Abstract
Background
Paraquat dichloride is currently among the most widely used commercial herbicides in the USA. In the present study, we provide epidemiological assessment of ambient paraquat exposure and Parkinson’s disease (PD) risk in a population-based study of PD in agricultural regions of Central California.
Methods
Based on 829 PD patients and 824 community controls, we assessed associations between ambient paraquat dichloride exposure and PD. We estimated residential and workplace proximity to commercial agricultural applications in three California counties since 1974 using the CA pesticide use reporting (PUR) data and land use maps. We evaluated any, duration and average intensity [pounds (0.45 kilograms) per acre per year] of exposure for paraquat in four time windows.
Results
Ambient paraquat exposure assessed at both residence and workplace was associated with PD, based on several different exposure measures. The PD patients both lived and worked near agricultural facilities applying greater amounts of the herbicide than community controls. For workplace proximity to commercial applications since 1974, working near paraquat applications every year in the window [odds ratio (OR) = 2.15, 95% confidence interval (CI) = 1.46, 3.19] and a higher average intensity of exposure [per 10 pounds (4.54 kilograms), OR = 2.08, 95% CI = 1.31, 3.38] were both associated with an increased odds of PD. Similar associations were observed for residential proximity (duration: OR = 1.91, 95% CI = 1.30, 2.83; average intensity: OR = 1.72, 95% CI = 0.99, 3.04). Risk estimates were comparable for men and women, and the strongest odds were observed for those diagnosed at ≤60 years of age.
Conclusion
This study provides further indication that paraquat dichloride exposure increases the risk of Parkinson’s disease.
Keywords: Parkinson’s disease, paraquat, California, epidemiology, PUR, agriculture
Key Messages.
This epidemiological study assessed ambient paraquat exposure based on historical pesticide application records and Parkinson’s disease risk, using a population-based case-control approach in agricultural central California.
We assessed associations between paraquat dichloride exposure, estimated based on residential and workplace proximity to commercial agricultural applications in three California counties since 1974, and Parkinson’s disease.
Higher levels of ambient paraquat exposure at either residence or workplace was associated with Parkinson’s disease risk, based on several different exposure measures and exposure windows.
This study provides further evidence that paraquat dichloride exposure increases the risk of Parkinson’s disease.
Introduction
Paraquat dichloride, commonly known as paraquat, is currently one of the most widely used commercial herbicides in the USA.1 It is a quick-acting weed killer also used for desiccation purposes, which acts by killing green plant tissue on contact through inhibiting photosynthesis with redox-cycling activity inducing necrosis.2 Its strong redox-cycling potential has been documented for nearly a century, an activity that is also highly toxic to animals and humans.3 Paraquat can undergo cyclic oxidation/reduction reactions, with each cycle generating a highly reactive superoxide radical.4
Paraquat was initially scrutinized for its potential to cause Parkinson’s disease (PD) due to its structural similarity to MPP+ (1-methyl-4-phenylpyridinium), the toxic metabolite of MPTP (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine), which was found to induce parkinsonism in humans in 1983.5 This was followed by an ecological study in Quebec in 1987, which attributed differences in regional PD prevalence to soil and water contamination from agricultural pesticides, with paraquat being among those most prominently used.6 Since then, at least 10 epidemiological studies have linked exposure to PD and a meta-analysis of 13 case-control studies with 3231 patients and 4901 controls reported exposure to be associated with a 1.64-fold increase in the risk of PD (95% CI = 1.27, 2.13).7 Epidemiological results, however, have not been unequivocal. A recent report from the Agricultural Health Study (AHS) suggested no association,8 contradicting positive associations previously reported in a nested case-control study from the same cohort.9
Experimental research has shown that paraquat crosses the blood-brain barrier10,11 and can enter and accumulate in dopaminergic neurons,12 the cells lost in PD. Furthermore, studies have demonstrated that paraquat can directly cause and exacerbate alpha-synuclein pathology13–17 and lead to degeneration of dopaminergic neurons,18 along with other toxic mechanisms. Yet, even the first study (Barbeau 1987)6 implicating paraquat in PD cautioned that it may not be necessary for environmental agents to reach the substantia nigra to be toxic to the dopaminergic system.
The current study provides a new and expanded epidemiological assessment of ambient paraquat exposure and PD risk in a large population-based case-control study in agricultural central California.
Methods
Study population
To re-assess paraquat and PD associations, we used the Parkinson’s Environment and Genes (PEG) study (n = 829 PD patients; n = 824 controls). PEG is a population-based case-control study conducted in three agricultural counties in central California (Kern, Fresno and Tulare).19 Participants were recruited in two waves: Wave 1 (PEG1): 2000–07, n = 357 patients, n = 400 population-based controls; and Wave 2 (PEG2): 2009–15, n = 472 patients, n = 424 population-based controls. Patients were enrolled early in disease course [mean PD duration at baseline, 3.0 years (SD = 2.6)] and all were seen by University of California, Los Angeles (UCLA) movement disorder specialists for in-person neurological examinations, many on multiple occasions, and confirmed as having probable idiopathic PD.20,21 More information on subject recruitment and PD diagnosis can be found in the Supplementary Material, available as Supplementary data at IJE online.
As shown in Table 1, PD patients were on average slightly older than controls and a higher proportion were men, had European ancestry, and were never smokers.
Table 1.
Study population characteristics
| Variable | Patients with PD (n=829) | Controls (n=824) |
|---|---|---|
| Age, years, mean (SD) | 67.7 (10.6) | 65.9 (11.6) |
| Male sex | 524 (63.2%) | 383 (46.5%) |
| Years of education, mean (SD) | 14 (4.6) | 14 (4.0) |
| European ancestry | 634 (76.5%) | 569 (69.2%) |
| Non-European ancestry | 195 (23.5%) | 253 (30.8%) |
| White | 631 (76.5%) | 569 (69.0%) |
| Latino | 137 (16.6%) | 155 (18.8%) |
| Asian | 22 (2.7%) | 26 (3.2%) |
| Other | 39 (4.7%) | 74 (9.0%) |
| Never smoker | 449 (54.4%) | 397 (48.2%) |
| Former smoker | 345 (41.8%) | 331 (40.2%) |
| Current smoker | 31 (3.8%) | 96 (11.7%) |
PD, Parkinson’s disease.
