Abstract
The Chinese mitten crab (Eriocheir sinensis) holds significant importance as a popular aquaculture food source; however, there are concerns about its potential contamination with polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs), polychlorinated biphenyls (PCBs) from both food and aquatic environment. To assess the associated health risks and identify potential sources of contamination in crabs, a comprehensive investigation was conducted, including a total of 70 samples from the crab food web. The results demonstrated that crabs predominantly exhibited elevated concentrations of PCBs and dl-PCBs, with mean concentrations of 12 207 ± 11 962 pg g-1 and 554 ± 203 pg g-1, respectively, while PCDD/Fs concentrations were comparatively lower at 20 ± 17 pg g-1. The accumulation of PCBs in crabs significantly surpassed that of PCDD/Fs. The material balance of PCDD/Fs and PCBs in the crab food web was estimated, indicating that sediments and feeds likely constitute the two primary sources of PCDD/Fs and PCBs in crabs. The monthly intake of PCDD/Fs and PCBs through crab consumption accounted for 30% of the dietary intake, which was well below the provisional tolerable monthly intake (PTMI) limit. The weekly intake of PCDD/Fs and PCBs for adults consuming one crab (100 g) does not pose health risks and the recommended weekly intake of white crabmeat and brown crabmeat is 443 g and 21 g, respectively.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-75660-2.
Subject terms: Environmental chemistry, Environmental impact
Introduction
Polychlorinated dibenzo-p-dioxins and dibenzofurans (PCDD/Fs) and polychlorinated biphenyls (PCBs) are typically generated as byproducts released from diverse human activities and natural sources1. These pollutants pervade the environment, existing in the air, soil, sediments, dust, and various organisms2. With the ability to accumulate in fat and undergo biomagnification through the consumption of animal-derived food, PCDD/Fs and PCBs pose a threat to human health by activating aryl hydrocarbon receptor3–5. The transfer of PCDD/Fs and PCBs from the environment and feed to organisms can lead to increased bioaccumulation over time5. Globally, instances of PCDD/Fs and PCBs pollution in pigs in Belgium and pork in Germany have been linked to contaminated feed6,7. The transfer of PCDD/Fs and PCBs from sediments, water, or food into fish and crabs has been investigated in the Passaic River estuary, where the depletion of polluted sediments directly contributed to the bioaccumulation and biomagnification of PCDD/Fs and PCBs in the food chain8. In our previous studies on crabs, sediments were also identified as the primary source of PCDD/Fs and PCBs9. In addition to sediments, water, and feedstuffs also play a crucial role in the presence of PCDD/Fs and PCBs in biota8,9. More than 90% of human exposure to PCDD/Fs and PCBs is through dietary intake, and even at low levels, these contaminations are associated with a range of health issues10,11. Therefore, joint Food and Agriculture Organization (FAO) and World Health Organization (WHO) have set strict limits, and Provisional Tolerable Monthly Intake (PTMI) on the level of dietary intake of PCDD/Fs and PCBs was set at 70 pg TEQ kg− 1 bw per month12. In 2018, this level was reevaluated by the European Food Safety Authority (EFSA), and a new Tolerable Weekly Intake (TWI) for PCDD/Fs and dioxin-like PCBs (dl-PCBs) in food has been set at 2 pg TEQ kg− 1 bw per week13.
The Chinese mitten crab (Eriocheir sinensis), a significant and popular food source in the aquaculture industry, may serve as a conduit for human exposure to PCDD/Fs and PCBs. China is the world’s largest consumer of crabs, and crab production was roughly the same as consumption, reaching about 800,000 tons in 2021, with more than 80% of that coming from the Yangtze Delta Region14–16. China’s crab production is concentrated in provinces with coastlines or major inland lakes, such as Jiangsu, with five provinces accounting for 74% of the total production17. Therefore, it is crucial to investigate the levels of PCDD/Fs and PCBs in Jiangsu and assess relevant health risks.
Crabs are benthic aquatic omnivores, thus they rapidly accumulate contaminants from the environment and feed. Recent reports from Germany highlighted elevated levels of PCDD/Fs and dl-PCBs in crab populations18. In the Netherlands, the toxic equivalent concentration (TEQ) of PCDD/Fs and PCBs in crabmeat was determined to be 43 pg g− 1 based on the wet weight (ww)4. In 2016, crabs from Jiangsu Province in China were found to contain 11.7 and 40.3 pg TEQ g− 1 of total PCDD/Fs and PCBs. Additionally, crabs have been found to harbor contaminants such as microplastics, heavy metals, short-chain and medium-chain chlorinated paraffins, as well as PCDD/Fs and PCBs18–21. A health risk of heavy metals in crabs and shrimps in Bangladesh showed that the consumption of these foods did not pose significant risks to human health either in terms of carcinogenic or non-carcinogenic effects22. Previous studies indicated that sediment and feed may be important sources of PCDD/Fs and dl-PCBs, however, little is known about the pathways by which they are transferred into farmed crabs. Understanding the occurrence, distribution, and accumulation of PCDD/Fs and dl-PCBs on various food webs can better predict the levels of these contaminants in farmed crabs. The selective enrichment of PCDD/Fs and PCBs in white crabmeat and brown crabmeat, together with different consumer preferences for crabmeat consumption, require separate assessments of their health risks.
This study focuses on assessing the health risks associated with consuming crabs by investigating the levels of PCDD/Fs and PCBs in representative crab samples and their surrounding ecological web. Crab samples containing both brown and white crabmeat were collected from four different inland lakes in Jiangsu. This study aims to (1) assess the levels of PCDD/Fs and PCBs in reared crabs and environments and their potential health risks; (2) analyze the material balance of PCDD/Fs and PCBs in food webs of crab farms; and (3) assess potential dietary risks from consumption of white crabmeat and brown crabmeat separately and inform dietary limits for consumers.
Materials and methods
Crab sampling
The study collected a total of 32 crabs from four different inland lakes in Jiangsu, China (Fig. 1). These lakes included Taihu Lake (8 crab samples from TH1, TH2, TH3, and TH4), Hongze Lake (4 crab samples from HZ1 and HZ2), Yangcheng Lake (4 crab samples from YC1 and YC2), and Gehu Lake (16 crab samples from GH1-GH8). Each crab, weighing approximately 100 g, underwent collection for analysis of PCDD/Fs and PCBs. The crabmeat was separated into brown crabmeat and white crabmeat, and subsequent individual analyses were conducted on these distinct samples. To ensure representative results, crabmeat from two crabs of the same sex and the specific crab part were obtained for each sampling site. These paired samples were then combined, resulting in 16 samples of brown crabmeat and 16 samples of white crabmeat. To facilitate identification based on sex, male crabs were labeled with odd numbers, while female crabs were assigned even numbers. This labeling system helps distinguish between the sexes of the collected crabs. All the crabmeat samples were stored at -20 ℃ until they were analyzed for PCDD/Fs and PCBs.
Fig. 1.
Sampling regions in the main producing area of crabs (depicted in ArcGIS sofeware (version 10.6; URL: https://www1.msc23.cn/arcgis/?bd_vid=18383096661822682606)).
