Abstract
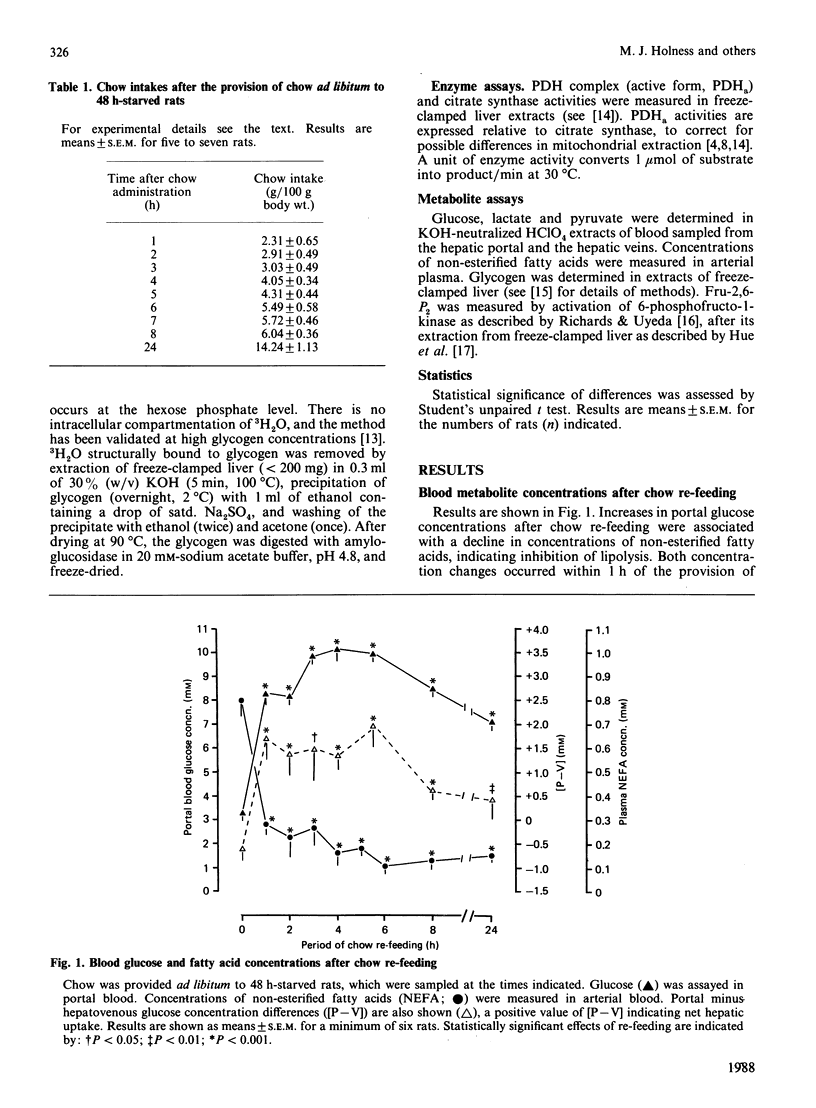
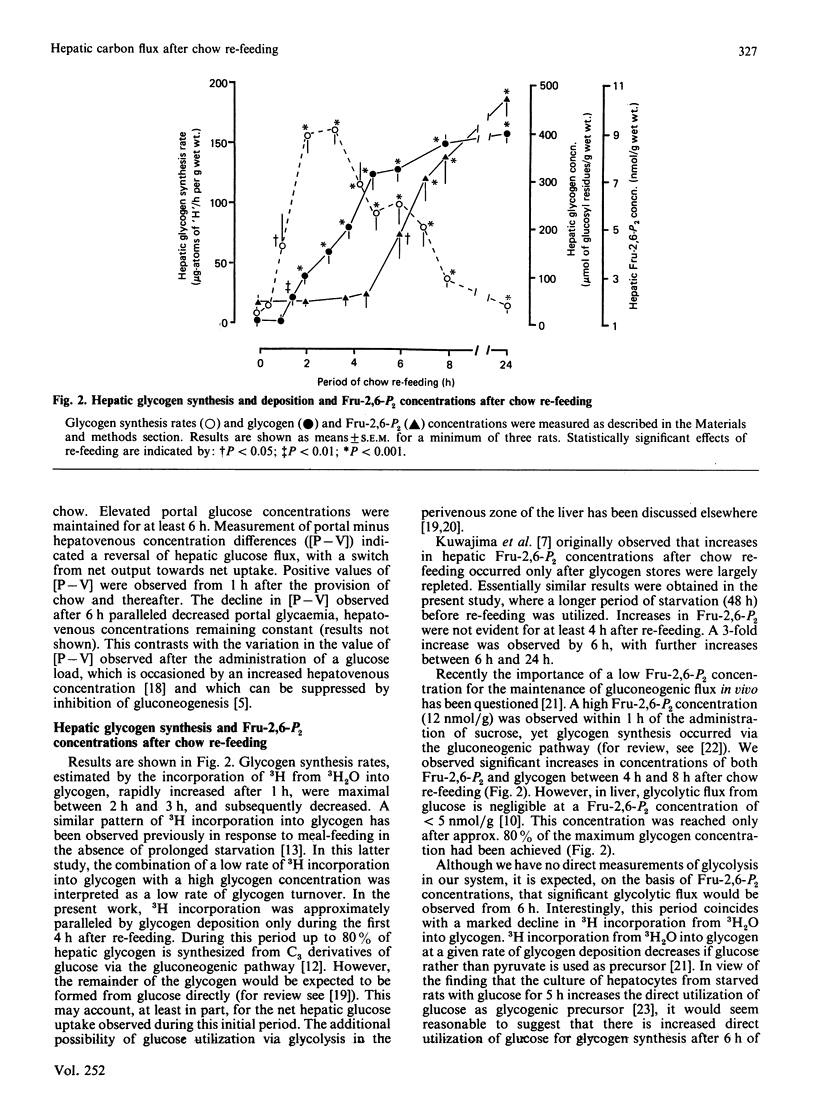
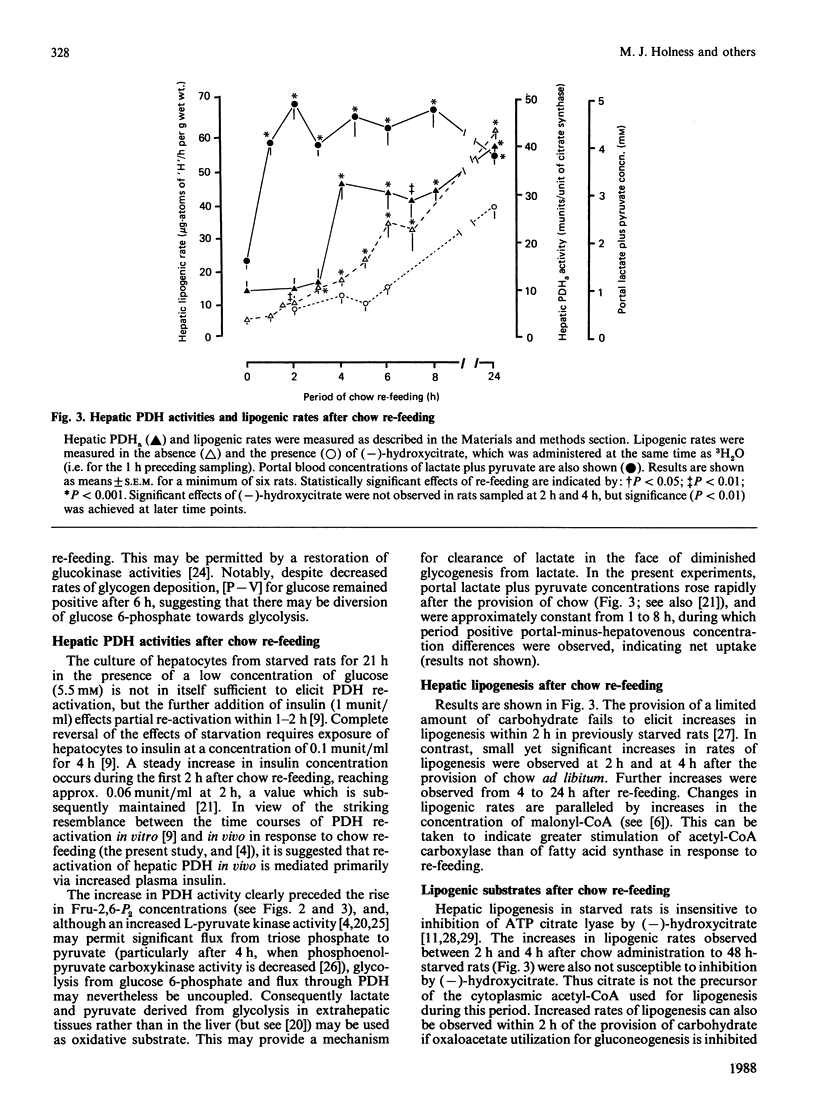
A comparison was made between the time courses of restoration of pyruvate dehydrogenase activities, fructose 2,6-bisphosphate concentrations and lipogenic rates, together with net hepatic glucose flux and glycogen synthesis/deposition in livers of 48 h-starved rats provided with laboratory chow ad libitum for up to 24 h. Increased glycogenesis, lipogenesis and net glucose uptake were observed after 1 h of re-feeding, preceding re-activation of pyruvate dehydrogenase, which occurred after 3-4 h. Increased concentrations of fructose 2,6-bisphosphate were only observed after 5-6 h. The implication of the temporal relationship between these parameters is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agius L., Williamson D. H. The utilization of ketone bodies by the interscapular brown adipose tissue of the rat. Biochim Biophys Acta. 1981 Oct 23;666(1):127–132. doi: 10.1016/0005-2760(81)90098-9. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid, 3-beta-hydroxysterol, and ketone synthesis in the perfused rat liver. Effects of (--)-hydroxycitrate and oleate. Eur J Biochem. 1978 Jan 16;82(2):373–384. doi: 10.1111/j.1432-1033.1978.tb12032.x. [DOI] [PubMed] [Google Scholar]
- Caterson I. D., Fuller S. J., Randle P. J. Effect of the fatty acid oxidation inhibitor 2-tetradecylglycidic acid on pyruvate dehydrogenase complex activity in starved and alloxan-diabetic rats. Biochem J. 1982 Oct 15;208(1):53–60. doi: 10.1042/bj2080053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus T. H., Nyfeler F., Muenkel H. A., Burns M. G., Pate T., Pilkis S. J. Changes in key regulatory enzymes of hepatic carbohydrate metabolism after glucose loading of starved rats. Biochem Biophys Res Commun. 1984 Dec 14;125(2):655–661. doi: 10.1016/0006-291x(84)90589-8. [DOI] [PubMed] [Google Scholar]
