Abstract
Small cell carcinoma of the bladder (SCCB) is a rare, highly malignant neuroendocrine tumor. This study attempted to analyze tumor characteristics, treatments and clinical outcomes in China. We conducted a retrospective analysis of patients diagnosed with non-metastatic SCCB at multi-institutions between January 2007 and January 2022. The Kaplan-Meier method was used to calculate survival. A total of 20 patients were included. 10 had localized disease (T1-2N0), and 10 had locally advanced disease (≥ T3 or N+). 13 received local treatment (partial cystectomy or transurethral resection of the bladder tumor) and 7 received radical treatment (radical cystectomy or radiotherapy). A total of 18 patients (90%) received chemotherapy (CT), either neoadjuvant CT (n = 5) or adjuvant CT (n = 13). The median OS for the receiving local treatment was 65.3 months (95% CI 0 to 138 months) and the corresponding 1-year, 2-year, and 3-year OS was 77%, 54%, and 54%, respectively. The median OS for the receiving radical treatment was not reached and the corresponding 1-year, 2-year, and 3-year OS was 100%, 100%, and 75%, respectively. The median PFS for receiving local treatment was 13.8 months (95% CI 9.3 to 18.3 months) and the corresponding 1-year, 2-year, and 3-year PFS was 46%, 31%, and 31%, respectively. The median PFS for the receiving radical treatment was not reached and the corresponding 1-year, 2-year, and 3-year PFS was 83%, 56%, and 56%, respectively. This study reported the largest cohort of non-metastatic SCCB among Chinese population. Given its metastatic potential, CT remained an essential part of the treatment. The survival outcomes of radical cystectomy and RT in non-metastatic SCCB were encouraging.
Keywords: Urinary bladder cancer, Small cell carcinoma, Cystectomy, Chemotherapy, Transurethral resection of bladder tumor, Radiation therapy
Subject terms: Bladder cancer, Bladder
Introduction
In 2016, bladder cancer ranked as the 16th most frequently diagnosed cancer in China, the incidence of bladder cancer was 5.95 cases per 100,000 population, 3.4 times higher in men than in women, an estimated 82,300 people were newly diagnosed with bladder cancer and about 33,700 of them died from this disease1. Over 90% of bladder cancers are transitional cell carcinoma of the urothelial tract, small cell carcinoma of the bladder (SCCB) is rare and accounts for only 0.5-1.0% of all bladder cancers2–4. Koay et al. analyzed 642 SCCB patients from the Surveillance, Epidemiology, and End Results (SEER) Database from 1991 to 2005, showing an increase in proportion of SCCB among all bladder cancers from 0.3 to 0.6%, with a male to female ratio of 3:1 and a median age of onset of 73 years2. Pure SCCB accounts for 12-60% of all SCCB, usually coexisting with non-small cell carcinoma components, such as transitional cell carcinoma, adenocarcinoma, squamous cell carcinoma or other variants2–8. Patients with pure SCCB showed poorer survival than those with mixed forms or pure urothelial carcinoma because of aggressive behavior and high metastatic potential4,6,7,9,10, but Chau et al.5 and Parimi et al.11 didn’t detect any survival difference on the basis of pure versus mixed SCCB histology. The most common sites of metastasis are pelvic and retroperitoneal lymph nodes (30-50%), liver (24-47%), and bone (24-33%)7,12. To our knowledge, since it was first reported in 1981, five largest patient-based studies (N > 400) from UK, SEER, and National Cancer Database (NCDB) showed a median overall survival (OS) of 11 to 20.7 months and 3-year OS of 33–37% for all stages combined2,5,13–15.
Unfortunately, the rarity of SCCB has precluded well-designed prospective randomized trials to compare different treatments, and most data comes from retrospective studies and 2 small prospective trials16,17. Generally, treatment strategies for SCCB are extrapolated and adapted from the management of small cell lung cancer (SCLC) and transitional cell bladder carcinoma. The national comprehensive cancer network (NCCN) guideline recommends concurrent chemoradiotherapy or neoadjuvant chemotherapy followed by local treatment (cystectomy or radiotherapy) for SCCB patients with limited stage disease. For extensive SCCB, similar regimens to SCLC, such as cisplatin or carboplatin in combination with etoposide, are recommended. In 2013, the Canadian Association of Genitourinary Medical Oncologists (CAGMO) published a consensus on the management of SCCB9. This included Gadolinium-enhanced MRI or contrast-enhanced CT examination as part of staging, adoption of a limited versus extensive disease staging system analogous to SCLC, and use of etoposide/platinum-based chemotherapy regimen. However, the level of evidence from these guidelines and consensus has been consistently assessed as low due to the lack of level I and II evidence.
Given the paucity of data from the Chinese population, this retrospective study was conducted to analyze the patient and tumor characteristics, received treatments, clinical outcomes and failure patterns of SCCB.
