, Bergmann MM, Bondonno NP, Braaten T, Colorado-Yohar SM, Crous-Bou M, Dahm CC, Fortner RT, Gram IT, Harlid S, Heath AK, Idahl A, Kvaskoff M, Nost TH, Overvad K, Palli D, Perez-Cornago A, Sacerdote C, Sanchez MJ, Schulze MB, Severi G, Simeon V, Tagliabue G, Tjonneland A, Truong T, Tumino R, Johansson M, Weiderpass E, Murphy N, Gunter MJ, Lacey B, Allen NE, Dossus L. Cigarette smoking and endometrial cancer risk: observational and mendelian randomization analyses. Cancer Epidemiol Biomarkers Prev. 2022;31(9):1839–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friberg E, Orsini N, Mantzoros CS, Wolk A. Alcohol intake and endometrial cancer risk: a meta-analysis of prospective studies. Br J Cancer. 2010;103(1):127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fedirko V, Jenab M, Rinaldi S, Biessy C, Allen NE, Dossus L, Onland-Moret NC, Schutze M, Tjonneland A, Hansen L, Overvad K, Clavel-Chapelon F, Chabbert-Buffet N, Kaaks R, Lukanova A, Bergmann MM, Boeing H, Trichopoulou A, Oustoglou E, Barbitsioti A, Saieva C, Tagliabue G, Galasso R, Tumino R, Sacerdote C, Peeters PH, Bueno-de-Mesquita HB, Weiderpass E, Gram IT, Sanchez S, Duell EJ, Molina-Montes E, Arriola L, Chirlaque MD, Ardanaz E, Manjer J, Lundin E, Idahl A, Khaw KT, Romaguera-Bosch D, Wark PA, Norat T, Romieu I. Alcohol drinking and endometrial cancer risk in the European prospective investigation into cancer and nutrition (EPIC) study. Ann Epidemiol. 2013;23(2):93–8. [DOI] [PubMed] [Google Scholar]
- 8.Yang TO, Crowe F, Cairns BJ, Reeves GK, Beral V. Tea and coffee and risk of endometrial cancer: cohort study and meta-analysis. Am J Clin Nutr. 2015;101(3):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crous-Bou M, Du M, Gunter MJ, Setiawan VW, Schouten LJ, Shu XO, Wentzensen N, Bertrand KA, Cook LS, Friedenreich CM, Gapstur SM, Goodman MT, Ibiebele TI, La Vecchia C, Levi F, Liao LM, Negri E, McCann SE, O’Connell K, Palmer JR, Patel AV, Ponte J, Reynolds P, Sacerdote C, Sinha R, Spurdle AB, Trabert B, van den Brandt PA, Webb PM, Petruzella S, Olson SH, De Vivo I, Epidemiology of Endometrial Cancer C. Coffee consumption and risk of endometrial cancer: a pooled analysis of individual participant data in the Epidemiology of Endometrial Cancer Consortium (E2C2). Am J Clin Nutr. 2022;116(5):1219–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen KE, Hannibal CG, Nielsen A, Jensen A, Nohr B, Munk C, Kjaer SK. Social inequality and incidence of and survival from cancer of the female genital organs in a population-based study in Denmark, 1994–2003. Eur J Cancer. 2008;44(14):2003–17. [DOI] [PubMed] [Google Scholar]
- 11.Laaksonen MA, Arriaga ME, Canfell K, MacInnis RJ, Byles JE, Banks E, Shaw JE, Mitchell P, Giles GG, Magliano DJ, Gill TK, Klaes E, Velentzis LS, Hirani V, Cumming RG, Vajdic CM. The preventable burden of endometrial and ovarian cancers in Australia: a pooled cohort study. Gynecol Oncol. 2019;153(3):580–8. [DOI] [PubMed] [Google Scholar]
- 12.Tavani A, Bravi F, Dal Maso L, Zucchetto A, Bosetti C, Pelucchi C, Montella M, Franceschi S, La Vecchia C. Physical activity and risk of endometrial cancer: an Italian case–control study. Eur J Cancer Prev. 2009;18(4):303–6. [DOI] [PubMed] [Google Scholar]
- 13.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30(5):397–412. [DOI] [PubMed] [Google Scholar]
- 14.Aune D, Sen A, Vatten LJ. Hypertension and the risk of endometrial cancer: a systematic review and meta-analysis of case–control and cohort studies. Sci Rep. 2017;7:44808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saed L, Varse F, Baradaran HR, Moradi Y, Khateri S, Friberg E, Khazaei Z, Gharahjeh S, Tehrani S, Sioofy-Khojine AB, Najmi Z. The effect of diabetes on the risk of endometrial cancer: an updated a systematic review and meta-analysis. BMC Cancer. 2019;19(1):527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lathigara D, Kaushal D, Wilson RB. Molecular mechanisms of western diet-induced obesity and obesity-related carcinogenesis-a narrative review. Metabolites. 