- Grantham B. D., Zammit V. A. Restoration of the properties of carnitine palmitoyltransferase I in liver mitochondria during re-feeding of starved rats. Biochem J. 1986 Oct 15;239(2):485–488. doi: 10.1042/bj2390485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Schofield P. S., Sugden M. C. The relationship between fat synthesis and oxidation in the liver after re-feeding and its regulation by thyroid hormone. Biochem J. 1987 Nov 1;247(3):621–626. doi: 10.1042/bj2470621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., French T. J., Sugden M. C. Hepatic glycogen synthesis on carbohydrate re-feeding after starvation. A regulatory role for pyruvate dehydrogenase in liver and extrahepatic tissues. Biochem J. 1986 Apr 15;235(2):441–445. doi: 10.1042/bj2350441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Continued glucose output after re-feeding contributes to glucose intolerance in hyperthyroidism. Biochem J. 1987 Nov 1;247(3):801–804. doi: 10.1042/bj2470801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness M. J., Sugden M. C. Hepatic carbon flux after re-feeding. Hyperthyroidism blocks glycogen synthesis and the suppression of glucose output observed in response to carbohydrate re-feeding. Biochem J. 1987 Nov 1;247(3):627–634. doi: 10.1042/bj2470627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopgood M. F., Ballard F. J. Synthesis and degradation of phosphoenolpyruvate carboxylase in rat liver and adipose tissue. Changes during a starvation-re-feeding cycle. Biochem J. 1973 Jun;134(2):445–453. doi: 10.1042/bj1340445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hue L., Blackmore P. F., Shikama H., Robinson-Steiner A., Exton J. H. Regulation of fructose-2,6-bisphosphate content in rat hepatocytes, perfused hearts, and perfused hindlimbs. J Biol Chem. 1982 Apr 25;257(8):4308–4313. [PubMed] [Google Scholar]