Potential source sampling in the crab food web
Crabs are biennial benthos that may be influenced by contaminants in the aquatic environment. In this study, we explored potential sources of PCDD/Fs and PCBs in crabs, aiming to comprehensively identify the primary contributors to their presence. The investigation encompassed commercial feed, feed-grade CuSO4 and ZnSO4, and sediments to discern the principal source of PCDD/Fs and PCBs in carb specimens. A total of 7 economical feeds (SL1-SL7), 11 commercial feed-grade CuSO4 (C1-C11), 10 feed-grade ZnSO4 (Z1-Z10), and 10 sediment samples (S1-S10) were collected from diverse crab ponds across the four inland lakes. The main raw materials for the feeds are fish meal, soybean meal, rapeseed meal, cottonseed meal, grains, grain processing products, soybean phospholipid oil, soybean oil, dicalcium phosphate, and compound premix feed, among others. Feed-grade CuSO4, in addition to CuSO4, also contains small amounts of copper, lead, and arsenic. The main component of feed-grade ZnSO4 is hydrated ZnSO4. The sediments (primarily consisting of clastic, organic matter, and chemicals) underwent natural drying, grinding into powders, and subsequent storage in cool, dry conditions. Feeds and chemicals were similarly preserved for analysis. This systematic approach was employed to shed light on the intricate pathways through which PCDD/Fs and PCBs may enter crab habitats and accumulate within these organisms.
Sample preparation and instrument analysis
The 32 crab samples collected from the four lakes for conducting the experiment analysis, we obtained ethical approval from the Animal Ethics and Welfare Committee of Research Center for Eco-Environmental Sciences, Chinese Academy of Sciences (Approval Number. AEWC-RCEES-2017002). The analysis of PCDD/Fs and PCBs in the samples was conducted using USEPA methods 1613 and 1668 A, as detailed in our previous publications23,24. Briefly, crabmeat samples were freeze-dried before being extracted. Each sample was spiked with 1 ng of13C-labeled surrogate internal standards (100 ng/mL of EPA-1613 LCS and 1 µg/mL of EPA-1668B LCS) (Wellington Laboratories, Guelph, Canada) and then extracted with Dionex accelerated solvent extraction 300 (Thermo Fisher Scientific, Waltham, MA, USA). The lipid level of crabmeat was determined by lipid removal, achieved by mixing silica gel with 44% of sulfuric acid with the crabmeat extract. Then the extract was added 100 mL of n-hexane and 10 mL of aliquot was rotated to dry. Each extract was cleaned by passing through an acidic silica gel column, a multilayer silica gel column, and a basic alumina column. Two fractions of PCB extract (5% of dichloromethane and 95% of hexane in 100 mL elution solvent) and PCDD/F extract (50% of dichloromethane and 50% of hexane in 50 mL elution solvent) were obtained. The extract was evaporated and then concentrated to 10 µL under nitrogen. Before instrumental analysis, 1 ng of13C-labeled surrogate recovery standards (200/400 ng/mL of EPA-1613 IS and 5 µg/mL of EPA-1668B IS) (Wellington Laboratories, Guelph, Canada) were added. The high-resolution gas chromatograph (HRGC, Agilent Technologies, Santa Clara, CA, USA) coupled with the high-resolution mass spectrometer (HRMS, Waters Corporation, Milford, MA, USA) was applied to determine PCDD/Fs and PCBs.
Quality control and quality assurance
During the analysis procedures for samples, one procedural blank sample was inserted into each batch of samples. The PCDD/Fs and PCBs method detection limits were 0.15–1.8 pg g− 1 and 0.063–0.72 pg g− 1, respectively. The recoveries of the cleanup standard for PCDD/Fs and labeled PCBs in all the samples were 42-118% and 31-126%, respectively. The 42% recovery rate was associated with 1,2,3,4,7,8-HxCDF and 118% recovery rate was associated with 1,2,3,6,7,8-HxCDD. The 31% recovery rate was for PCB-189 and 126% recovery rate was for PCB-105. The dominant congeners vary among different types of samples. However, they all fall within the recovery rate range. Less than 10% of the congeners’ concentration was below the LOD.
Risk assessment of PCDD/Fs and PCBs
The daily dietary intake of PCDD/Fs and PCBs for adults was calculated by multiplying estimated crab consumption by the levels of PCDD/Fs and PCBs determined in this study and then dividing by the body weight of the consumer. To assess the total risk associated with PCDD/Fs and PCBs, the Toxicity Equivalence Quantities (TEQs) were calculated using the Toxicity Equivalence Factors (TEFs) established by the World Health Organization (WHO) in 200525. The TEQs of the PCDD/Fs and PCBs in crabs were calculated according to formulas (1)-(4).
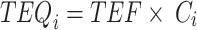 |
1 |
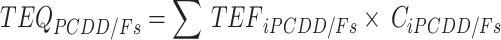 |
2 |
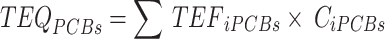 |
3 |
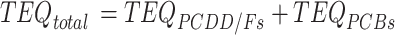 |
4 |
where TEQi represents TEQ of the congeners of PCDD/Fs or PCB in crabs; TEF refers to toxicity equivalence quantities of congeners in PCDD/Fs or PCBs; Ci denotes concentrations of PCDD/Fs and PCBs congeners in crabs; the subscripts i are specific combinations of PCDD/Fs or dl-PCBs.
In 2021, the total consumption of crabs in China was 800,000 tons, which means the per capita consumption is 5.0 g per day, including 2 g of brown crabmeat and 3 g of white crabmeat. The preference for brown crabmeat (hepatopancreas and gonads) with higher lipids content over white crabmeat (muscle tissues in the claws and legs) is evident among consumers4. Brown and white crabmeat collectively accounted for 11% and 16% of the average weight of a 104 g crab. For these calculations, the average body weight of 60 kg for Chinese adults was used.
Statistical analysis
All data are presented as mean ± standard error. Statistical analysis was performed with SPSS Statistics 27.0 (IBM, Armonk, NY, USA). The T-test was employed to evaluate the significance of the mean differences, and the effect size of mean differences is divided into low (< 0.5), medium (0.5–0.8), and large (> 0.8) according to Cohen’d value. Differences were significant at p < 0.05.
Results and discussion
PCDD/Fs and PCBs in crabs
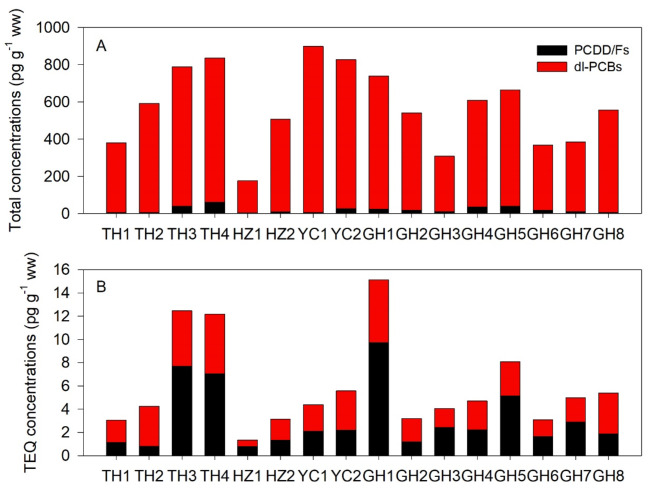
The concentrations of PCDD/Fs and PCBs showed significant differences between white crabmeat and brown crabmeat. The mean concentration of PCDD/Fs and PCBs in white crabmeat was 0.19 ± 0.41 pg TEQ g− 1 and 0.082 ± 0.096 pg TEQ g− 1 respectively, while the mean concentration of PCDD/Fs and PCBs in brown crabmeat was as high as 2.95 ± 2.73 pg TEQ g− 1 and 2.72 ± 1.38 pg TEQ g− 1, respectively. The mean concentration of 17 higher chlorinated PCDD/F (2,3,7,8-substituted PCDD/Fs) congeners in crabs was 20 ± 17 pg g− 1. Total PCB levels and dl-PCBs in crabs were 12 207 ± 11 962 pg g− 1 and 554 ± 203 pg g− 1, respectively. Concentrations of dl-PCBs in crabs were 48 times PCDD/Fs on average (Fig. 2A). However, the relative burden of PCDD/Fs and dl-PCBs for TEQs in crabs showed an almost equal proportion (Fig. 2B). Certain crab samples exhibited higher percentages of PCDD/Fs (ranging from 53 to 64%), including TH3, TH4, HZ1, GH1, GH3, GH5, GH6, and GH7. Conversely, the remaining crab samples observed higher percentages of dl-PCBs (ranging from 52 to 81%). Total TEQs of PCDD/Fs and dl-PCBs in crabs ranged from 1.4 pg g− 1 to 15 pg g− 1, with a mean TEQ of 5.9 ± 4.0 pg g− 1. A quarter of crab samples’ TEQs of PCDD/Fs and dl-PCBs exceeded the current European Union limits (3.5 pg g− 1 for PCDD/Fs and 6.5 pg g− 1 for PCDD/Fs and dl-PCBs)26. The TEQ ratio of PCDD/Fs to dl-PCBs was in the range of 0.24–1.81 with a mean ratio of 1.06 (Fig. S1). Spatial distributions of PCDD/Fs and PCBs in crabs were influenced by their differences in the brown and white crabmeat.
