Abstract
Background
Benzodiazepines are known for their possible risk of delirium, especially when administered to older adults or during the perioperative period. However, the risk of sudden discontinuation of benzodiazepines and subsequent withdrawal delirium seems to be underappreciated and not properly recognized in cancer treatment, even among healthcare workers.
Case Presentation
A man in his late 70s was diagnosed with rectal cancer and had a history of taking etizolam and alprazolam for over 15 years. After hospitalization for cancer, these benzodiazepines were discontinued abruptly 2 days before the surgery, due to concerns about benzodiazepine‐related delirium. After half a day, severe benzodiazepine withdrawal symptoms, such as disorientation, agitation, tachycardia, diaphoresis, and muscle twitching, appeared. The patient was diagnosed with delirium associated with benzodiazepine withdrawal, and his symptoms subsided soon after re‐administration of benzodiazepines. Despite development of mild postoperative delirium, the operation was performed safely.
Conclusion
The rapid and abrupt discontinuation of benzodiazepines before cancer surgery could impose a high risk of benzodiazepine withdrawal delirium in patients, which makes the management of psychiatric symptoms in the perioperative period more complex and difficult. We believe that it is necessary to highlight the risks of withdrawal delirium and abrupt benzodiazepine discontinuation in cancer treatment. Moreover, re‐education on this topic is essential for healthcare workers.
Keywords: abrupt discontinuation of benzodiazepine, benzodiazepine withdrawal delirium, cancer perioperative period, inappropriate benzodiazepine prescription, postoperative delirium
It is necessary to highlight the risks of abrupt benzodiazepine discontinuation in cancer treatment. Moreover, re‐education on this topic is essential for healthcare workers.
BACKGROUND
Benzodiazepines (BZDs) are used as hypnotics, anxiolytics, anticonvulsants, and muscle relaxants. Although short‐term use is recommended, BZDs are commonly, and in a sense, very easily, prescribed for a long time for various diseases and symptoms. Approximately 4% of the general population is prescribed BZDs, 1 implying that there are many possible risks associated with the long‐term use of BZDs, including addiction, withdrawal symptoms, cognitive dysfunction, and other side‐effects. Besides, it is pointed out that only 5% of all patients receiving BZDs eventually manage to discontinue their use once started, suggesting the difficulty of stopping BZDs on their own. 2 With all these facts, unnecessary and long‐term BZDs prescriptions are often seen in clinical situations, and at times, prescriptions based on inappropriate diagnoses are also recognized.
In clinical situations, the fact that BZDs are possible risk factors for delirium and that their risk becomes greater in the perioperative period is well known. 3 , 4 It is reported that as much as 20% of delirium in hospitalized patients is due to BZDs withdrawal. 5 Therefore, healthcare workers who are educated on the risks and side‐effects of BZDs generally avoid starting them in the perioperative period, especially in elderly patients with cognitive problems, who are known to be at high risk for postoperative delirium. This holds true in cancer treatment as well. However, in contrast to BZDs‐induced delirium, the risk of abrupt discontinuation of BZDs following the withdrawal of symptoms seems to be relatively underrated in cancer treatment. It is reported that abrupt BZDs cessation even cause death. 6 Notwithstanding this fact, to the best of our knowledge based on a literature review, there are no previous reports on the risk of BZDs discontinuation and withdrawal in cancer treatment.
Here, we report an accidental case of discontinuation of BZDs in the cancer perioperative period. We believe that underscoring the importance of BZDs management in the cancer perioperative period and re‐educating all healthcare workers on this issue is necessary.
CASE PRESENTATION
The patient was a man in his late 70s diagnosed with colon cancer (cT3N0M0, stage II) with a history of hypertension, trifascicular block, ventricular premature complex, arrhythmia, and chronic chest pain. The patient's life history is concisely summarized as follows: In childhood, he was diagnosed with an atrial septal defect and underwent heart surgery at the age of 15, with no aftereffects. After graduating from high school, he worked as a wholesaler in a fish market for more than 40 years. When he was 65 years old, he experienced shortness of breath with fatigue and was diagnosed with hypertension, trifascicular block, ventricular premature complex, and arrhythmia. Medications including antihypertensive and antiarrhythmic drugs were initiated, and his symptoms subsided. Around the same time, although the details are unknown, he was also diagnosed with chronic chest pain and had been prescribed etizolam (1.5 mg/day, orally) and alprazolam (0.4 mg/day, orally) by a primary care physician for more than 15 years. Etizolam 1.5 mg corresponds to 5 mg of diazepam and alprazolam 0.4 mg corresponds to 2.5 mg of diazepam, respectively, 7 meaning that the patient is considered to have taken the equivalent of 7.5 mg of diazepam. The patient did not have a diagnosis of psychiatric illness, including hypochondriacal disorder. Despite his pain symptoms and stable mental status, no attempts to reduce these BZDs were made, and the same prescription was continued.