- Hue L., Sobrino F., Bosca L. Difference in glucose sensitivity of liver glycolysis and glycogen synthesis. Relationship between lactate production and fructose 2,6-bisphosphate concentration. Biochem J. 1984 Dec 15;224(3):779–786. doi: 10.1042/bj2240779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwajima M., Golden S., Katz J., Unger R. H., Foster D. W., McGarry J. D. Active hepatic glycogen synthesis from gluconeogenic precursors despite high tissue levels of fructose 2,6-bisphosphate. J Biol Chem. 1986 Feb 25;261(6):2632–2637. [PubMed] [Google Scholar]
- Kuwajima M., Newgard C. B., Foster D. W., McGarry J. D. Time course and significance of changes in hepatic fructose-2,6-bisphosphate levels during refeeding of fasted rats. J Clin Invest. 1984 Sep;74(3):1108–1111. doi: 10.1172/JCI111479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchington D. R., Kerbey A. L., Jones A. E., Randle P. J. Insulin reverses effects of starvation on the activity of pyruvate dehydrogenase kinase in cultured hepatocytes. Biochem J. 1987 Aug 15;246(1):233–236. doi: 10.1042/bj2460233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry J. D., Kuwajima M., Newgard C. B., Foster D. W., Katz J. From dietary glucose to liver glycogen: the full circle round. Annu Rev Nutr. 1987;7:51–73. doi: 10.1146/annurev.nu.07.070187.000411. [DOI] [PubMed] [Google Scholar]
- Minderop R. H., Hoeppner W., Seitz H. J. Regulation of hepatic glucokinase gene expression. Role of carbohydrates, and glucocorticoid and thyroid hormones. Eur J Biochem. 1987 Apr 1;164(1):181–187. doi: 10.1111/j.1432-1033.1987.tb11009.x. [DOI] [PubMed] [Google Scholar]
- Newgard C. B., Hirsch L. J., Foster D. W., McGarry J. D. Studies on the mechanism by which exogenous glucose is converted into liver glycogen in the rat. A direct or an indirect pathway? J Biol Chem. 1983 Jul 10;258(13):8046–8052. [PubMed] [Google Scholar]