2023;13(5):675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baron JA, Nichols HB, Safe S. Cigarette smoking and estrogen-related cancer-reply. Cancer Epidemiol Biomarkers Prev. 2021;30(10):1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hazelwood E, Sanderson E, Tan VY, Ruth KS, Frayling TM, Dimou N, Gunter MJ, Dossus L, Newton C, Ryan N, Pournaras DJ, O’Mara TA, Davey Smith G, Martin RM, Yarmolinsky J. Identifying molecular mediators of the relationship between body mass index and endometrial cancer risk: a Mendelian randomization analysis. BMC Med. 2022;20(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nead KT, Sharp SJ, Thompson DJ, Painter JN, Savage DB, Semple RK, Barker A, Perry JR, Attia J, Dunning AM, Easton DF, Holliday E, Lotta LA, O’Mara T, McEvoy M, Pharoah PD, Scott RJ, Spurdle AB, Langenberg C, Wareham NJ, Scott RA. Evidence of a causal association between insulinemia and endometrial cancer: a Mendelian randomization analysis. J Natl Cancer Inst. 2015;107(9):djv178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitson SJ, Crosbie EJ. Endometrial cancer and obesity. Obstet Gynaecol. 2019;21(4):237–45. [Google Scholar]
- 22.Kho PF, Amant F, Annibali D, Ashton K, Attia J, Auer PL, Beckmann MW, Black A, Brinton L, Buchanan DD, Chanock SJ, Chen C, Chen MM, Cheng THT, Cook LS, Crous-Bous M, Czene K, De Vivo I, Dennis J, Dörk T, Dowdy SC, Dunning AM, Dürst M, Easton DF, Ekici AB, Fasching PA, Fridley BL, Friedenreich CM, García-Closas M, Gaudet MM, Giles GG, Goode EL, Gorman M, Haiman CA, Hall P, Hankinson SE, Hein A, Hillemanns P, Hodgson S, Hoivik EA, Holliday EG, Hunter DJ, Jones A, Kraft P, Krakstad C, Lambrechts D, Le Marchand L, Liang X, Lindblom A, Lissowska J, Long J, Lu L, Magliocco AM, Martin L, McEvoy M, Milne RL, Mints M, Nassir R, Otton G, Palles C, Pooler L, Proietto T, Rebbeck TR, Renner SP, Risch HA, Rübner M, Runnebaum I, Sacerdote C, Sarto GE, Schumacher F, Scott RJ, Setiawan VW, Shah M, Sheng X, Shu XO, Southey MC, Tham E, Tomlinson I, Trovik J, Turman C, Tyrer JP, Van Den Berg D, Wang Z, Wentzensen N, Xia L, Xiang YB, Yang HP, Yu H, Zheng W, Webb PM, Thompson DJ, Spurdle AB, Glubb DM, O’Mara TA. Mendelian randomization analyses suggest a role for cholesterol in the development of endometrial cancer. Int J Cancer. 2021;148(2):307–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Naqvi A, MacKintosh ML, Derbyshire AE, Tsakiroglou AM, Walker TDJ, McVey RJ, Bolton J, Fergie M, Bagley S, Ashton G, Pemberton PW, Syed AA, Ammori BJ, Byers R, Crosbie EJ. The impact of obesity and bariatric surgery on the immune microenvironment of the endometrium. Int J Obes (Lond). 2022;46(3):605–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, Auer PL, Beckmann MW, Black A, Bolla MK, Brauch H, Brenner H, Brinton L, Buchanan DD, Burwinkel B, Chang-Claude J, Chanock SJ, Chen C, Chen MM, Cheng THT, Clarke CL, Clendenning M, Cook LS, Couch FJ, Cox A, Crous-Bous M, Czene K, Day F, Dennis J, Depreeuw J, Doherty JA, Dörk T, Dowdy SC, Dürst M, Ekici AB, Fasching PA, Fridley BL, Friedenreich CM, Fritschi L, Fung J, García-Closas M, Gaudet MM, Giles GG, Goode EL, Gorman M, Haiman CA, Hall P, Hankison SE, Healey CS, Hein A, Hillemanns P, Hodgson S, Hoivik EA, Holliday EG, Hopper JL, Hunter DJ, Jones A, Krakstad C, Kristensen VN, Lambrechts D, Marchand LL, Liang X, Lindblom A, Lissowska J, Long J, Lu L, Magliocco AM, Martin L, McEvoy M, Meindl A, Michailidou K, Milne RL, Mints M, Montgomery GW, Nassir R, Olsson H, Orlow I, Otton G, Palles C, Perry JRB, Peto J, Pooler L, Prescott J, Proietto T, Rebbeck TR, Risch HA, Rogers PAW, Rübner M, Runnebaum I, Sacerdote C, Sarto GE, Schumacher F, Scott RJ, Setiawan VW, Shah M, Sheng X, Shu XO, Southey MC, Swerdlow AJ, Tham E, Trovik J, Turman C, Tyrer JP, Vachon C, VanDen BD, Vanderstichele A, Wang Z, Webb PM, Wentzensen N, Werner HMJ, Winham SJ, Wolk A, Xia L, Xiang YB, Yang HP, Yu H, Zheng W, Pharoah PDP, Dunning AM, Kraft P, De Vivo I, Tomlinson I, Easton DF, Spurdle AB, Thompson DJ. Identification of nine new susceptibility loci for endometrial cancer. Nat Commun. 2018;9(1):3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierce BL, Ahsan H, Vanderweele TJ. Power and instrument strength requirements for Mendelian randomization studies using multiple genetic variants. Int J Epidemiol. 2011;40(3):740–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3-9. [DOI] [PubMed] [Google Scholar]