Paraquat exposure assessment
We estimated ambient exposure due to living or working near agricultural paraquat dichloride application, using pesticide use report (PUR) pesticide application data within a geographical information system (GIS)-based model.22
Since 1972, California law mandates the recording of commercial pesticide use in a database maintained by the California Department of Pesticide Regulation (CA-DPR) that includes all commercial agricultural pesticide use by pest control operators and all restricted pesticide use until 1989, and afterwards (1990–current) all commercial agricultural pesticide use. This database records the location of applications, which can be linked to the Public Land Survey System (PLSS), and the poundage, type of crop, and acreage a pesticide has been applied on, as well as the method and date of application. Figure 1 shows all PUR-reported paraquat application across California and in the tri-county study area since 1974.
Figure 1.
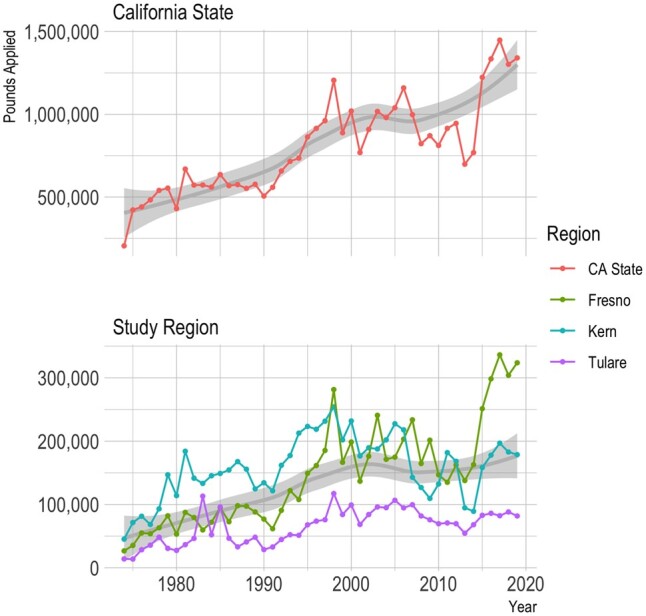
Pesticide Use Report (PUR)-based paraquat application since 1974 by pounds applied. CA, California
We combined the PUR with maps of land use and crop cover, providing a digital representation of historical land use, to determine pesticide applications at specific agricultural sites.23 PEG participants provided lifetime residential and workplace address information, which we geocoded in a multi-step process.24
For each pesticide active ingredient, including paraquat dichloride (CA-DPR ChemCode 1601), as well as glyphosate isopropylamine salt (1855), chlorpyrifos (253), diazinon (198), diquat (229) and dithiocarbamates (see Supplementary Material, available as Supplementary data at IJE online), we determined the pounds of pesticide active ingredient (AI) applied per acre within a 500-m buffer of the latitude and longitude representing each residential and workplace address per year since 1974, weighting the total poundage by the proportion of acreage treated (lbs/acre). For our study participants, this resulted in 57 435 annual records for residential and 44 138 for occupational site paraquat exposure. After we identified and removed several extreme outliers (values >99th percentile of the distribution; Supplementary Figure S1, available as Supplementary data at IJE online), the resulting data ranged from 0 to 41.47 lbs/acre (18.81 kg/acre). Figure 2 shows a scatter plot of the data (Figure 2a) and smoothed trend lines based on local regression (Figure 2b).
Figure 2.
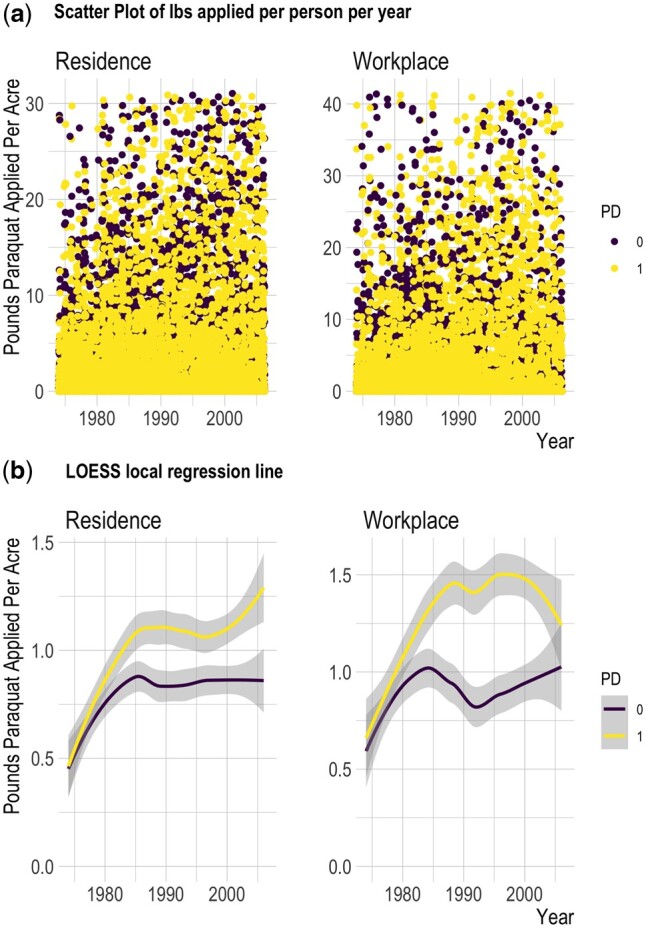
Pounds of agriculturally applied paraquat active ingredient per acre, per subject 1974–2007. (a) Scatter plot of pounds active ingredient applied per acre for each participant each year within 500 m of residential address and workplace address. (b) Smoothed trend line from loess local regression based on data shown in plots above. Lb, pounds; LOESS, locally estimated scatterplot smoothing
We report on the following exposure measures for both residential and workplace addresses:
any pesticide application within 500 m of the address within the exposure time window (yes/no);
duration of exposure: the number of years participants lived or worked within 500 m of agricultural applications as a proportion of the time covered by the window (i.e. no. of years with any application in buffer/no. of years in window);
-
average exposure intensity: yearly average pounds of active ingredient applied per acre in the time window (i.e.
where AI = active ingredient, y = the first year in the window, and t = last year in the window).
The denominator (no. of years in the exposure window) represents the number of years for which the participant provided an address that could be geocoded and linked to the pesticide exposure model (i.e. years with exposure information). We also log transformed the average exposure estimates, offset by one. We present associations for the average exposure intensity with and without log transformation. Duration of exposure is presented as a proportion.