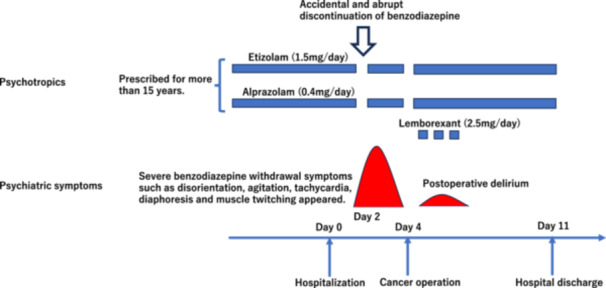
After the diagnosis of colon cancer, he was hospitalized as planned in our institution 3 days before the cancer surgery, and all medications, including BZDs, were continued. His vital signs were as follows: temperature 36.5°C, blood pressure 123/73 mmHg, pulse 85/min. A 12‐lead electrocardiogram showed a sinus rhythm, a QT correction of 444 msec, and a QRS of 148 msec. The patient was fully awake and alert, and no psychiatric problems were observed. Laboratory testing was unremarkable. However, the nurse discontinued the above BZDs after receiving instructions from the surgeon in charge 2 days before the cancer surgery, on the assumption that they are risk factors for postoperative delirium and that discontinuation itself is beneficial with no side‐effects. After half a day, however, BZDs withdrawal symptoms, such as disorientation, severe agitation, tachycardia, diaphoresis, and muscle twitching, appeared (Figure 1). The patient was very confused, agitated and disoriented; he walked restlessly around the hospital ward. His mental status was unstable and very agitated, and an EEG examination could not be performed. He was initially regarded as having delirium due to environmental changes after hospitalization by the surgeon in charge, and the use of antipsychotics, such as haloperidol dripping, was considered. However, because of his cardiovascular conditions, a liaison consultation was referred to us before the use of haloperidol. We diagnosed the patient with BZDs withdrawal delirium based on his clinical characteristics, and his symptoms subsided within several hours after re‐administration of BZDs (etizolam 1.5 mg/day and alprazolam 0.4 mg/day, orally). Subsequently, the patient became awake and alert again, and surgery was performed as planned. The surgery was carried out safely with no delay; mild postoperative delirium did develop, but there were no after‐effects. We were able to cope with his psychiatric symptoms of postoperative delirium, such as insomnia, using lemborexant (2.5 mg/day, orally) only when necessary. He was discharged 11 days after admission with no aftereffects. Etizolam and alprazolam were continued at the same dose until the time of discharge. Then we sent a medical information form to the patient's clinic, requesting that benzodiazepines be tapered off carefully in the near future.
Figure 1.
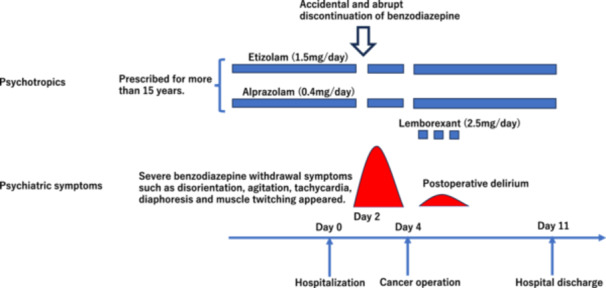
Clinical course of the patient.
After this accident, our psychiatric liaison team regularly visit hospital wards and conduct further education on BZDs withdrawal during the perioperative period. Specifically, we regularly check and make a list of patients prescribed BZDs before hospitalization and share the risks among doctors, nurses, and liaison teams. Besides, we regularly lecture on the dangers of BZDs withdrawal in our training sessions for nurses.
DISCUSSION
As our hospital specializes in cancer care, we often encounter patients with delirium due to environmental changes after hospitalization and postoperative delirium after cancer surgery. As in many other cases, the patient in this case was initially regarded as having delirium due to environmental changes, and antipsychotics were about to be administered. The patient had a history of cardiac disease and we considered the possible risk of QT elongation and worsening of arrhythmia by antipsychotics. Fortunately, in this case, BZDs withdrawal delirium was appropriately diagnosed and managed during the postoperative period, and the surgery was performed safely without delay.