Fig. 2.
The relative distribution of PCDD/Fs and dl-PCBs for concentrations (A) and TEQs (B) in crabs.
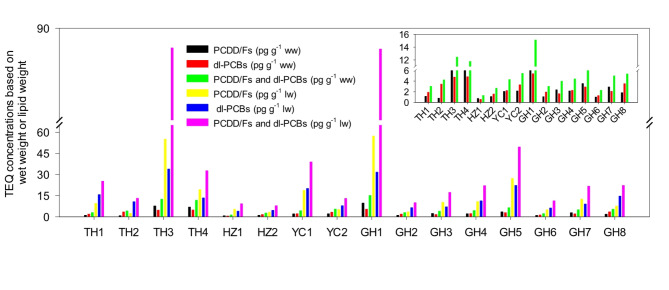
The lipid content in the brown crabmeat was 11-42%, which was higher than that in the white crabmeat. The fat content in female crabs was higher than in male crabs (Fig. S2). A significant difference could be found between total PCDD/F and dl-PCB TEQs and lipid levels in most male and female crabs (p < 0.01), and the effect size was obvious (Cohen’d 2.35). The TEQs of PCDD/Fs and dl-PCBs in most female crabmeat were higher than the male crabmeat from each lake. Most female crabs had more fat than male ones, which had the advantage of accumulating pollutants. The TEQ levels exhibited variability, ranging from 0.74 to 9.7 pg g− 1 for PCDD/Fs, 0.56 to 5.4 pg g− 1 for dl-PCBs, and 1.3 to 15 pg g− 1 for both PCDD/Fs and dl-PCBs on a wet weight in the brown crabmeat. Within the brown crabmeat, 25% exceeded relevant limits for PCDD/Fs, and 19% surpassed limits for total PCDD/Fs and dl-PCBs, thereby contributing a greater share of TEQs to the whole crab. The TEQ value was in the range of 2.5–57 pg g− 1 for PCDD/Fs, 4.0–34 pg g− 1 for dl-PCBs, and 7.8–89 pg g− 1 for both PCDD/Fs and dl-PCBs on a lipid weight in the brown crabmeat (Fig. 3). In comparison to the level of PCDDs and dl-PCBs found in brown crabmeat, the total TEQs in the white crabmeat for PCDD/Fs and dl-PCBs ranged from 0.0092 to 1.65 pg g− 1, consistently falling within the established European Union limits. The average TEQ value of PCDD/Fs and dl-PCBs in the white crabmeat, at 0.27 pg g− 1, was only 0.047 times that of PCDD/Fs and dl-PCBs TEQ in brown crabmeat (mean 5.7 pg g− 1), potentially influenced by the higher fat percentage in brown crabmeat.
Fig. 3.
Comparisons of PCDD/F and dl-PCB TEQs on the basis of wet weight and lipid weight in the brown crabmeat.
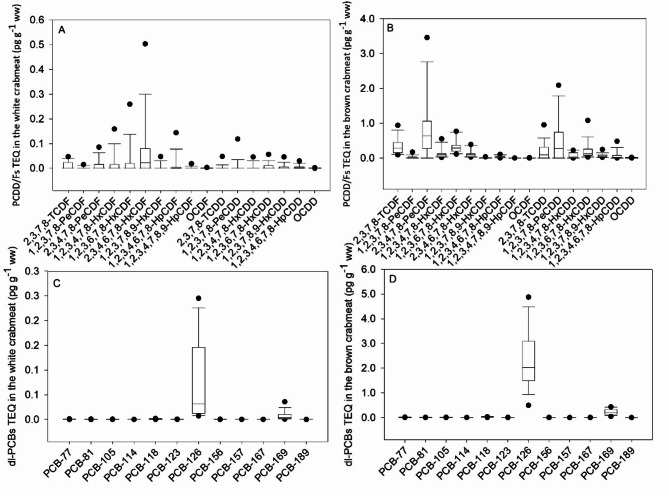
Concentrations and congeners of PCDD/Fs and PCBs in white crabmeat and brown crabmeat showed differences. The congeners of 2,3,4,6,7,8-HxCDF, 1,2,3,6,7,8-HxCDF, and 1,2,3,4,7,8-HxCDF were dominant TEQ contributors in white crabmeat, contributing 36%, 14%, and 10%, respectively. The concentrations of PCDD/Fs and PCBs in brown crabmeat were higher, and more types of congeners were accumulated, with 2,3,4,7,8-PeCDF, 1,2,3,7,8-PeCDD, 2,3,7,8-TCDF, and 1,2,3,6,7,8-HxCDF accounting for 29%, 17%, 12%, and 11% of total TEQ concentration, respectively (Fig. 4A,B). The PCB-126 contributed the most to dl-PCB congeners both in the white and brown crabmeat, accounting for 88% and 90%, respectively (Fig. 4C,D). The samples with higher concentrations of PCDD/Fs in white crabmeat and brown crabmeat were from GeHu Lake and Hongze Lake, with mean concentrations of 0.32 pg TEQ g− 1 ww and 0.13 pg TEQ g− 1 ww, respectively. Higher concentrations of PCBs in white crabmeat and brown crabmeat were found in Taihu Lake, with mean concentrations of 4.14 pg TEQ g− 1 ww and 3.72 pg TEQ g− 1 ww, respectively.
Fig. 4.
TEQ distributions of PCDD/F and dl-PCB congeners on the basis of wet weight in the white (A and C) and brown crabmeat (B and D).
PCDD/Fs and PCBs in sediments, commercial feeds, CuSO4 and ZnSO4
Crabs are bottom-dwelling aquatic animals, that may be exposed to PCDD/Fs and PCBs in the sediments from the aquaculture environment9. Individual PCDD/F and dl-PCB congener concentrations in the sediments collected from the crab ponds were presented in Figure S3. The total concentrations of the 17 target PCDD/F and 12 dl-PCB congeners were 87-1547 pg g− 1 dw (mean: 715 ± 629 pg g− 1 dw) and 36-1403 pg g− 1 dw (mean: 500 ± 389 pg g− 1 dw), respectively. Approximately 50% of the total concentrations fell below the mean value. OCDD and PCB-118 emerged as dominant contributors, constituting 64-88% of PCDD/Fs and 25-51% of dl-PCBs, respectively.