Materials and methods
Study design and patients
Between January 2007 and January 2022, patients with a diagnosis of SCCB, whose histology was either the predominant component (> 50%) or pure, were retrospectively reviewed at multi-institutions (National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and National Cancer Center/Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences). Patients must receive at least one of the following treatments as their primary treatment, such as transurethral resection of the bladder tumor (TURBT), partial cystectomy (PC), radical cystectomy (RC), radiotherapy, and chemotherapy. Pathological specimens of the primary tumor were obtained by cystoscopy and TURBT, PC or RC. All pathologic reports were reviewed by the authors through medical records. The study included patients with T1-4N0-3 disease and excluded distant metastases. Patients with small cell carcinoma from other organs were also excluded from this study. The tumor population was divided into two groups, including localized disease group and locally advanced disease group. Localized disease was defined as tumor confined to the bladder muscle (T1-2N0), and locally advanced disease was defined as tumor invaded perivesical tissue (pathologic T3-4) and/or pathologic node-positive bladder cancer18,19. Demographic and tumor characteristics, received treatments, and clinical outcomes were extracted from medical charts and correspondence. The data cutoff was May 31, 2022.
Ethics statement
This study was conducted in compliance with the ethical principles originating from the Declaration of Helsinki. This research has received approval and a waiver for participant informed consent from the Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital due to the retrospective nature of the study, where patient identities were deliberately anonymized, rendering individual informed consent unnecessary. All methods in this study were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
Descriptive data were expressed as frequency and percentages. Tumor response was assessed according to RECIST 1.1 criteria. Overall survival (OS) was defined as the time from the date of diagnosis to death by any cause or the final follow-up date, censoring at last known follow-up for living patients. Progression-free survival (PFS) was calculated from the day of first treatment to disease progression or death. The Kaplan-Meier method was used to calculate OS, PFS. Statistical analyses were performed using SPSS (version 26; IBM SPSS Statistics Inc, Chicago, IL, USA).
Results
Patient characteristics
A total of 23 patients met the enrollment criteria and were reviewed. 1 patient didn’t receive any treatment, 1 had no specific treatment details and 1 patient was diagnosed with large cell neuroendocrine carcinoma, all were excluded from the study. Consequently, 20 patients with SCCB were included in the analysis. Patient demographics, tumor characteristics, and staging are summarized in Table 1. The predominant gender was male, with a male to female ratio of 4:1. The median age of the patients was 61 years (range, 50–86 years). A total of 5 (25%) patients were older than 70 years. The ECOG PS ranged from 0 to 1. 6 patients (30%) had a history of cigarette smoking. The most common presenting symptoms were painless gross hematuria (75%), frequent urination (10%) and urinary tract obstruction (5%). Tumor histology was pure SCCB in 13 patients (65%), whereas the remaining 7 patients had mixed histological subtypes, including transitional cell carcinoma (30%) and adenocarcinoma (5%). At initial diagnosis, all patients were non-metastatic, 10 had localized disease (T1-2N0), and 10 had locally advanced disease (≥ T3 or N+). According to the TNM staging system, the proportions of patients with stage I-IVa disease were 20% (4/20), 30% (6/20), 45% (9/20), and 5% (1/20) respectively.
Table 1.
Baseline characteristics and treatment (n = 20).
| Characteristics | Overall (%) | Local treatment (%) | Radical treatment (%) |
|---|---|---|---|
| Age | |||
| < 60 | 8 (40%) | 5 (25%) | 3 (15%) |
| ≥60 | 12 (60%) | 8 (40%) | 4 (20%) |
| Gender | |||