- 27.Schaal C, Chellappan SP. Nicotine-mediated cell proliferation and tumor progression in smoking-related cancers. Mol Cancer Res. 2014;12(1):14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schuller HM. Regulatory role of the alpha7nAChR in cancer. Curr Drug Targets. 2012;13(5):680–7. [DOI] [PubMed] [Google Scholar]
- 29.Singh S, Pillai S, Chellappan S. Nicotinic acetylcholine receptor signaling in tumor growth and metastasis. J Oncol. 2011;2011: 456743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jeon SY, Go RE, Heo JR, Kim CW, Hwang KA, Choi KC. Effects of cigarette smoke extracts on the progression and metastasis of human ovarian cancer cells via regulating epithelial-mesenchymal transition. Reprod Toxicol. 2016;65:1–10. [DOI] [PubMed] [Google Scholar]
- 31.Raglan O, Kalliala I, Markozannes G, Cividini S, Gunter MJ, Nautiyal J, Gabra H, Paraskevaidis E, Martin-Hirsch P, Tsilidis KK, Kyrgiou M. Risk factors for endometrial cancer: an umbrella review of the literature. Int J Cancer. 2019;145(7):1719–30. [DOI] [PubMed] [Google Scholar]
- 32.Zhao LG, Li ZY, Feng GS, Ji XW, Tan YT, Li HL, Gunter MJ, Xiang YB. Coffee drinking and cancer risk: an umbrella review of meta-analyses of observational studies. BMC Cancer. 2020;20(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Q, Wang R, Chen C, Feng Y, Ye Z, Zhan M, Wen H, Guo K. Educational attainment and endometrial cancer: a Mendelian randomization study. Front Genet. 2022;13: 993731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedenreich CM, Ryder-Burbidge C, McNeil J. Physical activity, obesity and sedentary behavior in cancer etiology: epidemiologic evidence and biologic mechanisms. Mol Oncol. 2021;15(3):790–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Freuer D, Meisinger C, Linseisen J. Causal relationship between dietary macronutrient composition and anthropometric measures: a bidirectional two-sample Mendelian randomization analysis. Clin Nutr. 2021;40(6):4120–31. [DOI] [PubMed] [Google Scholar]
- 36.Shaw E, Farris M, McNeil J, Friedenreich C. Obesity and endometrial cancer. Recent Results Cancer Res. 2016;208:107–36. [DOI] [PubMed] [Google Scholar]
- 37.Painter JN, O’Mara TA, Marquart L, Webb PM, Attia J, Medland SE, Cheng T, Dennis J, Holliday EG, McEvoy M, Scott RJ, Ahmed S, Healey CS, Shah M, Gorman M, Martin L, Hodgson SV, Beckmann MW, Ekici AB, Fasching PA, Hein A, Rubner M, Czene K, Darabi H, Hall P, Li J, Dork T, Durst M, Hillemanns P, Runnebaum IB, Amant F, Annibali D, Depreeuw J, Lambrechts D, Neven P, Cunningham JM, Dowdy SC, Goode EL, Fridley BL, Winham SJ, Njolstad TS, Salvesen HB, Trovik J, Werner HM, Ashton KA, Otton G, Proietto A, Mints M, Tham E, Bolla MK, Michailidou K, Wang Q, Tyrer JP, Hopper JL, Peto J, Swerdlow AJ, Burwinkel B, Brenner H, Meindl A, Brauch H, Lindblom A, Chang-Claude J, Couch FJ, Giles GG, Kristensen VN, Cox A, Pharoah PD, Tomlinson I, Dunning AM, Easton DF, Thompson DJ, Spurdle AB, Group A, for R, National Study of Endometrial Cancer Genetics G, and Australian National Endometrial Cancer Study G. Genetic risk score Mendelian randomization shows that obesity measured as body mass index, but not waist: hip ratio, is causal for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2016;25(11):1503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Burgess S, Thompson SG. Interpreting findings from Mendelian randomization using the MR-Egger method. Eur J Epidemiol. 2017;32(5):377–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Burgess S, Davies NM, Thompson SG. Bias due to participant overlap in two-sample Mendelian randomization. Genet Epidemiol. 2016;40(7):597–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the manuscript or supplementary information files.