The total TEQs for both PCDD/F and PCB congeners in sediments ranged from 1.3 to 28 pg g− 1 dw, with a mean of 11 ± 9.5 pg g− 1 dw. PCDD/Fs TEQ varied from 1.2 pg g− 1 dw to 28 pg g− 1 dw (Figure S4A), while dl-PCBs TEQ ranged from 0.13 pg g− 1 dw to 2.4 pg g− 1 dw (Figure S4B). Following Hemming et al. (2003) risk estimate categorization, the sediment dioxin TEQ concentration was classified into five groups: no risk for 0–10 pg g− 1 dw, lowest possible risk for 10.01-20 pg g− 1 dw, possible risk for 20.01-30 pg g− 1 dw, possible/probable risk for 30.01-50 pg g− 1 dw, and special concern need to be noticed for 50.01-80 pg g− 1 dw27. Six sediment samples exhibited TEQ levels below 10 pg TEQ g− 1 dw, indicating no risk. Two sediment samples showed the lowest possible risk with 16 and 19 pg TEQ g− 1 dw for total PCDD/Fs and dl-PCBs. A possible risk could be found in another two sediments with 28 and 22 pg TEQ g− 1 dw for total PCDD/Fs and dl-PCBs. PCDD/Fs dominated as TEQ contributors, with an average TEQ ratio of PCDD/Fs to dl-PCBs at 16. The TEQ values of dl-PCBs in all samples remained within an acceptance range, signifying the absence of risk in sediments for dl-PCBs. Our results demonstrate that sediment is one of the main sources of PCDD/Fs and PCBs in cultured crabs, which is consistent with other findings28,29. Remediation of contaminated sites can effectively reduce the ongoing pollution of PCDD/Fs and PCBs in sediments and aquatic food web30.
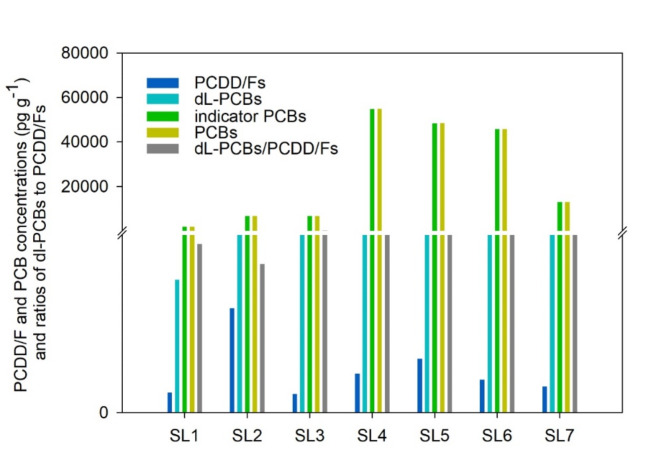
Commercial crab compound feeds are widely utilized in China for feeding crabs. A total of 7 commercial feed samples for feeding crabs were analyzed for the presence of PCDD/Fs and PCBs. The distribution of the results obtained for feeds is presented in Fig. 5. Concentrations of PCDD/Fs varied from 0.74 to 4.1 pg g− 1 dw, with a mean value of 1.7 ± 1.2 pg g− 1 dw. Total PCB and dl-PCB concentrations spanned from 1890 to 68 330 pg g− 1 dw (mean: 34 570 ± 25 930 pg g− 1 dw) and 5.2–152 pg g− 1 dw (mean: 74 ± 56 pg g− 1 dw), respectively. Dl-PCBs in feeds were on average 62 times PCDD/Fs higher than PCDD/Fs, aligning with the PCDD/Fs and dl-PCBs distribution observed in crabs. The maximum acceptable TEQ level of PCDD/Fs and dl-PCBs in accordance with regulation EC/277/2012 for animal feed was set at 1.5 ng kg− 1 (1500 fg g− 1)31. The results obtained for all feed samples were in compliance with the regulation. Total TEQs for PCDD/F and dl-PCB ranged from 16 fg g− 1 dw to 328 fg g− 1 dw (mean: 142 ± 113 fg g− 1 dw). The average TEQ concentrations for PCDD/Fs and dl-PCBs were 3.7 ± 3.1 fg g− 1 dw and 138 ± 112 fg g− 1 dw, respectively.
Fig. 5.
Concentrations of PCDD/Fs and PCBs and ratios of dl-PCBs to PCDD/Fs in feeds.
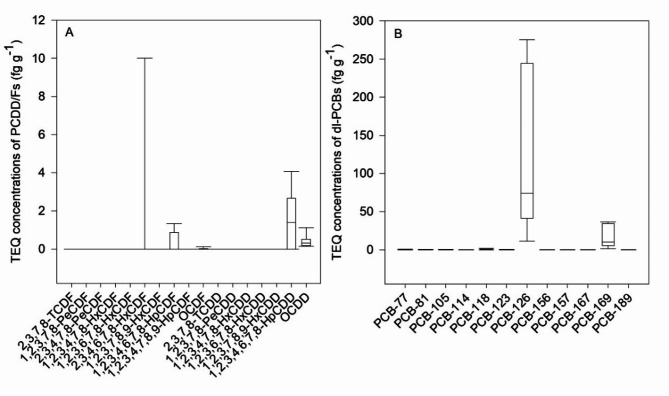
Among the PCDD/F congeners, OCDD, 1,2,3,4,6,7,8-HpCDD, 2,3,4,6,7,8-HxCDF, and 1,2,3,4,6,7,8-HpCDF were the main TEQ contributors, accounting for 33%, 37%, 14%, and 13%, respectively (Fig. 6A). PCB-126 and PCB-169 contributed the most to dl-PCB TEQs, which occupied 86% and 12%, respectively (Fig. 6B).
Fig. 6.
TEQ concentrations of PCDD/Fs and dl-PCBs in feeds.
CuSO4 was sprayed into the crab ponds as an antimicrobial agent and ZnSO4 was applied to clear ciliates. In China, the production of feed-grade CuSO4 reached 27,000 tons in 2013 and 8.6–41 pg g− 1 of PCDD/Fs was detected32. The feed-grade CuSO4 is also a key substance for PCDD/Fs and PCBs to enter the aquatic food web. In this study, 11 CuSO4 samples were analyzed, revealing PCDD/Fs concentrations of 6.0 ± 5.5 pg g− 1 dw and TEQ of PCDD/Fs at 0.56 ± 0.49 pg g− 1 dw (Figure S5). Total PCBs and dl-PCBs were 1677 ± 1001 pg g− 1 dw and 281 ± 267 pg g− 1 dw, respectively. Dl-PCBs TEQ was 7.7 ± 8.0 pg g− 1 dw (Figure S6). Among the 10 ZnSO4 samples, the concentration of PCDD/Fs and TEQ of PCDD/Fs were 21 ± 35 pg g− 1 dw and 0.59 ± 1.3 pg g− 1 dw, respectively (Figure S7). Total PCBs and dl-PCBs were 2804 ± 5225 pg g− 1 dw and 12 ± 20 pg g− 1 dw, respectively. Dl-PCBs TEQ was 0.035 ± 0.061 pg g− 1 dw (Figure S8). PCDD/Fs and total PCB levels in ZnSO4 were higher than those in CuSO4. In PCB homologs of ZnSO4, indicator PCBs constituted a larger proportion than in CuSO4. The PCDD/Fs TEQ contents in CuSO4 and ZnSO4 were similar, whereas the TEQ of dl-PCBs in CuSO4 exceeded that in ZnSO4 by several orders of magnitude due to higher dl-PCB concentrations.