| Male | 16 (80%) | 9 (45%) | 7 (35%) |
| Female | 4 (20%) | 4 (20%) | 0 (0%) |
| Smoking | |||
| Yes | 6 (30%) | 3 (15%) | 3 (15%) |
| No | 14 (70%) | 10 (50%) | 4 (20%) |
| Histology | |||
| Pure small cell(SC) | 13 (65%) | 8 (40%) | 5 (25%) |
| SC + transitional cell | 6 (30%) | 5 (25%) | 1 (5%) |
| SC + adenocarcinoma cell | 1 (5%) | 0 (0%) | 1 (5%) |
| T Stage | |||
| 1 | 4 (20%) | 4 (20%) | 0 (0%) |
| 2 | 6 (30%) | 5 (25%) | 1 (5%) |
| 3 | 9 (45%) | 3 (15%) | 6 (30%) |
| 4b | 1 (5%) | 1 (5%) | 0 (0%) |
| N Stage | |||
| 0 | 18 (90%) | 13 (65%) | 5 (25%) |
| 1 | 1 (5%) | 0 (0%) | 1 (5%) |
| 2 | 1 (5%) | 0 (0%) | 1 (5%) |
| Stage(TNM 8th) | |||
| I (T1N0) | 4 (20%) | 4 (20%) | 0 (0%) |
| II (T2N0) | 6 (30%) | 5 (25%) | 1 (5%) |
| III (T3N0-2) | 9 (45%) | 3 (15%) | 6 (30%) |
| IVa (T4bN0) | 1 (5%) | 1 (5%) | 0 (0%) |
Treatments
13 received local treatment and 7 received radical treatment, and the treatment characteristics for patients included in this analysis are shown in Table 1. Local treatment includes PC and TURBT, and radical treatment includes RC and RT. Treatment according to staging is listed in Table 2. In the localized disease group, 9 patients received local treatment and 1 patient received radical treatment. 6 patients (4T1N0, 2T2N0) were treated with TURBT, of which only 4 received adjuvant chemotherapy (CT) or intravesical CT, reasons for 2 patients not receiving CT (T1N0) were advanced age, patient decisions and rapid progression of disease. 3 patients with T2N0 disease received PC and adjuvant CT who refused RC. 1 patient (T2N0) received neoadjuvant CT with complete response (CR) confirmed by cystoscopy, followed by bladder hypofractionated radiation therapy (RT) with a regimen of 45 Gy at 3.0 Gy per fraction over 3 weeks, analogous to RT for limited stage SCLC20. In the locally advanced disease group, 4 received local treatment and 6 received radical treatment. Specifically, 3 underwent PC, 2 underwent RC, 4 received neoadjuvant CT and radical chemoradiation therapy (CRT), and 1 patient received TURBT and CT as primary treatment due to intolerance of cystectomy and RT. In the locally advanced disease group, all patients received CT, either neoadjuvant (n = 4) or adjuvant (n = 6). Among the 4 patients receiving CRT, 3 achieved partial response (PR) confirmed by TURBT prior to radical RT. Pelvic cavity received a total dose of 45 Gy at 1.8 Gy per fraction, and a boost to the gross tumor and lymph node of 15–20 Gy at 2.0–2.4 Gy per fraction was planned for each patient using intensity-modulated radiotherapy (IMRT) or volume-modulated radiotherapy (VMAT). Another patient (T3N0) underwent CR after CT confirmed by cystoscopy, followed by bladder hypofractionated RT with a regimen of 45 Gy at 3.0 Gy per fraction.
Table 2.
Treatment according to staging (n = 20).
| Local treatments ± CT | Radical treatments + CT | |||
|---|---|---|---|---|
| TURBT ± CT | Partial cystectomy + CT | Radical cystectomy + CT | RT + CT | |
| Localized disease | 6 (30%) | 3 (15%) | 0 (0%) | 1 (5%) |
| Locally advanced disease | 1 (5%) | 3 (15%) | 2 (10%) | 4 (20%) |
TURBT, transurethral resection of the bladder tumor; RT, radiation therapy; CT, chemotherapy.
A total of 18 patients (90%) received CT. The most commonly used CT regimen was etoposide and cisplatin (45%), with cisplatin given at 75 mg/m2 on day 1 and etoposide 100 mg/m2 on day 1, 2, 3 intravenously every 3 weeks for 4 to 6 cycles, which was based on the regimen of SCLC. A single instillation of epirubicin was administered in one patient within 24 h of TURBT. Other regimens are shown in Table 3.
Table 3.
Chemotherapy regimens.
| Chemotherapy | Number (%) |
|---|---|
| Cisplatin + etoposide | 9 (45%) |
| Carboplatin + etoposide | 2 (10%) |
| Cisplatin + gemcitabine | 3 (15%) |
| Gemcitabine | 1 (5%) |
| Cisplatin + cyclophosphamide + doxorubicin | 1 (5%) |
| Cisplatin + albumin paclitaxel | 1 (5%) |
| Epirubicin | 1 (5%) |
In 5 patients who received CRT, the concurrent CT regimens included etoposide and cisplatin (n = 2) and cisplatin (n = 1), the rest 2 patients received sequential RT and CT (etoposide and cisplatin). No patient received prophylactic brain irradiation.