We considered four exposure windows for risk assessment: (i) 1974 to index date (PD diagnosis for cases or interview for controls); (ii) 1974 to 10 years prior to index date, i.e. lagged 10 years; (iii) 20 years to 10 years prior to index date; (iv) 10 years prior to index date. By design, the exposure windows covered a very similar length and calendar period on average for patients and controls of each wave (Supplementary Table S1, available as Supplementary data at IJE online). The exposure windows ranged from 8.3 years on average (SD = 2.4) to 31.3 years (SD = 5.2). There was some correlation between exposures across these windows (Supplementary Figure S2, available as Supplementary data at IJE online). Thus, we present 1974–index year exposure results; the 1974–index year with 10-y lag window results are shown in the Supplement only.
Statistical methods
To assess exposure associations, we conducted univariate, unconditional logistic regression to calculate odds ratios (ORs) and 95% confidence intervals (CIs) for PD and estimated paraquat exposure for each time window and each location separately. We controlled for age, gender, race/ethnicity, study wave and index year (year of diagnosis or interview) to account for temporal trends in pesticide use. We also estimated associations stratified by gender (men and women) and index age (≤60 and >60 years at diagnosis for cases or interview for controls).
We conducted sensitivity analyses to adjust for potential co-exposures. As most participants did not solely live or work near facilities solely applying paraquat, we also controlled for exposure to chlorpyrifos, glyphosate isopropylamine salt and diazinon (yearly average lbs/acre). These pesticides were selected for their widespread agricultural use and/or because the PEG study has associated them with PD. We also controlled for occupational use of pesticides and co-adjusted for ambient workplace paraquat exposures in the residential models and residential exposures in the workplace models. We additionally provide results from sensitivity analyses both controlling for and stratifying by smoking status, county, dithiocarbamate exposure, diquat exposure, household pesticide use based on self-report, and results stratifying by study wave.
Results
We observed positive associations between paraquat and PD across exposure measures and time windows (Table 2). For example, for paraquat exposure assessed at residential addresses between 1974 and index year (a 31.3-year exposure window on average), living near paraquat applications every year in the exposure window was associated with an 91% increase in the odds of PD (95% CI = 1.30, 2.83) and a yearly average exposure intensity of 10 pounds (4.54 kilograms) of paraquat applied per acre per year increased the odds by 72% (OR = 1.72, 95% CI = 0.99, 3.04; log transformed average exposure, OR = 1.23, 95% CI = 1.04, 1.46). Somewhat stronger associations were observed for workplace proximity to paraquat applications, with higher duration of exposure (exposed every year in exposure window, OR = 2.15, 95% CI = 1.46, 3.19) and exposure intensity (OR = 2.08 per 10 lbs/acre, 95% CI = 1.31, 3.38; log transformed exposure, OR = 1.32, 95% CI = 1.12, 1.55) also increasing the odds of PD.
Table 2.
ORs (95% CI) for paraquat exposure and Parkinson’s disease estimated with logistic regression, according to type of exposure assessment
| Exposure assessment and time window | Patients with PD | Controls | OR (95% CI) | P | Patients with PD | Controls | OR (95% CI) | P | |
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| n (%) or mean (SD) | n (%) or mean (SD) | ||||||||
|
| |||||||||
| Application near | Residence | Workplace | |||||||
| Any application, yes | |||||||||
| 1974–index year | 591 (71.7) | 577 (70.3) | 1.10 (0.88, 1.37) | 0.41 | 551 (68.9) | 500 (63.9) | 1.23 (0.99, 1.53) | 0.06 | |
| 20y–10y prior to index year | 423 (51.6) | 363 (44.4) | 1.40 (1.14, 1.72) | 0.001 | 382 (49.9) | 305 (42.8) | 1.38 (1.11, 1.72) | 0.004 | |
| 10 y prior to index year | 347 (42.3) | 290 (35.5) | 1.37 (1.11, 1.69) | 0.003 | 230 (34.2) | 201 (33.8) | 1.14 (0.89, 1.46) | 0.48 | |
| Duration of exposure, per one (equivalent to living near paraquat application within buffer every year in window) | |||||||||
| 1974–index year | 0.23 (0.28) | 0.19 (0.24) | 1.91 (1.30, 2.83) | 0.001 | 0.24 (0.29) | 0.18 (0.24) | 2.15 (1.46, 3.19) | 1.3E-04 | |
| 20y–10y prior to index year | 0.25 (0.33) | 0.19 (0.30) | 1.83 (1.32, 2.53) | 3.0E-04 | 0.25 (0.34) | 0.18 (0.29) | 2.15 (1.53, 3.06) | 1.5E-05 | |
| 10y prior to index year | 0.20 (0.31) | 0.15 (0.28) | 1.67 (1.18, 2.36) | 0.004 | 0.19 (0.33) | 0.14 (0.27) | 1.86 (1.27, 2.77) | 0.002 | |
| Average exposure, per 10 pounds paraquat active ingredient applied per acre per year within buffera | |||||||||
| 1974–index year | 1.02 (1.92) | 0.83 (1.72) | 1.72 (0.99, 3.04) | 0.06 | 1.25 (2.52) | 0.88 (2.01) | 2.08 (1.31, 3.38) | 0.002 | |
| 20y–10y prior to index year | 1.20 (2.70) | 0.90 (2.36) | 1.61 (1.07, 2.44) | 0.02 | 1.51 (3.52) | 0.90 (2.56) | 1.96 (1.54, 2.89) | 5.2E-04 | |
| 10y prior to index year | 1.20 (3.00) | 0.91 (2.55) | 1.35 (0.94, 1.97) | 0.11 | 1.61 (4.26) | 1.08 (3.64) | 1.49 (1.11, 2.05) | 0.01 | |
| Average exposure, log transformed, per oneb | |||||||||
| 1974–index year | 0.46 (0.62) | 0.39 (0.56) | 1.23 (1.04, 1.46) | 0.02 | 0.49 (0.69) | 0.37 (0.60) | 1.32 (1.12, 1.55) | 8.6E-04 | |
| 20y–10y prior to index year | 0.44 (0.71) | 0.35 (0.62) | 1.24 (1.07, 1.45) | 0.006 | 0.48 (0.78) | 0.32 (0.64) | 1.37 (1.18, 1.60) | 6.6E-05 | |
| 10y prior to index year | 0.40 (0.72) | 0.32 (0.65) | 1.19 (1.03, 1.38) | 0.02 | 0.42 (0.83) | 0.30 (0.69) | 1.27 (1.09, 1.49) | 0.003 | |
Models control for age, race/ethnicity, sex, index year and study wave.