Toxicity potential and risk assessment of PCDD/Fs and PCBs
The ranges of PCDD/F TEQs and dl-PCB TEQs in the brown crabmeat were 0.74–9.7 pg g− 1 and 0.56–5.4 pg g− 1, respectively. The ranges of PCDD/F TEQs and dl-PCB TEQs in the white crabmeat were 0.00025-1.6 pg g− 1 and 0.0082-0.29 pg g− 1, respectively, which accounted for only 6.4% of PCDD/Fs and 3.0% of dl-PCBs in brown crabmeat (Fig. 7). Risk assessment is conducted by comparing the results with PTMI (70 pg TEQ kg− 1 bw m− 1) established by the WHO. For an individual consuming 5 g crabmeat, the estimated intake of PCDD/Fs and PCBs was 3.23 (0.82–9.73) pg TEQ kg− 1 bw m− 1 and 2.84 (0.59–5.39) pg TEQ kg− 1 bw m− 1, respectively (Fig. 7). Consequently, the estimated monthly intake of PCDD/Fs and PCBs for the average adult by eating crab was 6.07 pg TEQ kg− 1 bw m− 1 based on calculations using mean levels from the four sample lakes, accounting for 9% of PTMI. When this data is considered in view of the revised tolerable dioxin-like dietary intake published by EFSA in 2018, the estimated weekly intake of PCDD/Fs and PCBs for an average adult consuming crab was 1.42 pg TEQ kg− 1 bw w− 1, accounting for 71% of the TWI (2 pg TEQ kg− 1 bw w− 1). This result is higher than a study conducted on the Yangtze River, which found that the intake of PCDD/Fs and PCBs for an average adult via crab consumption was 1.05 pg TEQ kg− 1 bw w− 1, accounting for 53% of the TWI11.
Fig. 7.
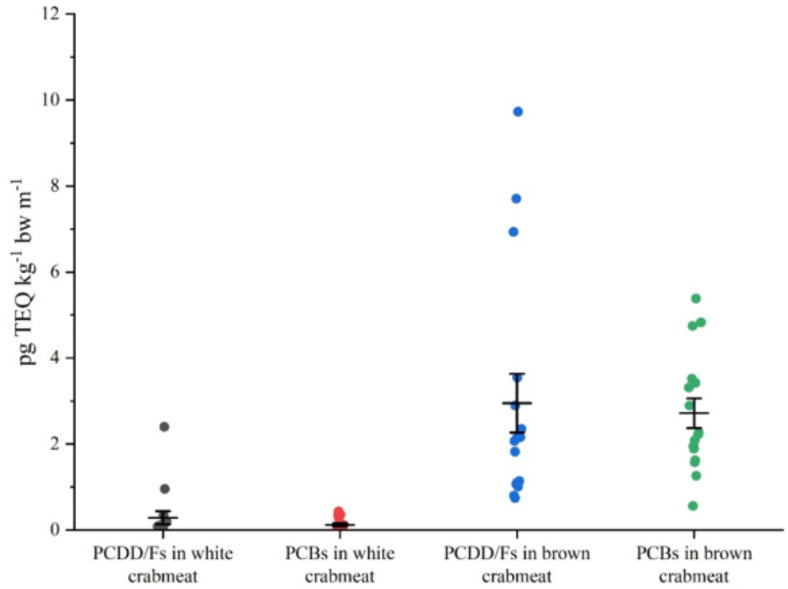
Monthly intake of PCDD/Fs and PCBs in white crabmeat and brown crabmeat (each data point represents a sample from four lakes. The median and standard error are shown as bars).
An extensive survey in China revealed that the average dietary intake of PCDD/Fs and PCBs for the general population was 20.1 (15.4–38.7) pg TEQ kg− 1 bw m− 1. The dietary intake of PCDD/Fs and PCBs in China was 29% of the PTMI, and the intake of PCDD/Fs and PCBs through crab consumption was 30% of the dietary intake, both of which were well below the PTMI values. These results suggest that consuming framed crabs from the Jiangsu Province does not pose significant health risks to the public. A survey in Guangdong Province showed that freshwater fish, beef, and pork were not seriously contaminated and the dietary exposure to PCDD/Fs and PCBs was below the PTMI, with the highest proportion of 20% being consumed via freshwater fish33. The spatial distribution results showed that the southern coastal regions were higher exposure regions of dietary intakes of these contaminants34. The dietary intake of PCDD/Fs and PCBs for adults in China is higher than intake levels reported in France, Italy, and Thailand. A dietary survey in France revealed that milk and meat products were major sources of dioxin-like contaminants, with intake levels exceeding 0.2 pg TEQ kg− 1 bw d− 1, and fish products accounting for 16% of the intake35. In Thailand, the dietary intakes of PCDD/Fs and dl-PCBs were estimated at 8.09 and 4.93 pg TEQ kg− 1 bw m− 1, respectively, with marine animals (26%), milk products (22%), and freshwater animals (21%) being the major contributors36. In Italy, dietary exposure to these contaminants was estimated to range from 0.17 to 0.42 pg TEQ kg− 1 bw d− 1 depending on the population subgroup37. Notably, dietary intake of PCDD/Fs and PCBs in China, Thailand, and Italy exceeded the TWI limits.
We assessed the risks arising from the consumption of white crabmeat and brown crabmeat, which had been neglected in previous dietary surveys. The weekly intake of PCDD/Fs and PCBs for the average adult consuming one crab (weight 100 g) is 1.12 pg TEQ kg− 1 bw m− 1, accounting for 56% of the TWI limit. This suggests that consuming up to 1.7 crabs per week does not pose potential health risks under the TWI. In contrast, under the PTMI limit, an adult could safely consume up to 62 crabs per month. If only white crabmeat is consumed, the weekly limit under the TWI is 26 crabs, while the limit for brown crabmeat is 1.9 crabs per week. The recommended weekly intake of white crabmeat and brown crabmeat for an adult is 443 g and 21 g, respectively. Therefore, the general population should consume more white crabmeat and less brown crabmeat to reduce potential risks of PCDD/Fs and PCBs. Overall, there is no need for the general population to be alarmed regarding crab consumption, and moderate consumption is beneficial for the crab market. Nevertheless, given the adverse effects of PCDD/Fs and PCBs on human development, regulations targeting crab consumption should be revisited to ensure public safety.