Survival outcomes and failure patterns
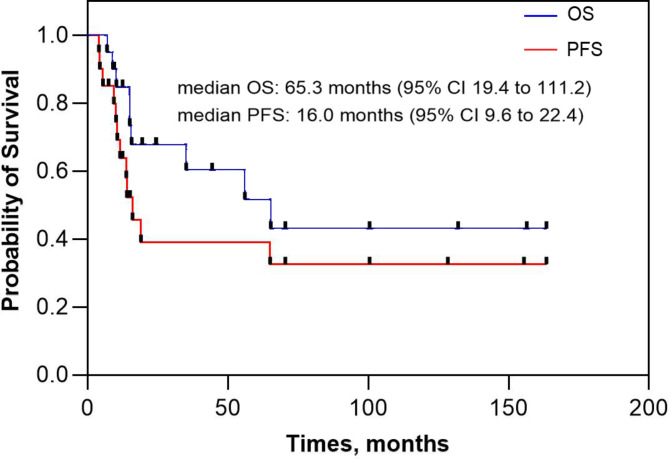
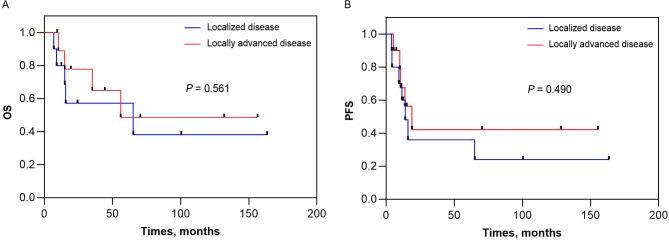
During a median follow-up period of 65.8 months (range, 0-137.7 months), locoregional recurrence occurred in 2 patients, distant metastasis developed in 3 patients, and both developed in 6 patients. The median OS for the entire cohort was 65.3 months (95% CI 19.4 to 111.2 months) and the corresponding 1-year, 2-year, and 3-year OS was 85%, 68%, and 60%, respectively. The median PFS for the entire cohort was 16.0 months (95% CI 9.6 to 22.4 months) and the corresponding 1-year, 2-year, and 3-year PFS was 58%, 39%, and 39%, respectively (Fig. 1). The median OS for the localized disease group was 65.3 months (95% CI 0 to 162.2 months) and the corresponding 1-year, 2-year, and 3-year OS was 80%, 57%, and 57%, respectively. The median OS for the locally advanced disease group was not reached and the corresponding 1-year, 2-year, and 3-year OS was 89%, 78%, and 65%, respectively (Fig. 2A). The median PFS for the localized disease group was 14.0 months (95% CI 8.1 to 19.9 months) and the corresponding 1-year, 2-year, and 3-year PFS was 60%, 36%, and 36%, respectively. The median PFS for the locally advanced disease group was 19.0 months (95% CI 6.4 to 31.6 months) and the corresponding 1-year, 2-year, and 3-year PFS was 56%, 42%, and 42%, respectively (Fig. 2B).
Fig. 1.
Kaplan–Meier curve of overall survival and progression-free survival for patients with non-metastatic small cell carcinoma of the bladder (SCCB).
Fig. 2.
Kaplan–Meier curve of overall survival and progression-free survival stratified according to disease staging, (A) OS and (B) PFS.
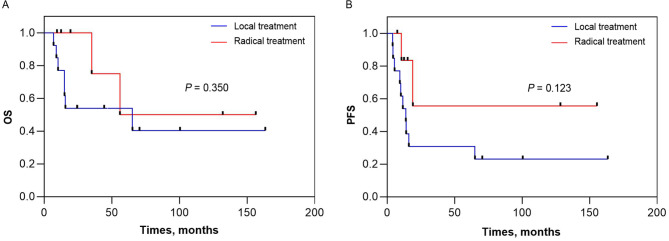
This study also analyzed survival outcomes according to local and radical treatment. The median OS for the receiving local treatment was 65.3 months (95% CI 0 to 138 months) and the corresponding 1-year, 2-year, and 3-year OS was 77%, 54%, and 54%, respectively. The median OS for the receiving radical treatment was not reached and the corresponding 1-year, 2-year, and 3-year OS was 100%, 100%, and 75%, respectively (Fig. 3A). The median PFS for the receiving local treatment was 13.8 months (95% CI 9.3 to 18.3 months) and the corresponding 1-year, 2-year, and 3-year PFS was 46%, 31%, and 31%, respectively. The median PFS for the receiving radical treatment was not reached and the corresponding 1-year, 2-year, and 3-year PFS was 83%, 56%, and 56%, respectively (Fig. 3B). 13 patients received with local treatment (7 TURBT and 6 PC), 3 patients had rapid disease progression with PFS of 4.0, 4.4 and 5.5months, and OS of 9.1, 6.9 and 10.3 months, respectively. 7 patients received radical treatment (5 CRT and 2 RC), 5 remain alive and free of SCCB, of which 4 patients have survived for more than 3, 4, 10 and 13 years respectively.
Fig. 3.
Kaplan–Meier curve of overall survival and progression-free survival stratified according to treatment, (A) OS and (B) PFS.