PD, Parkinson’s disease; CI, confidence interval; OR, odds ratio; y, years.
After outlier removal, the pounds of paraquat applied per acre in any given year ranged from 0 to 41.47 (Figure 2).
Average exposure (year average paraquat applied per acre exposure assessment) was offset by 1 and log transformed. Values ranged from 0 to 3.4.
Overall, most of the study population lived and worked within 500 m of at least one paraquat application since 1974 (residence: 72% of patients and 70% of controls; workplace: 69% of patients and 64% of controls; Table 2). The difference in the proportion of patients who lived near agricultural paraquat applications versus controls was greater in the 10 years prior to index (42% versus 36%; OR = 1.37, 95% CI = 1.11, 1.69) and between 20 and 10 years prior to index (52% versus 44%; OR = 1.40, 95% CI = 1.14, 1.72). Also, a higher proportion of patients worked near paraquat applications than controls in all windows except the 10 years prior to diagnosis. This includes the period 20 to 10 years prior to diagnosis (50% versus 43%; OR = 1.38, 95% CI = 1.11, 1.72). Over this time window, patients lived near agricultural fields or facilities applying paraquat for 25% of the years on average compared with the controls’ 19%. Living near paraquat applications every year in the exposure window was associated with an 83% increase in the odds of PD (95% CI = 1.32, 2.53; Table 2). Similar results were observed based on workplace exposure assessments for other time windows and for average exposure intensity (pounds applied per acre).
Risk estimates stratified by gender were generally similar, with somewhat stronger associations for workplace exposures in men (Table 3). Risk estimates were higher among those ≤60 than >60 years of age at index date. For example, living near paraquat applications every year from 1974 to index year increased the odds 3.78-fold among those ≤60 (95% CI = 1.52, 9.56) and 1.68-fold among those >60 years (95% CI = 1.09, 2.60). Such differences were also observed for other exposure measures and time windows (Table 3). Supplementary Table S2, available as Supplementary data at IJE online, shows results for the lagged exposure time window.
Table 3.
ORs (95% CI) for paraquat exposure and Parkinson’s disease estimated with logistic regression, stratified by sex and age
| Men |
Women |
Age at index ≤60 years |
Age at index >60 years |
|||||
|---|---|---|---|---|---|---|---|---|
| Exposure assessment and time window | Patients with PD (n=519) | Controls (n=380) | Patients with PD (n=301) | Controls (n=437) | Patients with PD (n=195) | Controls (n=260) | Patients with PD (n=625) | Controls (n=557) |
|
|
|
|
|
|||||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |||||
| Application near | Residence | |||||||
|
| ||||||||
| Any application, yes | ||||||||
| 1974–index year | 1.00 (0.74, 1.35) | 1.22 (0.88, 1.71) | 1.58 (0.99, 2.55) | 1.01 (0.78, 1.30) | ||||
| 20y–10y prior to index year | 1.33 (1.02, 1.76) | 1.48 (1.09, 2.02) | 1.72 (1.13, 2.62) | 1.34 (1.06, 1.70) | ||||
| 10y prior to index year | 1.27 (0.96, 1.68) | 1.54 (1.12, 2.12) | 2.17 (1.42, 3.35) | 1.18 (0.92, 1.50) | ||||
| Duration of exposure, per one unit (equivalent to living near paraquat application within buffer every year in window) | ||||||||
| 1974–index year | 1.76 (1.07, 2.92) | 2.26 (1.20, 4.26) | 3.78 (1.52, 9.56) | 1.68 (1.09, 2.60) | ||||
| 20y–10y prior to index year | 1.78 (1.16, 2.74) | 1.96 (1.17, 3.30) | 2.46 (1.18, 5.18) | 1.71 (1.19, 2.49) | ||||
| 10y prior to index year | 1.55 (1.00, 2.41) | 1.98 (1.11, 3.54) | 2.99 (1.43, 6.36) | 1.44 (0.97, 2.16) | ||||
| Average exposure, per 10 pounds on average paraquat active ingredient applied per acre per year within buffer | ||||||||
| 1974–index year | 1.79 (0.90, 3.68) | 1.73 (0.65, 4.59) | 4.29 (1.15, 16.71) | 1.49 (0.81, 2.79) | ||||
| 20y–10y prior to index year | 1.58 (0.94, 2.73) | 1.77 (0.90, 3.50) | 2.83 (1.21, 6.92) | 1.44 (0.91, 2.32) | ||||
| 10y prior to index year | 1.48 (0.96, 2.36) | 1.11 (0.55, 2.23) | 3.29 (1.38, 8.76) | 1.13 (0.75, 1.71) | ||||
| Average exposure, log transformed, per one unit | ||||||||
| 1974–index year | 1.21 (0.97, 1.51) | 1.29 (0.97, 1.72) | 1.49 (1.03, 2.18) | 1.18 (0.97, 1.44) | ||||
| 20y–10 y prior to index year | 1.22 (1.00, 1.49) | 1.32 (1.03, 1.69) | 1.36 (0.99, 1.88) | 1.22 (1.02, 1.46) | ||||
| 10y prior to index year | 1.19 (0.99, 1.44) | 1.20 (0.94, 1.54) | 1.54 (1.13, 2.13) | 1.11 (0.94, 1.32) | ||||
|
| ||||||||
| Application near | Workplace | |||||||
|
| ||||||||
| Any application, yes | ||||||||
| 1974–index year | 1.27 (0.94, 1.70) | 1.19 (0.86, 1.66) | 1.44 (0.92, 2.27) | 1.14 (0.88, 1.47) | ||||
| 20y–10y prior to index year | 1.45 (1.09, 1.94) | 1.31 (0.94, 1.85) | 1.39 (0.90, 2.14) | 1.33 (1.02, 1.72) | ||||
| 10y prior to index year | 1.37 (0.99, 1.96) | 0.91 (0.61, 1.35) | 1.08 (0.70, 1.68) | 1.24 (0.91, 1.69) | ||||
| Duration of exposure, per one unit (equivalent to living near paraquat application within buffer every year in window) | ||||||||
| 1974–index year | 2.45 (1.47, 4.11) | 1.84 (0.99, 3.43) | 3.61 (1.42, 9.39) | 1.84 (1.19, 2.86) | ||||
| 20y–10y prior to index year | 2.43 (1.56, 3.87) | 1.92 (1.10, 3.35) | 3.84 (1.83, 8.23) | 1.77 (1.19, 2.64) | ||||
| 10y prior to index year | 2.33 (1.40, 3.95) | 1.38 (0.74, 2.58) | 1.96 (0.92, 4.21) | 1.92 (1.21, 3.09) | ||||
| Average exposure, per 10 pounds on average paraquat active ingredient applied per acre per year within buffer | ||||||||
| 1974–index year | 2.42 (1.33, 4.58) | 1.78 (0.82, 3.93) | 3.92 (1.43, 11.46) | 1.71 (1.01, 2.99) | ||||
| 20y–10y prior to index year | 2.01 (1.27, 3.32) | 1.96 (1.04, 3.81) | 4.06 (1.84, 9.85) | 1.57 (1.04, 2.45) | ||||
| 10y prior to index year | 1.86 (1.21, 3.00) | 1.24 (0.80, 1.94) | 1.63 (0.96, 2.86) | 1.49 (1.02, 2.22) | ||||
| Average exposure, log transformed, per one unit | ||||||||
| 1974–index year | 1.40 (1.14, 1.72) | 1.23 (0.94, 1.61) | 1.47 (1.06, 2.05) | 1.26 (1.05, 1.53) | ||||
| 20y–10y prior to index year | 1.42 (1.17, 1.73) | 1.32 (1.02, 1.72) | 1.69 (1.24, 2.32) | 1.26 (1.06, 1.51) | ||||
| 10y prior to index year | 1.42 (1.16, 1.76) | 1.10 (0.84, 1.42) | 1.30 (0.99, 1.72) | 1.29 (1.06, 1.57) | ||||
Models control for age, race/ethnicity, sex (except when sex stratified), index year and study wave.