There are very few reports discussing cancer treatment and BZDs withdrawal, and currently only a case report in terminal care is known based on a literature review. 8 This is the first case report discussing the problem of BZDs withdrawal in the cancer perioperative period. However, as in this case, it is not rare that inappropriate, long‐term use of BZDs is revealed to doctors and nurses for the first time when a patient is hospitalized for a cancer operation. Biringen et al. reported that 89.2% of psychotropic drugs prescribed by oncologists were BZDs. 9 A third of long‐term (beyond 6 months) BZDs users experience symptoms and signs of withdrawal such as anxiety, insomnia, muscle spasms, tension, and perceptual hypersensitivity. 10 In the cancer perioperative period, patients with many doses of BZDs have to quit oral medications, and consequently, the risk of BZDs withdrawal syndrome increases. This situation often leads to trouble and confusion amongst the hospital doctors and nurses regarding the choice of whether to administer BZDs in the perioperative period, especially when the patient is at a high risk of delirium. Moreover, in planned cancer surgeries, hospitalization days are often limited, and the tapering and discontinuation of BZDs, which require long‐term adjustment, are difficult. Omichi et al. suggested that the possibility of postoperative delirium itself increases even if BZDs are discontinued before surgery, and they recommend to avoid the abrupt discontinuation of BZDs in the perioperative period. 11 Therefore, at this point, it follows that a relatively safe BZDs measurement in the perioperative period is to keep administering BZDs as just before the surgery and restart after the operation to avoid aggravation of withdrawal symptoms. However, many healthcare workers would at first see this measurement as a possible worsening cause of perioperative delirium, and thus abrupt cessation of BZDs could occur in the perioperative period as in this case, because we are generally taught that BZDs themselves are direct risk factors for delirium. Therefore, we believe that re‐education focusing on BZDs measurements during the postoperative period is essential for all healthcare workers. We underscore that the abrupt BZDs discontinuation should be avoided in the cancer perioperative period, even if BZDs themselves are direct risk factors for perioperative delirium.
Additionally, the presented case was associated with cancer, a life‐threatening event. Although there are several recommended measures for BZDs withdrawal, such as gradual tapering, drug replacement, and long‐term cognitive behavioural therapy, 12 , 13 these all require at least several months, which is not always appropriate for cancer patients in terms of the time limitation of prognosis. Moreover, as other possible alternatives for BZDs, such as carbamazepine, 14 valproic acid, 15 imipramine, 16 pregabalin, gabapentin, and buspirone 12 , 16 have many drug interactions with anticancer medications, 17 these are often difficult to use simultaneously during cancer treatment. On this point, it is possible that lemborexant might be effective in the treatment of delirium and sleep disorder in cancer patients, as in this case. The efficacy rate of lemborexant is reported as 70% for patients with insomnia and hyperactive delirium in cancer patients by regulating sleep–wake rhythms. 18
Currently, we advocate that as soon as cancer is diagnosed, an attempt may well be made to reduce the BZDs dose in collaboration with the family doctor, taking into account the surgical schedule. Furthermore, the most important aspect of BZDs use is avoiding unnecessary prescriptions as much as possible, and based on an appropriate diagnosis, prescribing them for a limited and short period of time. Besides, we believe that re‐education regarding BZDs for all healthcare workers is essential to prevent the aggravation of delirium and avoid severe accidents during the cancer perioperative period.
AUTHOR CONTRIBUTIONS
J.Y. treated the patient, acquired data, drafted the manuscript, and supervised the study. All the authors have read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
ETHICS APPROVAL STATEMENT
Ethics committee approval was not required for this case report.
PATIENT CONSENT STATEMENT
The patient provided written informed consent for the submission of this case report for publication.
CLINICAL TRIAL REGISTRATION
N/A.
ACKNOWLEDGMENTS
The authors thank the patient and his family for their participation in this study.
Yamaguchi J, Sadahiro R, Wada S, Nishikawa E, Terada T, Nakahara R, et al. A case report of benzodiazepine withdrawal delirium due to accidental discontinuation of benzodiazepines in cancer perioperative period. Psychiatry Clin Neurosci Rep. 2024;3:e70026. 10.1002/pcn5.70026
DATA AVAILABILITY STATEMENT
All the data supporting the finding of this article are available within the report.