By the end of the follow-up period, 11 patients experienced disease progression, 8 (40%) had a locoregional recurrence, 9 (45%) exhibited distant metastasis and 6 (30%) showed both diseases. 8 patients died of distant metastasis, whereas the remaining one patient died of nonneoplastic causes. The most common locoregional recurrence sites were bladder (35%) and pelvic lymph nodes (5%). Distant metastases mainly developed in the liver (25%), brain (15%), bone (15%), and retroperitoneal lymph nodes (10%) (Table 4). The vast majority (89%) of distant metastases occurred within 14 months of diagnosis. Of the 7 patients treated with TURBT, 1 had bladder recurrence only, and 4 developed distant metastases, including 2 liver metastases with bladder recurrence synchronously (2 received no CT), 1 brain metastasis, 1 bone metastasis with bladder recurrence synchronously. Of the 6 patients who underwent PC and CT, 3 experienced distant metastases, including 1 with bone and liver metastases, 1 with brain and bone metastases, and 1 with bladder recurrence and multiple retroperitoneal lymph node metastases. Another patient experienced only bladder relapse 9.5 months after PC and CT, followed by TURBT and maintenance treatment with albumin paclitaxel + bevacizumab + tislelizumab, and remained cancer-free confirmed by cystoscopy with OS of 24.4 months at last follow-up. Among the 5 patients who received chemoradiotherapy, 1 case experienced bladder and retroperitoneal lymph node recurrence, as well as liver metastases. Of the 2 patients who underwent RC and CT, 1 experienced brain and liver metastases.
Table 4.
Clinical outcomes.
| Progression | 11 (55%) |
| Distant failure | 9 (45%) |
| Locoregional failure | 8 (40%) |
| Local | 7 (35%) |
| Nodal | 1 (5%) |
| Death | 9 (45%) |
Five patients received effective salvage therapy, such as TURBT, cystectomy, CT, immunotherapy (anti-PD-1) and RT, and achieved long-term survival of 8, 15, 16, 34, and 43 months after disease progression, respectively. At cut-off, three patients are still alive, and one remains cancer free.
Discussion
Given its rarity, a total of 880 cases of SCCB were registered in SEER database from 1975 to 201821. To our knowledge, this study reported the largest cohort of SCCB among Chinese populations, providing clinical characteristics of patients and tumors as well as detailed treatment strategies and clinical outcomes. The median age of all SCCB patients in this study was 61 years and 80% of the patients were male, indicating that SCCB predominantly occurs in elderly men. In the present study, the patients were slightly younger compared with other series that reported a median age of more than 70 years2,14. The pathogenesis of SCCB is not yet clear, although several theories have been proposed. The dominant theory is that SCCB originates from cancer stem cells that can differentiate into diverse cell types, such as urothelial carcinoma, squamous cell carcinoma or adenocarcinoma8. This theory was confirmed by Cheng et al. who found consistent genetic alterations between small cell carcinoma and coexisting uroepithelial carcinoma, suggesting that both tumor components were derived from multipotent, undifferentiated cells or stem cells in the uroepithelium22. Other theories include malignant transformation of neuroendocrine cells in the bladder to small cell carcinoma, origin from extreme metaplasia of urothelial cells23, and urothelial-to-neural plasticity24.
The diversity of treatment strategies for SCCB in this study reflects the lack of standard treatment, regardless of localized or locally advanced stage. In clinical practice, monotherapy is often inadequate to control tumor due to its aggressive behavior leading to local spread and distant metastasis. As in this study, 3 patients receiving local treatment had rapid disease progression with PFS of 4.0, 4.4 and 5.5months, and OS of 9.1, 6.9 and 10.3 months, respectively. Multiple factors contribute to this dilemma, such as lack of insight into the pathogenesis of SCCB and the rarity of the disease preventing prospective randomized trials to aid clinical decision making. Therefore, researchers have proposed cystectomy plus multimodal treatment (CT, RT) as well as bladder preservation therapy. The survival outcomes of radical treatment in our study were encouraging. 7 (35%) patients had RC or RT, 4 of them have survived for more than 3 years and 5 patients remain cancer free. Several retrospective studies and only 2 small prospective trials have shown that both bladder preservation strategies (CT and RT) and cystectomy combined with CT, especially neoadjuvant CT, play a major role in the management of SCCB. In a prospective study, Bex et al.16 found that of 17 patients with limited stage SCCB, 11 (64.7%) achieved clinical CR by bladder preservation treatment, including all 8 patients who received CRT and 3 received TURBT and radiotherapy, with a median duration of CR of 15 months. Furthermore, no patients died from locoregional progression, which was consistent with our findings. The authors concluded that cystectomy can be avoided in the majority of patients undergoing CRT16.
A phase 2 clinical trial conducted by Siefker-Radtke et al. evaluated neoadjuvant CT in 18 patients with surgically resectable SCCB (≤ cT4aN0M0) who underwent alternating double neoadjuvant CT followed by radical cystectomy (RC)17. 78% of the patients achieved pathologic downstaging to ≤ pT1N0M0, and the median OS for the surgically resectable cohort was 58 months with 13 patients remaining alive and cancer free17. In Lynch et al.’s retrospective study of 95 patients with resectable SCCB (≤ cT4aN0M0), the reported median OS was 159.5 months for neoadjuvant CT followed by cystectomy (n = 48) and 18.3 months for cystectomy followed by adjuvant CT (n = 47) (p < 0.001), and pathologic downstaging to ≤ pT1N0 occurred in 62% of tumors after neoadjuvant CT25. Therefore, in 2016, The National Comprehensive Cancer Network recommends that patients with non-metastatic SCCB receive neoadjuvant cisplatin-based combination chemotherapy followed by radical treatment (cystectomy or radiotherapy).