PD, Parkinson’s disease; CI, confidence interval; OR, odds ratio; y, years.
Associations were generally robust to including other pesticide exposures in the models, including adding an indicator for occupational pesticide use, adjusting for residential or workplace proximity to chlorpyrifos, diazinon and glyphosate applications and co-adjusting for ambient workplace paraquat exposures in the residential models and residential exposures in the workplace models, though estimates showed some variability (Table 4). There was an attenuation of residential paraquat exposure odds ratios when including ambient workplace exposures in the model; for residential average exposure estimates when adjusting for proximity to chlorpyrifos, diazinon and glyphosate applications; and for workplace, but not residential, exposure estimates when adjusting for dithiocarbamate exposure (Supplementary Table S3, available as Supplementary data at IJE online).
Table 4.
ORs (95% CI) for paraquat exposure and Parkinson’s disease estimated with logistic regression, with different multi-pesticide co-exposure adjustments
| Exposure assessment and time window | Adjusted for any reported occupational pesticide use |
Adjusted for ambient chlorpyrifos, diazinon and glyphosate |
Mutually adjusted for paraquat co-exposure at the other location |
|||
|---|---|---|---|---|---|---|
| Residence | Workplace | Residence | Workplace | Residence | Workplace | |
| OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Any application, yes | ||||||
| 1974–index year | 1.07 (0.85, 1.32) | 1.19 (0.95, 1.48) | 0.99 (0.78, 1.26) | 1.12 (0.88, 1.42) | 1.01 (0.79, 1.29) | 1.21 (0.96, 1.53) |
| 20y–10y prior to index year | 1.36 (1.11, 1.67) | 1.34 (1.08, 1.67) | 1.31 (1.03, 1.67) | 1.32 (1.01, 1.71) | 1.36 (1.07, 1.73) | 1.21 (0.95, 1.54) |
| 10y prior to index year | 1.32 (1.07, 1.64) | 1.10 (0.85, 1.42) | 1.28 (0.99, 1.64) | 1.04 (0.78, 1.38) | 1.46 (1.13, 1.89) | 0.99 (0.76, 1.30) |
| Duration of exposure, per one unit | ||||||
| 1974–index year | 1.75 (1.18, 2.61) | 1.98 (1.33, 2.97) | 1.92 (1.10, 3.38) | 2.24 (1.31, 3.88) | 1.27 (0.77, 2.08) | 1.85 (1.14, 3.03) |
| 20y–10y prior to index year | 1.72 (1.24, 2.40) | 2.01 (1.41, 2.87) | 1.82 (1.17, 2.85) | 2.69 (1.66, 4.41) | 1.33 (0.88, 2.02) | 1.82 (1.21, 2.76) |
| 10 y prior to index year | 1.55 (1.09, 2.20) | 1.74 (1.17, 2.60) | 1.50 (0.97, 2.35) | 1.96 (1.22, 3.20) | 1.44 (0.95, 2.22) | 1.62 (1.06, 2.50) |
| Average exposure, per 10 pounds | ||||||
| 1974–index year | 1.50 (0.85, 2.67) | 1.95 (1.22, 3.19) | 1.21 (0.60, 2.50) | 1.89 (1.06, 3.43) | 1.07 (0.56, 2.06) | 2.03 (1.20, 3.50) |
| 20 y–10y prior to index year | 1.50 (1.00, 2.29) | 1.85 (1.27, 2.74) | 1.30 (0.80, 2.15) | 2.06 (1.33, 3.30) | 1.24 (0.77, 2.00) | 1.83 (1.23, 2.77) |
| 10y prior to index year | 1.25 (0.87, 1.83) | 1.43 (1.06, 1.97) | 1.11 (0.72, 1.71) | 1.47 (1.05, 2.08) | 1.14 (0.75, 1.77) | 1.46 (1.07, 2.04) |
| Average exposure, log transformed, per one unit | ||||||
| 1974–index year | 1.18 (0.99, 1.40) | 1.28 (1.09, 1.51) | 1.13 (0.89, 1.44) | 1.30 (1.04, 1.63) | 1.05 (0.86, 1.29) | 1.29 (1.07, 1.55) |
| 20y–10y prior to index year | 1.21 (1.04, 1.41) | 1.33 (1.14, 1.56) | 1.17 (0.96, 1.43) | 1.47 (1.20, 1.81) | 1.10 (0.92, 1.32) | 1.31 (1.11, 1.56) |
| 10y prior to index year | 1.15 (0.99, 1.34) | 1.24 (1.05, 1.45) | 1.10 (0.92, 1.32) | 1.28 (1.06, 1.54) | 1.10 (0.92, 1.31) | 1.24 (1.05, 1.47) |
Models control for age, race/ethnicity, sex, index year and study wave.