Material balance of PCDD/Fs and PCBs in the crab food web
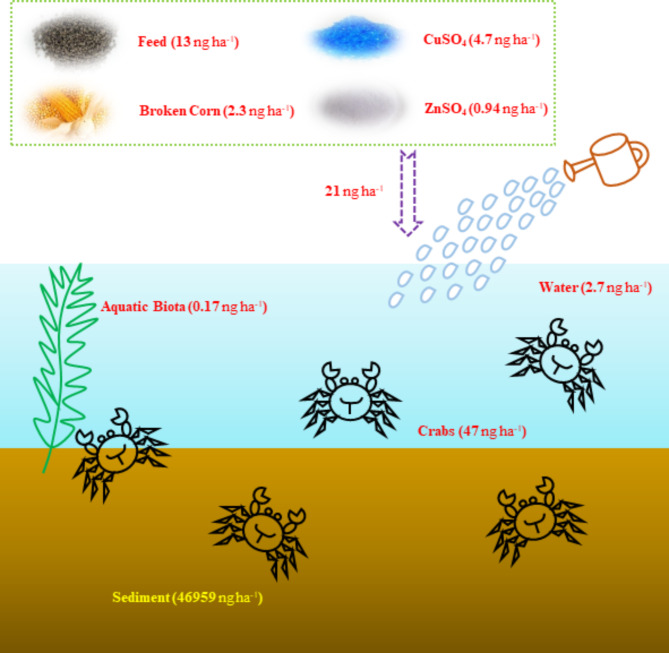
The material balance of PCDD/Fs and PCBs in the crab food web was estimated (Fig. 8). A hectare (ha) of crab pond was estimated to produce approximately 50 kg of crabs9. Production rates for the brown crabmeat (constituting 11% of the whole crab weight) and white crabmeat (constituting 16% of the whole crab weight) were 5.5 and 8.0 kg ha− 1, respectively. The average TEQ values of PCDD/Fs and PCBs in the brown crabmeat and white crabmeat were 5.7 and 0.27 pg g− 1, respectively. Consequently, the total TEQ of PCDD/Fs and PCBs in brown crabmeat was 31 ng ha− 1 and in white crabmeat was 2.2 ng ha− 1. Assuming a recapture rate of 70%, it was estimated that there would be 13 ng TEQ in brown crabmeat and 0.93 ng TEQ in white crabmeat from decreased crabs. Taking into account this factor, a total of 47 ng ha− 1 of PCDD/F and PCB TEQ was considered in crabs. 95 kg ha− 1 of commercial feed dose, 0.57 kg ha− 1 of CuSO4 and 1.52 kg ha− 1 of ZnSO4 application were assumed and 4269 kg ha− 1 of sediment was included in the crab pond13. Accordingly, the total TEQs of PCDD/Fs and PCBs for feed, CuSO4, ZnSO4, and sediment were 13, 4.7, 0.94, and 46,959 ng ha− 1, respectively. In addition, other potential sources of PCDD/Fs and PCBs in crabs in our previous studies were investigated for water (2.7 ng ha− 1), aquatic biota (0.17 ng ha− 1), and broken corn (2.3 ng ha− 1)9.
Fig. 8.
Material balance of PCDD/Fs and PCBs in the crab food web.
Considering the material balance of PCDD/Fs and PCBs in the crab food web, the TEQ inputs significantly exceeded the TEQ outputs regardless of the biological processes. The TEQ input from feed, CuSO4, ZnSO4, water, aquatic biota, and broken corn to the crab pond was estimated at 24 ng ha− 1, equivalent to 0.51 times the TEQ value of crabs. Among these potential sources, the input from feed culturing dominated, constituting 54% of the cumulative TEQ from all six sources. Notably, the TEQ input from sediment was 999 times higher than that observed in crabs. Sediments, recognized as a sink and preserver of pollutants, have been identified as reservoirs for PCDD/Fs and PCBs due to their stability and propensity to adsorb onto suspended particles and then deposit in the sediments38–40. Higher concentrations of estimated PCDD/Fs and PCBs TEQ in the potential source of sediments discovered may be substantially influenced by the historical depositions of pollutants. Taken together, sediments and feeds may be the most two important sources of PCDD/Fs and PCBs in crabs. The treatment, elimination, and replacement of sediments periodically could improve the surviving environment of crabs. Moreover, scientific crab culture especially for the restriction of feeding volumes could decrease the exposure of PCDD/Fs and PCBs in crabs.
Analysis of the sources of PCDD/Fs and PCBs in the crab food web
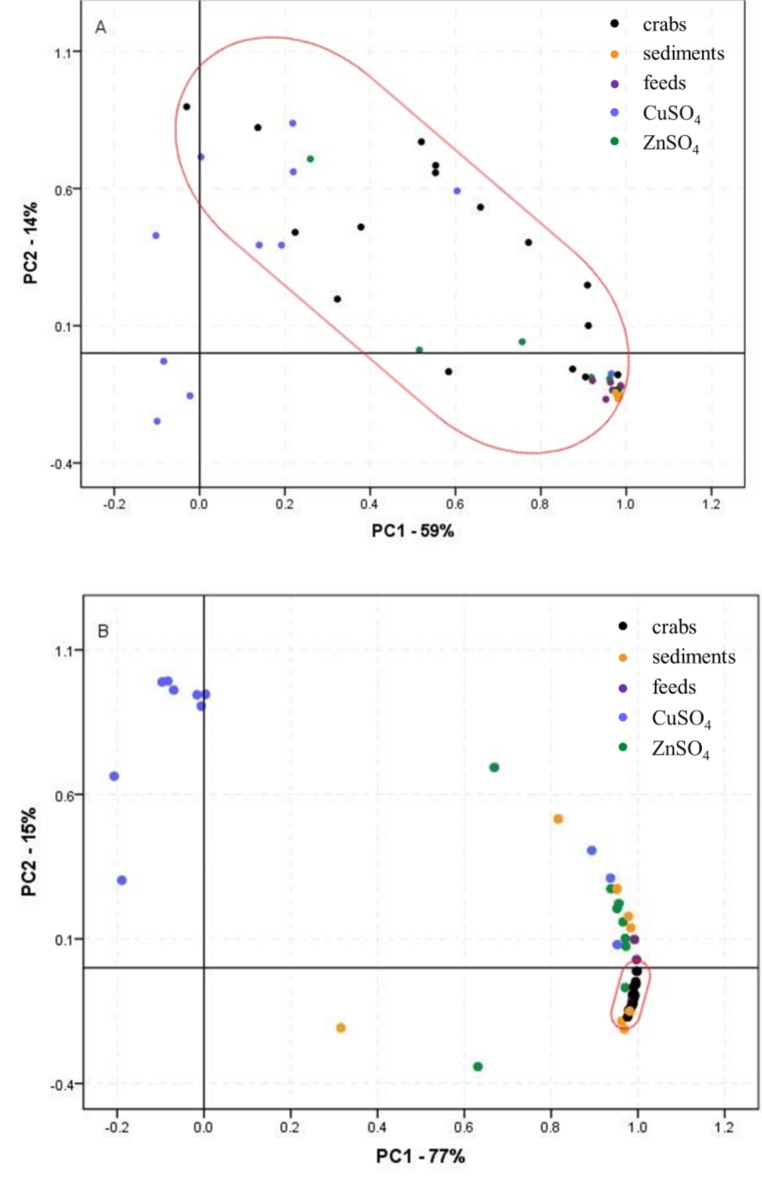
Principal component analysis (PCA) was employed to evaluate the sources of and correlations between the PCDD/F and PCB toxic congeners in the crab food web samples using SPSS 22.0, obtaining scores plots. As shown in Fig. 9A, the two components occupied 59% and 14% separately. The PCA analysis results for PCDD/Fs did not exhibit distinct separation in crabs, commercial feeds, sediments, CuSO4, and ZnSO4 samples, suggesting that the PCDD/Fs in these samples had intricate and overlapping sources. In contrast, PCB patterns displayed notable differences (Fig. 9B), with the two components explaining 77% and 15% of the variance, respectively. The PCB analysis results indicated that crabs were primarily clustered with sediments and compound feeds. Consequently, both sediments and feeds emerged as the primary sources of PCBs in crabs. Besides, the stable isotope ratios for nitrogen (δ15N) (ranged from – 0.70 to 7.71) and carbon (δ13C) (ranged from − 26.6 to − 12.2) were applied to access the trophic position and food sources in the crab food web. The δ15N and δ13C results for crabs and sediments were clustered together, consistent with the PCA analysis results.
Fig. 9.
