Abstract
Background
We tested whether proton pump inhibitors (PPIs) are associated with enteric infections among those with inflammatory bowel disease (IBD), after adequately accounting for baseline differences between PPI users and nonusers.
Methods
This was a self-controlled case series, with each patient serving as their own control. Ambulatory patients with IBD were included if they were tested for enteric infection by multiplex polymerase chain reaction testing panel (GIPCR) and/or Clostridoides difficile toxin PCR from 2015 to 2019 and received PPIs for some but not all of this period. Rates of enteric infections were compared between the PPI-exposed period vs pre- and post-PPI periods identical in duration to the exposed period. Conditional Poisson regression was used to adjust for time-varying factors.
Results
Two hundred twenty-one IBD patients were included (49% ulcerative colitis, 46% Crohn’s disease, and 5% indeterminate colitis). The median PPI duration was 7 months (interquartile range 4 to 11 months). A total of 25 (11%) patients had a positive GIPCR or C. difficile test in the PPI period, 9 (4%) in the pre-PPI period, and 8 (4%) in the post-PPI period. Observed incidence rates for enteric infections were 2.5, 7.4, and 2.2 per 100 person years for the pre-PPI, PPI, and post-PPI periods, respectively (adjusted incidence rate ratios, 2.8; 95% confidence interval [CI] 1.3-6.0) for PPI vs pre-PPI and 2.9 (95% CI, 1.3-6.4) for PPI vs post-PPI). The adjusted absolute excess risk associated with PPIs was 4.9 infections per 100 person years.
Conclusions
Proton pump inhibitors were associated with a 3-fold increased risk for enteric infection among those with IBD but had a modest absolute risk.
Keywords: inflammatory bowel disease, proton pump inhibitors, enteric infection, Clostridioides difficile
Key Messages.
Although the use of PPIs has been associated with risk enteric infection, prior studies have been plagued by baseline differences between PPI users and nonusers, which can cause residual confounding that usually biases “against” PPIs.
To address the problem of baseline differences between PPI users and nonusers, we employed a self-controlled case series study design and found that IBD patients had a nearly 3-fold increased risk for enteric infection during the period of PPI exposure compared with the periods before or after PPI exposure.
PPIs should be aggressively discontinued in those with IBD and should guide a thoughtful risk-benefit assessment in those for whom PPI is indicated.
Introduction
Proton pump inhibitors (PPIs) are among the most frequently prescribed drug classes globally. Proton pump inhibitors have clear benefits when prescribed appropriately. However, they are frequently prescribed and continued for long periods without an evidence-based indication.1,2 Prior studies have suggested that among the many adverse effects attributed to PPIs, the evidence might be the strongest for enteric infections.1,3–5
Proton pump inhibitors are thought to alter the gut microbiome through their direct effect on gastric acid secretion.6 Gastric acid is one of the main defenses against ingested bacteria, and the reduction in gastric acidity caused by PPIs changes microbial composition.7–9 The most profound physiologic changes related to PPIs are within the upper gastrointestinal tract before gastric acid is buffered away. However, PPIs also appear to have a modest but significant downstream effect on the colonic microbiota.10 Enteric infections, including C. difficile infection (CDI), Salmonella, and Campylobacter, have previously been associated with PPI use.3
The intestinal microbiota regulates mucosal immunity and dysbiosis and is thought to be a major factor in the pathogenesis and maintenance of inflammatory bowel disease (IBD).11,12 Enteric infection can cause dysbiosis within the microbiota and is common in patients with IBD.13 Host immune defense mechanisms work synergistically with the native gut microbiota to maintain intestinal homeostasis, but this process may be disrupted in those who are receiving acid suppression; and such disruption may be particularly relevant for those with IBD.14,15
Prior studies of IBD adverse effects have been plagued by baseline differences between PPI users and nonusers, which can cause residual confounding that usually biases “against” PPIs.16 This study sought to determine whether PPI exposure is associated with enteric infections among those with IBD. To address the problem of baseline differences between PPI users and nonusers, we employed a self-controlled case series study design with each patient serving as his or her own control.