CI, confidence interval; OR, odds ratio; y, years.
Results were also generally similar when controlling for smoking and across strata of smoking status (Supplementary Table S4), study waves (Supplementary Table S5), when controlling for county of residence (Supplementary Table S6), diquat pesticide exposure (Supplementary Table S7) or household pesticide use (Supplementary Table S8, all Supplementary tables available as Supplementary data at IJE online).
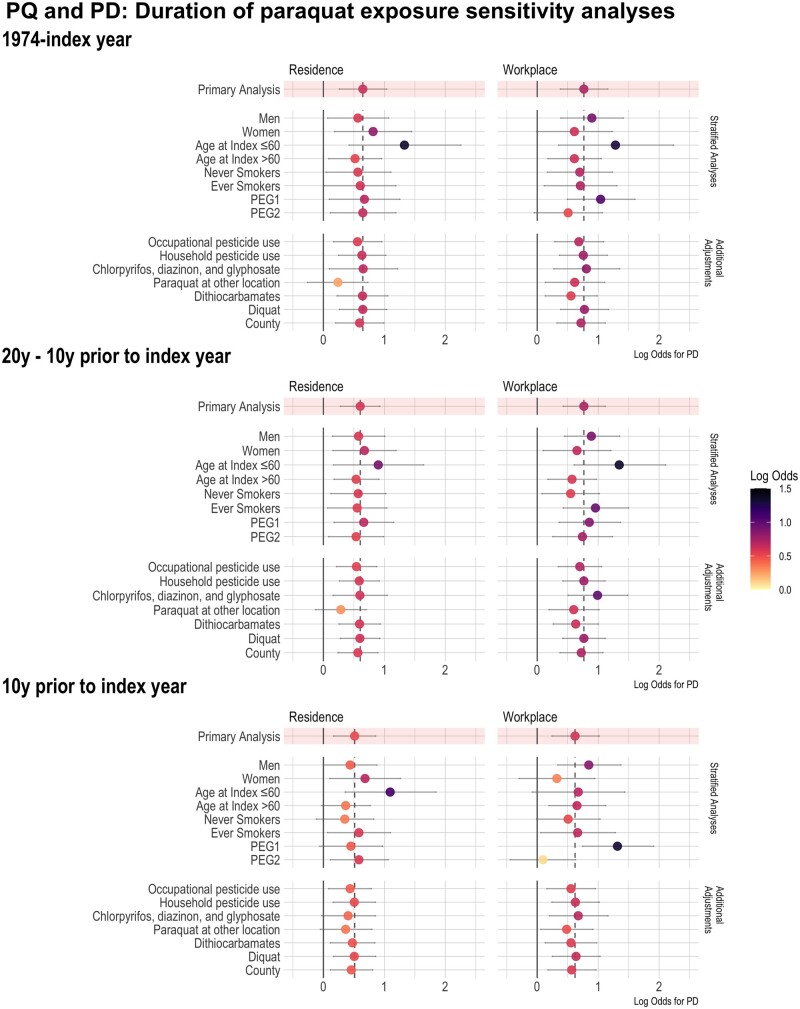
Figure 3 shows the log odds for the duration of paraquat exposure and PD across all time windows, including all sensitivity analyses to visualize similarities to and differences from results shown in Table 2.
Figure 3.
Results of all sensitivity analyses across all time windows and locations (residence or workplace) with paraquat exposure duration and Parkinson’s disease (PD) risk. PQ, paraquat; PD, Parkinson’s disease; PEG, Parkinson’s Environment & Genes Study; y, year
Discussion
In this population-based case-control study of Parkinson’s disease, we investigated ambient paraquat exposure estimated via residential and workplace proximity to agricultural paraquat application. Paraquat was associated with an increased risk of PD for multiple exposure assessment measures (any exposure, duration of exposure and average exposure intensity) and across multiple exposure windows, ranging from 8 to 31 years on average. Overall, relative to controls, a higher proportion of PD patients either lived and/or worked near commercial paraquat application, which was predominantly related to agriculture in our study region. Applications near patients’ addresses were on average greater in terms of the amount applied (pounds of active ingredient applied per acre) and took place for a longer duration (proportion of years in the time windows with exposure) than controls. Exposure associations were strongest for younger-onset patients (≤60 at diagnosis, replicating previous results seen in the PEG1 study with data from the PEG2 study wave).25 Furthermore, while risks were increased in all exposure windows, exposures in the decades prior to diagnosis, including the period 10–20 years prior, were associated with the strongest risks, perhaps suggesting this is a relevant time period in exposure-related PD pathogenesis.
We performed additional sensitivity analyses, such as controlling for pesticide co-exposures and occupational or household pesticide use. Across the time windows and exposure measures we considered, there were some fluctuations from attenuation to strengthening, but overall minimal change in most effect estimates. We have previously reported positive associations between diquat exposure and PD,26 and we find both bipyridyl herbicides (diquat and paraquat) to be independently associated with PD in this population.
This research adds to an existing body of literature that has connected paraquat to PD. Since initial scrutiny due to structural similarity to MPP+, which was found to induce parkinsonism in humans in 19835 and the Quebec ecological study linking agricultural use to PD,6 at least 13 case-control studies and one prospective cohort have investigated paraquat exposure. A recent systematic review and meta-analysis reported a summary estimate for PD and paraquat of OR = 1.64 (95% CI = 1.27, 2.13) in the 13 case-control studies.7 At the same time, experimental research has linked exposure to dopaminergic neuron toxicity and α-synuclein related biology among other mechanisms.10–18 Still, epidemiological results have not been unanimous and a recent report from the longitudinal Agricultural Health Study (AHS) has suggested no association.8 Loss to follow-up, however, may have affected the AHS results, as identification of PD cases mainly depended on self-report or death records (known for under-reporting PD) and only 46% of initially enrolled male farmers had not dropped out by the end of follow-up.8 The AHS cohort study results also contradict strongly positive associations reported for paraquat and PD by the Farming and Movement Evaluation study (FAME) PD case-control study nested within the AHS,9 leaving open questions about accurate self-reporting of pesticide use and selection bias.