- Postle A. D., Bloxham D. P. The use of tritiated water to measure absolute rates of hepatic glycogen synthesis. Biochem J. 1980 Oct 15;192(1):65–73. doi: 10.1042/bj1920065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards C. S., Uyeda K. Changes in the concentration of activation factor for phosphofructokinase in hepatocytes in response to glucose and glucagon. Biochem Biophys Res Commun. 1980 Dec 31;97(4):1535–1540. doi: 10.1016/s0006-291x(80)80040-4. [DOI] [PubMed] [Google Scholar]
- Spence J. T., Koudelka A. P. Pathway of glycogen synthesis from glucose in hepatocytes maintained in primary culture. J Biol Chem. 1985 Feb 10;260(3):1521–1526. [PubMed] [Google Scholar]
- Stansbie D., Brownsey R. W., Crettaz M., Denton R. M. Acute effects in vivo of anti-insulin serum on rates of fatty acid synthesis and activities of acetyl-coenzyme A carboxylase and pyruvate dehydrogenase in liver and epididymal adipose tissue of fed rats. Biochem J. 1976 Nov 15;160(2):413–416. doi: 10.1042/bj1600413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Marshall C. E., McCormack J. G. Brown-adipose-tissue lipogenesis in starvation: effects of insulin and (-) hydroxycitrate. Biosci Rep. 1982 May;2(5):289–297. doi: 10.1007/BF01115114. [DOI] [PubMed] [Google Scholar]
- Sugden M. C., Watts D. I., Palmer T. N., Myles D. D. Direction of carbon flux in starvation and after refeeding: in vitro and in vivo effects of 3-mercaptopicolinate. Biochem Int. 1983 Sep;7(3):329–337. [PubMed] [Google Scholar]
- Triscari J., Sullivan A. C. Comparative effects of (--)-hydroxycitrate and (+)-allo-hydroxycitrate on acetyl CoA carboxylase and fatty acid and cholesterol synthesis in vivo. Lipids. 1977 Apr;12(4):357–363. doi: 10.1007/BF02533638. [DOI] [PubMed] [Google Scholar]