Scores plot of PCA analysis for toxic congener contributors of PCDD/Fs (A) and PCBs (B) in the crab food web.
Furthermore, the T-test was applied to compare TEQs of PCDD/Fs and PCBs across crabs, sediments, feeds, CuSO4, and ZnSO4. A highly significant difference was observed between PCDD/Fs TEQ in crabs and that in sediments or feeds (p < 0.01), and the effect size was low (Cohen’d 0.25) and large (Cohen’d 1.04), respectively. The PCDD/Fs TEQ in crabs also showed a significant difference when compared to CuSO4 or ZnSO4 (p < 0.05), and the effect size was large (Cohen’d 1.12 and 1.03). Additionally, a significant difference was found between the PCBs TEQ in crabs and that in CuSO4, (p < 0.05), ZnSO4 (p < 0.01), sediment (p < 0.01), and feeds (p < 0.01). The effect size of PCBs TEQ in crab compared to that in CuSO4 (Cohen’d 0.96), ZnSO4 (Cohen’d 2.45), sediment (Cohen’d 1.19), and feeds (Cohen’d 2.21) were large. Consequently, highly significant differences were found in PCDD/Fs and PCBs TEQ in crabs differed markedly from that in sediments and feeds (p < 0.01), which is generally consistent with the results from PCA analysis and elemental analysis.
In a concurrent study, Dong et al.21 explored the sources of chlorinated paraffins (CPs) in crabs collected from three Chinese provinces, suggesting that sediment, crab feed, and aquatic plants could contribute to CPs sources21. In a separate investigation, De Jesus et al.20 reported correlations between heavy metals detected in crab muscles collected from the Amazon coast, Brazil, and metals in sediments20. These results indicate that the crab farming enterprises concerned should carry out regular cleaning of the substrate to reduce the level of contaminants in crabs.
Limitations and perspectives
The potential limitations and uncertainties associated with environmental sample collection, extraction techniques, and analytical methods for PCDD/Fs and PCBs in a crab food web in this work could be summarized to several points.
Sample collection (a) Spatial and Temporal Variability: Factors such as location, seasonal changes, and local pollution sources can influence contaminant levels, potentially leading to inconsistent or non-representative data. (b) Sample Contamination: There is a risk of contaminating samples with external PCDD/Fs and PCBs, which can skew results. (c) Sample Size and Representativeness: Inadequate sample sizes may not capture the full variability in contaminant concentrations across the crab food web.
Extraction Techniques (a) Matrix Interferences: Sample matrix effects need to be carefully managed to ensure reliable results. (b) Method Validation: Without rigorous validation, there may be uncertainties about the accuracy and precision of the extraction process.
Analytical Methods (a) Instrumentation and Detection Limits: Ensuring that instrumentation is properly calibrated and maintained is crucial for reliable analysis. (b) Quantification and Identification: Using appropriate standards and performing rigorous quality control checks are necessary. (c) Data Interpretation: Clear documentation of methods and results, along with appropriate statistical analyses, helps in mitigating the uncertainties.
To address the limitations and enhance the study’s robustness and credibility, it is essential to: (1) Implement comprehensive sample collection protocols to ensure representativeness and minimize contamination; (2) Utilize validated extraction techniques tailored to the specific matrices and target compounds; (3) Employ well-maintained and properly calibrated analytical instruments, along with rigorous quality control measures; (4) Provide detailed documentation and transparent reporting of methodologies and results to allow for reproducibility and critical evaluation.
By addressing these aspects, the reliability and accuracy of the findings can be improved, leading to a more robust and credible assessment of PCDD/Fs and PCBs in the crab food web.
Conclusions
The results indicated a certain level of contamination by PCDD/Fs and PCBs in the crab food web. PCA analysis revealed that PCDD/Fs in crabs had complex sources. Sediments in aquaculture environments and commercial crab feeds may contribute PCBs to crabs. In China, the monthly intake of PCDD/Fs and PCBs through consumption of crabs was 6.07 TEQ kg− 1 bw m− 1, representing 30% of the dietary intake, which was 9% and 71% of the PTMI and TWI limits. These results suggested that the consumption of crabs does not pose risks to human health. More attention should be paid to PCDD/Fs and PCBs in the crab food web because a certain amount of PCDD/Fs and PCBs in all samples were detected. Relatively high PCDD/F and PCB levels were found in sediment samples. Consequently, PCDD/Fs and PCBs contamination of the aquaculture environment in which crabs are cultured should not be neglected.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was supported by the National Natural Science Foundation of China [grant number 42407654, 21906009, 22076207], the Postgraduate Research & Practice Innovation Program of Jiangsu Province [SJCX24_1649], the Science and Technology Research Award Package of Changzhou University [grant number KYP2202117C, KYP2402150C], and the Fundamental Research Funds for the Central Universities.
Abbreviations
- dl-PCBs
dioxin-like polychlorinated biphenyls
- EDIs
Estimated daily intakes
- FAO
Food and agriculture organization
- LOD
Limit of detection
- PCA
Principal component analysis
- PCBs
Polychlorinated biphenyls
- PCDDs
Polychlorinated dibenzo-p-dioxins
- PCDFs
Polychlorinated dibenzofurans
- PTMI
Provisional tolerable monthly intake
- TEFs
Toxicity equivalence factors
- TEQ
Toxicity equivalence quantity
- TWI
Tolerable weekly intake
- WHO
World Health Organization
Author contributions
Y.H.: Conceptualization, Methodology, Software, Validation, Investigation, Data curation, Writing—original draft, Writing—review and editing, Visualization, and Funding acquisition. C.C.: Supervision, Writing—original draft, Writing—review and editing, and Funding acquisition. W.L.: Supervision, Writing—original draft, Writing—review and editing, and Funding acquisition. Y.H.: Investigation. F.Y.: Investigation. Q.C.: Investigation. All authors have read and agreed to the published version of the manuscript.
Data availability
Data is provided within the supplementary information files.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunci Chen, Email: chenchunci@ucas.ac.cn.
Wenbin Liu, Email: wbliu@ucas.ac.cn.
References
- 1.Terzaghi, E. et al. New data set of polychlorinated dibenzo-p-dioxin and dibenzofuran half-lives: Natural attenuation and rhizoremediation using several common plant species in a weathered contaminated soil. Environ. Sci. Technol.54, 10000–10011 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niu, S. et al. Using polychlorinated naphthalene concentrations in the soil from a Southeast China E-waste recycling area in a novel screening-level multipathway human cancer risk assessment. Environ. Sci. Technol.55, 6773–6782 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Whitehead, T. P. et al. Concentrations of persistent organic pollutants in California children’s whole blood and residential dust. Environ. Sci. Technol.49, 9331–9340 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Hoogenboom, R. L. et al. PCBs and heavy metals in Chinese mitten crabs from Dutch rivers and lakes. Chemosphere123, 1–8 (2015). Dioxins. [DOI] [PubMed] [Google Scholar]
- 5.Li, X. et al. Application of gas chromatography coupled to triple quadrupole mass spectrometry (GC-(APCI)MS/MS) in determination of PCBs (mono-to deca-) and PCDD/Fs in Chinese mitten crab food webs. Chemosphere265, 129055 (2021). [DOI] [PubMed] [Google Scholar]
- 6.Bernard, A. et al. Food contamination by PCBs and dioxins. Nature401, 231–232 (1999). [DOI] [PubMed] [Google Scholar]
- 7.Weber, R. et al. Reviewing the relevance of dioxin and PCB sources for food from animal origin and the need for their inventory, control and management. Environ. Sci. Eur.30, 1e42 (2018). [DOI] [PMC free article] [PubMed]
- 8.Khairy, M. A., Weinstein, M. P. & Lohmann, R. Trophodynamic behavior of hydrophobic organic contaminants in the aquatic food web of a tidal river. Environ. Sci. Technol.48, 12533–12542 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Han, Y. et al. Sources of polychlorinated dibenzo-p-dioxins and dibenzofurans, and biphenyls in Chinese mitten crabs. Chemosphere196, 522–530 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Sun, S. et al. Levels and patterns of polychlorinated dibenzo-p-dioxins and dibenzofurans and polychlorinated biphenyls in foodstuffs of animal origin from Chinese markets and implications of dietary exposure. Environ. Pollut.273, 116344 (2021). [DOI] [PubMed] [Google Scholar]
- 11.Rao, Q. et al. New insights into the transfer and accumulation of dioxins and dioxin-like PCBs in the food web of farmed Chinese mitten crabs: A typical case from the Yangtze River area. J. Hazard. Mater.436, 129178 (2022). [DOI] [PubMed] [Google Scholar]
- 12.Joint, F. A. O. & WHO Expert Committee on Food Addtives. Evaluation of certain food additives and contaminants. WHO technical report series: 909, p. 144. (Rome, Italy, 2001).