Our study makes use of agricultural pesticide application records with use of reporting required by law in California to determine the amount of paraquat active ingredient applied within 500 m of participants’ lifetime reported residential and workplace addresses. This allowed us to assess exposures as far back as 1974 without relying on participant recall. This is particularly important for PD, where exposures decades prior to diagnosis are likely relevant. Although paraquat was introduced in the 1960s, we did not extrapolate backwards past 1974 due to concerns about generating exposure misclassification and relying on unfounded assumptions about application patterns in the 1960s. With our record-based exposure assessment, we were able to greatly minimize recall bias and differential exposure misclassification, while still investigating historical exposures. Furthermore, PD is a commonly misdiagnosed disorder with estimates of misdiagnosis ranging 20–30%.27 All our patients were seen in person by the same UCLA movement disorder specialists to confirm diagnosis through clinical characteristics, thus minimizing outcome misclassification which is more common when relying on self-report of PD or medical records.
Historically, paraquat has been applied by ground, aerial and backpack sprayer methods. It is persistent in the soil environment, with field studies reporting a half-life ranging from 1.4 to 7.2 years.28 It also strongly binds to certain soils, which limits the threat of groundwater contamination.28 However, this property may also increase the risk of contaminated soil being blown or tracked to nearby locations, such as homes. Furthermore, prior to any recent United States Environmental Protection Agency restrictions of aerial applications and during the exposure windows of interest for the PEG study, paraquat has been detected in airborne samples up to 1600 m from single field aerial applications in the San Joaquin Valley in California.29,30 Other studies of paraquat drift have demonstrated foliage damage extending over a 805 m (0.5 mile) from fields into a neighbouring community.31 Our exposure buffer was limited to 500 m. We previously conducted a validation study demonstrating that PUR model-derived organochlorine estimates of exposure within 500 m of homes had high specificity in predicting blood-measured DDE (dichlorodiphenyldichloroethylene) levels.32 Pesticide air monitoring studies conducted by California Environmental Protection Agency in a small community over a 1-year period have also shown very high overlap between the PUR reported poundage applied for several different pesticides and the concurrently measured values obtained from monitors placed on the rooftops of elementary schools in these communities.33
Still, it should be noted that our exposure assessment method did not account for potentially relevant factors, such as wind patterns at the time of application, geographical features that may influence pesticide drift, or type of residence or workplace. Our method also assumes that the participant was at the recorded location during the exposure relevant time or that the agent was still active and exposed residents after application had occurred. Exposure misclassification would likely be non-differential to case status, however, and the resulting bias moves estimates towards the null.
Additionally, the reality of commercial agriculture is that many different pesticides are applied on the same fields in seasonal patterns year after year. Thus, participants with residential or workplace proximity to paraquat application also lived and worked in proximity to other pesticide applications. We performed a series of co-adjustments for; (i) other commonly applied pesticides, including chlorpyrifos, diazinon and glyphosate; (ii) pesticides previously related to PD risk (dithiocarbamates); (iii) diquat, another bipyridyl herbicide belonging to quaternary ammonium compounds and structurally similar34; (iv) occupational pesticide use; and (v) household pesticide use. Although there was some attenuation of risk estimates, particularly for some residential exposures with chlorpyrifos, diazinon and glyphosate, and ambient workplace exposures with dithiocarbamate exposure, paraquat was consistently positively associated with Parkinson’s disease for different exposure measures and windows. In fact, duration and average intensity of ambient exposure at workplaces even showed stronger associations in multi-pesticide adjusted models. There are several possibilities which may lead to attenuation of estimates aside from confounding, including a reduced ability to estimate single exposure effects due to strong collinearity, a concern here as the correlation between paraquat and other pesticides was often >0.5, and sample size-related random fluctuation of estimates when adding covariates. As real-world co-exposures are the norm and not the exception, due to intensive and changing agricultural pesticide use, co-exposure mixtures should be investigated to evaluate their toxicity as a part of policy and regulation.
Conclusion
Overall, this study provides further evidence that paraquat exposure increases the risk of Parkinson’s disease.
Ethics approval
The study is approved by the UCLA IRB (IRB#11–001530).
Supplementary Material
Acknowledgements
The authors thank the staff members and participants in the Parkinson’s Environment and Genes (PEG) Study.
Contributor Information
Kimberly C Paul, Department of Neurology, UCLA David Geffen School of Medicine, Los Angeles, CA, USA.
Myles Cockburn, Department of Population and Public Health Sciences, Keck School of Medicine, University of Southern California, Los Angeles, CA, USA.
Yufan Gong, Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, USA.
Jeff Bronstein, Department of Neurology, UCLA David Geffen School of Medicine, Los Angeles, CA, USA.
Beate Ritz, Department of Neurology, UCLA David Geffen School of Medicine, Los Angeles, CA, USA; Department of Epidemiology, UCLA Fielding School of Public Health, Los Angeles, CA, USA.
Data availability
The data underlying this article will be shared on reasonable request to the corresponding author. Analysis code is provided on github: [https://github.com/KCPaul-lab/IJE_PD-PQ.git].
Supplementary data
Supplementary data are available at IJE online.
Author contributions
Concept and design: K.C.P.., B.R., J.B., M.C. Acquisition, analysis, or interpretation of data: all authors. Drafting of the manuscript: K.C.P. Critical revision of the manuscript for important intellectual content: all authors. Statistical analysis: K.C.P., Y.G. Obtained funding: K.C.P., B.R., J.B., M.C. Supervision: K.C.P., B.R.. Final approval of the version to be published: all authors. Agreement to be accountable for all aspects of the work: all authors.
Funding
National Institute of Environmental Health Science (grant number 2R01ES010544, R56ES026600).
Conflict of interest
B.R. has been retained as an expert consultant for the plaintiff in a lawsuit against Syngenta Crop Protection LLC on the role of paraquat in Parkinson’s disease causation. M.C. has been retained as an expert consultant for the plaintiff in a lawsuit against Syngenta Crop Protection LLC on the role of paraquat in Parkinson’s disease causation, and on methods for assessing pesticide exposure. The remaining authors declare no competing interests.
References
- 1. United States Environmental Protection Agency. Paraquat Dichloride. 2022. https://www.epa.gov/ingredients-used-pesticide-products/paraquat-dichloride (10 January 2024, date last accessed).