Unfortunately, the rarity of SCCB precludes prospective comparison of RT and RC as radical treatment options. The largest retrospective study of all-stage SCCB was reported by Chau C et al. in 20215. The median OS was 28.3 months for patients with bladder-confined disease (N0M0), versus a median OS of 12.7 months for patients with N + and/or M + disease (HR, 2.03; 95% CI, 1.58–2.60; P < 0.001). Regardless of disease stage, the use of CT was associated with improved OS (HR, 0.46; 95% CI, 0.37–0.59; P < 0.001). The authors found no significant difference in survival outcomes between RC and RT in patients with organ-confined disease (26.7 months vs. 30.0 months; P = 0.726)5. Schreiber et al.14 used the SEER database to evaluate characterization and outcomes of SCCB and also showed no significant difference in survival between RC and RT, and both treatments improved patient survival compared to TURBT alone.
In bladder-only disease (cT1-4a N0, M0), Fischer-Valuck et al. investigated the relationship between OS and treatment strategy in 856 patients, reporting a similar OS between CRT and RC + CT (34.1 months vs. 32.4 months; P = 0.42). For patients receiving neoadjuvant CT and RC, the median OS was 56.2 months. On multivariable analysis, the best OS was associated with CRT (HR, 0.41; 95% CI 0.32–0.53; P < 0.0001) and RC + CT (HR, 0.45; 95% CI 0.34–0.59; p < 0.0001) compared to monotherapy15.
Retrospective and randomized phase II studies suggest that hypofractionated thoracic RT with dose schedule of 40–42 Gy in 3 weeks given in once-daily fractionation produce similar outcomes and toxicity as 45 Gy in 3 weeks in bid fractionation in limited stage SCLC20. In our study, two patients (T2N0, T3N0) underwent neoadjuvant CT with CR confirmed by cystoscopy and then received bladder hypofractionated RT with a regimen of 45 Gy at 3.0 Gy per fraction over 3 weeks, analogous to RT for limited stage SCLC. The two patients currently remain alive and cancer free with normal bladder function. Three other patients received conventional fractionated RT at a total dose of 60–66 Gy, of which two patients have survived for more than 4 and 10 years respectively. The most commonly used radiation dose regimens in locally advanced bladder cancer (adenocarcinoma, transitional or squamous cell carcinoma) were 64 Gy in 32 fractions and 55 Gy in 20 fractions. Choudhury A et al. conducted an individual patient data (n = 782) meta-analysis of the BC2001 and BCON trials and found that 55 Gy in 20 fractions (n = 406) was superior to 64 Gy in 32 fractions (n = 376) with regard to invasive locoregional control (adjusted HR, 0.71; 95% CI 0.52–0.96) and non-inferior to 64 Gy in 32 fractions with regard to toxicity (adjusted RD -3.37%; 95% CI -11.85 to 5.10)26. However, there is no standard regimen for RT of SCCB. Germino et al.27 used the NCDB to evaluate the relationship between OS and total radiation dose in a large SCCB cohort, the authors showed that total dose of 54 Gy or greater was associated with better OS compared to lower doses (< 54 Gy) with a median OS of 58.9 months versus 21.5 months for patients aged 41–79. But the total dose did not affect OS in patients over 79 years of age. Other retrospective studies have reported median/mean doses of RT ranging from 59 to 64.8 Gy28. The optimal dose schedule remains to be further identified in prospective studies.
Although SCCB is often associated with poor survival outcome, the response rate for salvage treatments such as CT, cystectomy, TURBT and BCG reached 64%29. Owing to effective salvage treatments, 5 patients still have a considerable survival time after progression in this study, leading to a more durable OS. 3 of them received salvage anti-PD-1 therapy and achieved long-term survival of 15, 34, and 43 months after progression, respectively. At cut-off, 2 patients are still alive and 1 remains cancer free. It is uncertain whether immune checkpoint therapies already approved for uroepithelial cancer will also be as effective in SCCB. Mandelkow et al. found that SCCB often exhibited an immune-excluded phenotype compared with uroepithelial cancers30. But case series showed that SCCB were sensitive to second line and beyond immune checkpoint inhibitor therapy and 60% of the patients showed radiographic and clinical benefit31.