REFERENCES
- 1. Baandrup L, Ebdrup BH, Rasmussen JØ, Lindschou J, Gluud C, Glenthøj BY. Pharmacological interventions for benzodiazepine discontinuation in chronic benzodiazepine users. Cochrane Database Syst Rev. 2018;3(3):CD011481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chapoutot M, Peter‐Derex L, Bastuji H, Leslie W, Schoendorff B, Heinzer R, et al. cognitive behavioral therapy and acceptance and commitment therapy for the discontinuation of long‐term benzodiazepine use in insomnia and anxiety disorders. Int J Environ Res Public Health. 2021;18(19):10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vasilevskis EE, Han JH, Hughes CG, Ely EW. Epidemiology and risk factors for delirium across hospital settings. Best Pract Res Clin Anaesthesiol. 2012;26(3):277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez NM, Cunningham DJ, Hinton ZW, Wu CJ, Seyler TM. Are patients taking benzodiazepines at increased risk for complications following primary total knee arthroplasty? J Arthroplasty. 2021;36(5):1611–1616. [DOI] [PubMed] [Google Scholar]
- 5. Moss JH, Lanctôt KL. Iatrogenic benzodiazepine withdrawal delirium in hospitalized older patients. J Am Geriatr Soc. 1998;46:1020–1022. [DOI] [PubMed] [Google Scholar]
- 6. Fluyau D, Revadigar N, Manobianco BE. Challenges of the pharmacological management of benzodiazepine withdrawal, dependence, and discontinuation. Ther Adv Psychopharmacol. 2018;8(5):147–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Inada T, Inagaki A. Psychotropic dose equivalence in Japan. Psychiatry Clin Neurosci. 2015;69(8):440–447. [DOI] [PubMed] [Google Scholar]
- 8. Kim S, Haider A, Reddy A, Bruera E. Management challenges at end‐of‐life in a patient with agitated delirium and benzodiazepine withdrawal at comprehensive cancer care center. Ann Palliat Med. 2021;10(6):6979–6983. [DOI] [PubMed] [Google Scholar]
- 9. Biringen EK, Cox‐Martin E, Niemiec S, Wood C, Purcell WT, Kolva E. Psychotropic medications in oncology. Supp Care Cancer. 2021;29(11):6801–6806. [DOI] [PubMed] [Google Scholar]
- 10. Lader M. Benzodiazepine harm: how can it be reduced? Br J Clin Pharmacol. 2014;77(2):295–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Omichi C, Ayani N, Oya N, Matsumoto Y, Tanaka M, Morimoto T, et al. Association between discontinuation of benzodiazepine receptor agonists and post‐operative delirium among inpatients with liaison intervention: a retrospective cohort study. Compr Psychiatry. 2021;104:152216. [DOI] [PubMed] [Google Scholar]
- 12. Soyka M. Treatment of benzodiazepine dependence. N Engl J Med. 2017;376(12):1147–1157. [DOI] [PubMed] [Google Scholar]
- 13. Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med. 2014;174(6):890–898. [DOI] [PubMed] [Google Scholar]
- 14. Denis C, Fatséas M, Lavie E, Auriacombe M. Pharmacological interventions for benzodiazepine mono‐dependence management in outpatient settings. Cochrane Database Syst Rev. 2006;(3):CD005194. 10.1002/14651858.CD005194.pub2 [DOI] [PubMed] [Google Scholar]
- 15. Rickels K, DeMartinis N, Rynn M, Mandos L. Pharmacologic strategies for discontinuing benzodiazepine treatment. J Clin Psychopharmacol. 1999;19(6 Suppl 2):12S–16S. [DOI] [PubMed] [Google Scholar]
- 16. Rickels K, DeMartinis N, García‐España F, Greenblatt DJ, Mandos LA, Rynn M. Imipramine and buspirone in treatment of patients with generalized anxiety disorder who are discontinuing long‐term benzodiazepine therapy. Am J Psychiatry. 2000;157(12):1973–1979. [DOI] [PubMed] [Google Scholar]
- 17. Yap KYL, Tay WL, Chui WK, Chan A. Clinically relevant drug interactions between anticancer drugs and psychotropic agents. Eur J Cancer Care. 2011;20(1):6–32. [DOI] [PubMed] [Google Scholar]
- 18. Terada T, Hirayama T, Sadahiro R, Wada S, Nakahara R, Matsuoka H. Pilot study of lemborexant for insomnia in cancer patients with delirium. J Palliat Med. 2022;25(5):797–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data supporting the finding of this article are available within the report.