- 2. Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect 1984;55:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dinis-Oliveira RJ, Duarte JA, Sánchez-Navarro A, Remião F, Bastos ML, Carvalho F. Paraquat poisonings: mechanisms of lung toxicity, clinical features, and treatment. Crit Rev Toxicol 2008;38:13–71. [DOI] [PubMed] [Google Scholar]
- 4. Peterson ME, Talcott PA. Small Animal Toxicology, 3rd ed. St. Louis, Missouri, USA: Elsevier Saunders, 2012;756–58. [Google Scholar]
- 5. Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic parkinsonism in humans due to a product of meperidine-analog synthesis. Science 1983;219:979–80. [DOI] [PubMed] [Google Scholar]
- 6. Barbeau A, Roy M, Bernier G, Campanella G, Paris S. Ecogenetics of Parkinson’s disease: prevalence and environmental aspects in rural areas. Can J Neurol Sci 1987;14:36–41. [DOI] [PubMed] [Google Scholar]
- 7. Tangamornsuksan W, Lohitnavy O, Sruamsiri R et al. Paraquat exposure and Parkinson’s disease: a systematic review and meta-analysis. Arch Environ Occup Health 2019;74:225–38. [DOI] [PubMed] [Google Scholar]
- 8. Shrestha S, Parks CG, Umbach DM et al. Pesticide use and incident Parkinson’s disease in a cohort of farmers and their spouses. Environ Res 2020;191:110186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tanner CM, Kame F, Ross GW et al. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect 2011;119:866–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McCormack AL, Monte DD. Effects of L-dopa and other amino acids against paraquat-induced nigrostriatal degeneration. J Neurochem 2003;85:82–86. [DOI] [PubMed] [Google Scholar]
- 11. Shimizu K, Ohtaki K, Matsubara K et al. Carrier-mediated processes in blood-brain barrier penetration and neural uptake of paraquat. Brain Res 2001;906:135–42. [DOI] [PubMed] [Google Scholar]
- 12. Rappold PM, Cui M, Chesser AS et al. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci USA 2011;108:20766–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of α-synuclein fibril formation: a possible factor in Parkinson’s disease. FEBS Lett 2001;500:105–108. [DOI] [PubMed] [Google Scholar]
- 14. Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Monte DD. The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice: paraquat and α-synuclein. J Biol Chem 2002;277:1641–44. [DOI] [PubMed] [Google Scholar]
- 15. Wills J, Credle J, Oaks AW et al. Paraquat, but not maneb, induces synucleinopathy and tauopathy in striata of mice through inhibition of proteasomal and autophagic pathways. PLoS One 2012;7:e30745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Naudet N, Antier E, Gaillard D et al. Oral exposure to paraquat triggers earlier expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. J Neuropathol Exp Neurol 2017;76:1046–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Musgrove RE, Helwig M, Bae EJ et al. Oxidative stress in vagal neurons promotes parkinsonian pathology and intercellular α-synuclein transfer. J Clin Invest 2019;129:3738–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCormack AL, Thiruchelvam M, Manning-Bog AB et al. Environmental risk factors and Parkinson’s disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis 2002;10:119–27. [DOI] [PubMed] [Google Scholar]
- 19. Ritz BR, Paul KC, Bronstein JM. Of pesticides and men: a California story of genes and environment in Parkinson’s disease. Curr Environ Health Rep 2016;3:40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hughes AJ, Ben-Shlomo Y, Daniel SE, Lees A J. What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 1992;42:1142–46. [DOI] [PubMed] [Google Scholar]
- 21. Postuma RB, Berg D, Stern M et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30:1591–601. [DOI] [PubMed] [Google Scholar]
- 22. Cockburn M, Mills P, Zhang X, Zadnick J, Goldberg D, Ritz B. Prostate cancer and ambient pesticide exposure in agriculturally intensive areas in California. Am J Epidemiol 2011;173:1280–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. CDWR. California Department of Water Resources Land Use Surveys. 2013. https://water.ca.gov/programs/water-use-and-efficiency/land-and-water-use/land-use-surveys (28 July 2015, date last accessed).
- 24. McElroy JA, Remington PL, Trentham-Dietz A, Robert SA, Newcomb PA. Geocoding addresses from a large population-based study: lessons learned. Epidemiology 2003;14:399–407. [DOI] [PubMed] [Google Scholar]
- 25. Costello S, Cockburn M, Bronstein J, Zhang X, Ritz B. Parkinson’s disease and residential exposure to Maneb and Paraquat from agricultural applications in the central valley of California. Am J Epidemiol 2009;169:919–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Paul KC, Krolewski RC, Lucumi Moreno E et al. A pesticide and iPSC dopaminergic neuron screen identifies and classifies Parkinson-relevant pesticides. Nat Commun 2023;14:2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Poewe W, Wenning G. The differential diagnosis of Parkinson’s disease. Eur J Neurol 2002;9(Suppl 3):23–30. [DOI] [PubMed] [Google Scholar]
- 28. Roede JR, Miller GW. Paraquat. In: Wexler P (ed). Encyclopedia of Toxicology . 3rd ed. London, UK: Academic Press, 2014. [Google Scholar]
- 29. Chester G, Ward RJ. Occupational exposure and drift hazard during aerial application of paraquat to cotton. Arch Environ Contam Toxicol 1984;13:551–63. [DOI] [PubMed] [Google Scholar]
- 30. Seiber JN, Woodrow JE. Sampling and analysis of airborne residues of paraquat in treated cotton field environments. Arch Environ Contam Toxicol 1981;10:133–49. [DOI] [PubMed] [Google Scholar]
- 31. Ames RG, Howd RA, Doherty L. Community exposure to a paraquat drift. Arch Environ Health 1993;48:47–52. [DOI] [PubMed] [Google Scholar]
- 32. Ritz B, Costello S. Geographic model and biomarker-derived measures of pesticide exposure and Parkinson’s disease. Ann N Y Acad Sci 2006;1076:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wofford P, Segawa R, Schreider J, Federighi V, Neal R, Brattesani M. Community air monitoring for pesticides. Part 3: using health-based screening levels to evaluate results collected for a year. Environ Monit Assess 2014;186:1355–70. [DOI] [PubMed] [Google Scholar]
- 34. Eddleston M. Paraquat and diquat. In: Brent J, et al. (eds). Critical Care Toxicology: Diagnosis and Management of the Critically Poisoned Patient. Cham, Switzerland: Springer, 2017;38:1855–74. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author. Analysis code is provided on github: [https://github.com/KCPaul-lab/IJE_PD-PQ.git].