Overall, SCCB is a rare, highly malignant neuroendocrine tumor that is prone to distant metastases. Given its highly metastatic potential, CT remains an important part of the treatment strategy. Neoadjuvant CT followed by radical treatments (RC or RT) for SCCB patients are effective means of cure. In the future, better understanding of the genetic landscape and molecular alterations driving SCCB may facilitate the development of new therapeutic strategies (such as immunotherapy) and improve survival outcomes. There were some limitations in our study. It was a retrospective study of with inevitable selection bias. The ability to identify prognostic factors was limited by the small sample size of the study and the variety of treatment strategies. Detailed information on the percentage of non-small cell carcinoma was missing in some cases. Thus, more prospective studies with large samples are needed to establish an optimal treatment strategy in the future.
In conclusion, to our knowledge, this study reported the largest cohort of non-metastatic SCCB among Chinese population, providing detailed information on patient and tumor characteristics, treatments and outcomes. TURBT and partial cystectomy were inadequate to control tumor. Given its metastatic potential, chemotherapy remained an essential part of the treatment strategy. The survival of cystectomy and radiotherapy in the non-metastatic SCCB were encouraging. Distant metastases were more common and fatal.
Author contributions
LJW collected and analyzed the data and wrote the manuscript. ZJM collected and analyzed the data. LYP, LYX, TY, LN, WSL, SYW, ZWJ, and XXY participated patient treatments. JJ participated research design and edited the manuscript. All authors reviewed the manuscript.
Funding
This study was supported by Shenzhen High-level Hospital Construction Fund, Shenzhen Key Medical Discipline Construction Fund (SZXK013), and Hospital Research Project (E010321004).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Competing interests
The authors declare no competing interests.
Ethics approval
This retrospective study was performed on existing data and was exempted from ethics by the Ethics Committee of National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital & Shenzhen Hospital.
Informed consent
The requirement for informed consent from the study subjects was waived by the IRB of Cancer Hospital & Shenzhen Hospital, Chinese Academy of Medical Sciences due to the retrospective study design.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zheng, R. et al. Cancer incidence and mortality in China, 2016. J. Natl. Cancer Cent.2, 1–9. 10.1016/j.jncc.2022.02.002 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koay, E. J., Teh, B. S., Paulino, A. C. & Butler, E. B. A surveillance, epidemiology, and end results analysis of small cell carcinoma of the bladder: Epidemiology, prognostic variables, and treatment trends. Cancer. 117, 5325–5333. 10.1002/cncr.26197 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Park, S., Reuter, V. E. & Hansel, D. E. Non-urothelial carcinomas of the bladder. Histopathology. 74, 97–111. 10.1111/his.13719 (2019). [DOI] [PubMed] [Google Scholar]
- 4.Erdem, G. U. et al. Small cell carcinoma of the urinary bladder: Changing trends in the current literature. Curr. Med. Res. Opin.32, 1013–1021. 10.1185/03007995.2016.1155982 (2016). [DOI] [PubMed] [Google Scholar]
- 5.Chau, C. et al. Treatment outcomes for small cell carcinoma of the bladder: Results from a UK patient retrospective cohort study. Int. J. Radiat. Oncol. Biol. Phys.110, 1143–1150. 10.1016/j.ijrobp.2021.02.003 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Cheng, L. et al. Small cell carcinoma of the urinary bladder: A clinicopathologic analysis of 64 patients. Cancer. 101, 957–962. 10.1002/cncr.20456 (2004). [DOI] [PubMed] [Google Scholar]
- 7.Choong, N. W., Quevedo, J. F. & Kaur, J. S. Small cell carcinoma of the urinary bladder. The Mayo Clinic experience. Cancer. 103, 1172–1178. 10.1002/cncr.20903 (2005). [DOI] [PubMed] [Google Scholar]
- 8.Abrahams, N. A., Moran, C., Reyes, A. O., Siefker-Radtke, A. & Ayala, A. G. Small cell carcinoma of the bladder: A contemporary clinicopathological study of 51 cases. Histopathology. 46, 57–63. 10.1111/j.1365-2559.2004.01980.x (2005). [DOI] [PubMed] [Google Scholar]
- 9.Moretto, P. et al. Management of small cell carcinoma of the bladder: Consensus guidelines from the Canadian Association of Genitourinary Medical oncologists (CAGMO). Can. Urol. Assoc. J.7, E44–56. 10.5489/cuaj.220 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geynisman, D. M. et al. Advanced small cell carcinoma of the bladder: Clinical characteristics, treatment patterns and outcomes in 960 patients and comparison with urothelial carcinoma. Cancer Med.5, 192–199. 10.1002/cam4.577 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parimi, V. et al. Comparison of clinicopathological characteristics, gene expression profiles, mutational analysis, and clinical outcomes of pure and mixed small-cell carcinoma of the bladder. Histopathology. 10.1111/his.14883 (2023). [DOI] [PubMed] [Google Scholar]