- 13.EFSA Panel on Contaminants in the Food Chain. Scientific opinion on the risk for animal and human health related to the presence of dioxins and dioxin-like PCBs in feed and food. EFSA J.16, e05333 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luo, R. et al. Determination of geographic origin of Chinese mitten crab (Eriocheir sinensis) using integrated stable isotope and multi-element analyses. Food Chem.274, 1–7 (2019). [DOI] [PubMed] [Google Scholar]
- 15.Zhang, C. et al. Genetic diversity and genetic structure of farmed and wild Chinese mitten crab (Eriocheir sinensis) populations from three major basins by mitochondrial DNA COI and cyt b gene sequences. Mitochondrial DNA Part. A29, 1081–1089 (2017). [DOI] [PubMed] [Google Scholar]
- 16.Association, C. F. China Fishery Statistical Yearbook. (2022).
- 17.Zhang, Z., Chen, L., Cheng, M., Liu, M. & Wang, X. Biotransport of mercury and human methylmercury exposure through crabs in China—A life cycle-based analysis. J. Hazard. Mater.415, 125684 (2021). [DOI] [PubMed] [Google Scholar]
- 18.Clark, P. F. et al. Dioxin and PCB contamination in Chinese Mitten crabs: Human consumption as a control mechanism for an invasive species. Environ. Sci. Technol.43, 1624–1629 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Zhang, T. et al. Microplastics in different tissues of wild crabs at three important fishing grounds in China. Chemosphere271, 129479 (2021). [DOI] [PubMed] [Google Scholar]
- 20.de Jesus, W. B. et al. Biomarkers and occurrences of heavy metals in sediment and the bioaccumulation of metals in crabs (Ucides cordatus) in impacted mangroves on the Amazon coast, Brazil. Chemosphere271, 129444 (2021). [DOI] [PubMed] [Google Scholar]
- 21.Dong, S. et al. Concentrations and sources of short- and medium-chain chlorinated paraffins in farmed Chinese mitten crabs in China. J. Hazard. Mater.411, 125076 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Ahmed, S. et al. Heavy metals contamination in shrimp and crab from southwest regions in Bangladesh: Possible health risk assessment. Toxicol. Rep.10, 580–588 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han, Y. et al. Formation pathways of mono- to octa-chlorinated dibenzo-p-dioxins and dibenzofurans in main organochemical industries. Environ. Sci. Technol.49, 10945–10950 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Han, Y. et al. Influence of long-range atmospheric transportation (LRAT) on mono-to octa-chlorinated PCDD/Fs levels and distributions in soil around Qinghai Lake, China. Chemosphere156, 143–149 (2016). [DOI] [PubMed] [Google Scholar]
- 25.Van den Berg, M. et al. The 2005 World Health Organization reevaluation of human and mammalian toxic equivalency factors for dioxins and dioxin-like compounds. Toxicol. Sci.93, 223–241 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.COMMISSION REGULATION (EU). 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No. 1881/2006.
- 27.Hemming, J. M., Brim, M. S. & Jarvis, R. B. A survey of dioxin and furan compounds in sediments of Florida Panhandle Bay systems. Mar. Pollut Bull.46, 512–521 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Sobek, A. et al. Coastal sediments in the Gulf of Bothnia as a source of dissolved PCDD/Fs and PCBs to water and fish. Sci. Total Environ.487, 463–470 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Wang, Y. et al. PCDD/Fs and DL-PCBs in Chinese mitten crab (Eriocheir sinensis) and its farming environment in Shanghai, China. Foods11, 2556 (2022). [DOI] [PMC free article] [PubMed]
- 30.Taylor, M. et al. Polychlorinated dibenzodioxins/furans and dioxin-like polychlorinated biphenyls in fish and crustaceans of a recreationally fished estuary, following targeted remediation. Sci. Total Environ.921, 17089 (2024). [DOI] [PubMed] [Google Scholar]
- 31.Husain, A. et al. Screening for PCDD/Fs and dl-PCBs in local and imported food and feed products available across the state of Kuwait and assessment of dietary intake. Ecotoxicol. Environ. Saf.100, 27–31 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Wang, P. et al. Dioxins contamination in the feed additive (feed grade cupric sulfate) tied to chlorine industry. Sci. Rep.4, 5975 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wu, W. L. et al. Levels, congener profiles, and dietary intake assessment of polychlorinated dibenzo-p-dioxins/dibenzofurans and dioxin-like polychlorinated biphenyls in beef, freshwater fish, and pork marketed in Guangdong province, China. Sci. Total Environ.615, 412–421 (2018). [DOI] [PubMed] [Google Scholar]
- 34.Zheng, W., Zhao, H., Liu, Q., Crabbe, M. J. C. & Qu, W. Spatial-temporal distribution, cancer risk, and disease burden attributed to the dietary dioxins exposure of Chinese residents. Sci. Total Environ.832, 154851 (2022). [DOI] [PubMed] [Google Scholar]
- 35.Godéré, M. et al. Polychlorinated naphthalenes in foods from the French market: Occurrence, dietary exposure, and evaluation of relative contributions to dioxin-like contaminants. Environ. Sci. Technol.58, 1721–1730 (2024). [DOI] [PubMed] [Google Scholar]
- 36.Sunya, S. et al. Estimation of dietary intake of polychlorinated dibenzo-p-dioxins and dibenzofurans and dioxin-like polychlorinated biphenyls from the Thai total diet study in 2019. Food Chem. Toxicol.182, 114154 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Diletti, G. et al. Polybrominated dibenzo-p-dioxins and furans (PBDD/Fs) in Italian food: Occurrence and dietary exposure. Sci. Total Environ.741, 139916 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Baran, A. et al. An assessment of the concentrations of PCDDs/Fs in contaminated bottom sediments and their sources and ecological risk. J. Soils Sediments20, 2588–2597 (2020). [Google Scholar]
- 39.Pizzini, S. et al. Occurrence and source apportionment of organic pollutants in deep sediment cores of the Venice Lagoon. Mar. Pollut Bull.164, (2021). [DOI] [PubMed]
- 40.Lei, R. et al. A review of levels and profiles of polychlorinated dibenzo-p-dioxins and dibenzofurans in different environmental media from China. Chemosphere239, 9 (2020). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is provided within the supplementary information files.