- 12.Boyer, A. C., Jafri, S. Z., Jafri, S. M. & Amin, M. B. Neuroendocrine carcinoma of the urinary bladder: A retrospective study of CT findings. Abdom. Imaging. 38, 870–876. 10.1007/s00261-012-9971-6 (2013). [DOI] [PubMed] [Google Scholar]
- 13.Patel, S. G. et al. Locoregional small cell carcinoma of the bladder: Clinical characteristics and treatment patterns. J. Urol.191, 329–334. 10.1016/j.juro.2013.09.009 (2014). [DOI] [PubMed] [Google Scholar]
- 14.Schreiber, D. et al. Characterization and outcomes of small cell carcinoma of the bladder using the surveillance, epidemiology, and end results database. Am. J. Clin. Oncol.36, 126–131. 10.1097/COC.0b013e3182438c71 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Fischer-Valuck, B. W. et al. Treatment patterns and survival outcomes for patients with small cell carcinoma of the bladder. Eur. Urol. Focus. 4, 900–906. 10.1016/j.euf.2017.09.001 (2018). [DOI] [PubMed] [Google Scholar]
- 16.Bex, A. et al. Small cell carcinoma of bladder: A single-center prospective study of 25 cases treated in analogy to small cell lung cancer. Urology. 65, 295–299. 10.1016/j.urology.2004.09.049 (2005). [DOI] [PubMed] [Google Scholar]
- 17.Siefker-Radtke, A. O. et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J. Clin. Oncol.27, 2592–2597. 10.1200/JCO.2008.19.0256 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Galsky, M. D. et al. Effectiveness of adjuvant chemotherapy for locally advanced bladder Cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.34, 825–832. 10.1200/jco.2015.64.1076 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Sternberg, C. N. et al. Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N + M0 urothelial carcinoma of the bladder (EORTC 30994): An intergroup, open-label, randomised phase 3 trial. Lancet Oncol.16, 76–86. 10.1016/s1470-2045(14)71160-x (2015). [DOI] [PubMed] [Google Scholar]
- 20.Gronberg, B. H. et al. Randomized phase II trial comparing twice daily hyperfractionated with once daily hypofractionated thoracic radiotherapy in limited disease small cell lung cancer. Acta Oncol.55, 591–597. 10.3109/0284186X.2015.1092584 (2016). [DOI] [PubMed] [Google Scholar]
- 21.Mollica, V. et al. Prognostic factors of survival for high-grade neuroendocrine neoplasia of the bladder: A SEER database analysis. Curr. Oncol.29, 5846–5854. 10.3390/curroncol29080461 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cheng, L. et al. Molecular Genetic evidence for a common clonal origin of urinary bladder small cell carcinoma and coexisting Urothelial Carcinoma. Am. J. Pathol.166, 1533–1539. 10.1016/s0002-9440(10)62369-3 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thota, S., Kistangari, G., Daw, H. & Spiro, T. A clinical review of small-cell carcinoma of the urinary bladder. Clin. Genitourin. Cancer. 11, 73–77 (2013). [DOI] [PubMed] [Google Scholar]
- 24.Yang, G. et al. Urothelial-to-neural plasticity drives progression to small cell bladder cancer. iScience23, 101201 (2020). 10.1016/j.isci.2020.101201 [DOI] [PMC free article] [PubMed]
- 25.Lynch, S. P. et al. Neoadjuvant chemotherapy in small cell urothelial cancer improves pathologic downstaging and long-term outcomes: Results from a retrospective study at the MD Anderson Cancer Center. Eur. Urol.64, 307–313. 10.1016/j.eururo.2012.04.020 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Choudhury, A. et al. Hypofractionated radiotherapy in locally advanced bladder cancer: An individual patient data meta-analysis of the BC2001 and BCON trials. Lancet Oncol.22, 246–255. 10.1016/s1470-2045(20)30607-0 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Germino, E. et al. Radiation Therapy as definitive local treatment in patients with limited-stage small cell carcinoma of the bladder: Does total dose matter? Bladder Cancer. 4, 311–317. 10.3233/BLC-180165 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang, Y. et al. Small cell carcinoma of the bladder: The characteristics of molecular alterations, treatment, and follow-up. Med. Oncol.36, 98. 10.1007/s12032-019-1321-x (2019). [DOI] [PubMed] [Google Scholar]
- 29.van de Kamp, M. et al. Intravesical recurrence after bladder sparing treatment of small cell carcinoma of the bladder: Characteristics, treatment, and outcome. Urol. Oncol.36 (307 e301-307 e308). 10.1016/j.urolonc.2018.02.015 (2018). [DOI] [PubMed]
- 30.Mandelkow, T. et al. Immune exclusion is frequent in small-cell carcinoma of the bladder. Dis Markers 2532518 (2019). 10.1155/2019/2532518 (2019). [DOI] [PMC free article] [PubMed]
- 31.Hoffman-Censits, J. et al. Small cell bladder Cancer response to second-line and beyond checkpoint inhibitor therapy: Retrospective experience. Clin. Genitourin. Cancer. 19, 176–181. 10.1016/j.clgc.2020.10.009 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.