Materials and Methods
Study Design
This was a self-controlled case series. In this study design, outcomes during the exposed period (on PPIs) are compared with outcomes during the unexposed period (off PPIs) within each individual. The chief virtues of this study design are (1) all individuals in this study received PPIs at some point so there are no baseline differences between PPI users and nonusers to act as residual confounders; (2) crude and adjusted incidence rate ratios (IRRs) can be calculated for the exposed and unexposed study periods, which permits estimation of absolute (as opposed to relative) risk.17,18
Study Population
In this self-controlled case series study, we used the electronic medical records of patients at NewYork-Presbyterian Columbia University Irving Medical Center. Ambulatory patients of any age were eligible for this study if they were tested for enteric infection by multiplex polymerase chain reaction testing (PCR) panel and/or CDI toxin PCR from 2015 to 2019. Patients received PPIs for a defined duration during some but not all of this period. Patients were excluded if they were exposed to PPIs for >20 months or if they died during the follow-up time.
PPI Exposure
Proton pump inhibitor exposure was ascertained from our institution’s outpatient medication prescription writer. Proton pump inhibitor exposure was defined based on the start and end dates of the prescription. Patients were excluded if there was a lack of clear start and/or end dates of the PPI prescription. Charts were reviewed manually to confirm accuracy of the exposed and unexposed periods.
PPI Risk Periods
Three unique PPI risk periods were created to assess the impact of PPI exposure on the incidence of enteric infections (Figure 1). The PPI exposed period was defined as the duration of the PPI prescription (as delineated previously) plus an additional 90 days. This 90-day period was selected because prior studies indicate that the risk for CDI associated with PPIs persists for up to 90 days after PPI cessation.19,20 This exposure period was unique in duration for each individual but was no more than 20 months. The length of each patient’s PPI-exposed period was then used to define pre-PPI and post-PPI periods for the same patient. The duration of these unexposed periods was identical to the duration of the PPI-exposed period within an individual and varied from 3 to 20 months across individuals. The pre-PPI period began immediately before the PPI-exposed period and the post-PPI period began immediately after the PPI-exposed period (Figure 1). Last, a 15-day window was created before and after the start date of the PPI to avoid the possibility that the PPI was initiated for symptoms related to enteric infection (ie, to diminish any protopathic bias). A 15-day window was also built into the beginning of the post-PPI period so that all 3 periods remained identical in duration. Outcomes during the window periods did not contribute to the calculated incidence rates.
Figure 1.
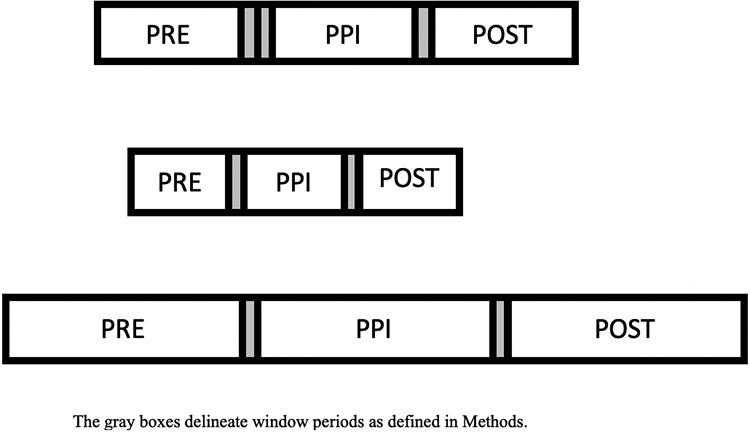
Pre-defined PPI risk periods. The gray boxes delineate window periods as defined in Methods.
Primary Outcome
The primary outcome was enteric infection, defined as either a positive gastrointestinal polymerase chain reaction test (GI PCR) or a positive CDI stool test. The FilmArray GIPCR panel (BioFire Diagnostics) tests for 22 organisms including 13 bacteria, 5 viruses, and 4 parasites (Table 4 for specific organisms), with a reported sensitivity and specificity of 95% to 100% for each individual pathogen.21,22 During the study period, all C. difficile tests were performed using a PCR for the C. difficile toxin B gene (Cepheid Xpert).23 Our laboratory performs CDI testing only on unformed stool specimens, defined as those that take the shape of the specimen container.
Table 4.
Types of enteric infections among IBD patients prescribed PPIs (N = 221).a
| Enteric Infection | N (%) |
|---|---|
| Bacteria | 54 (83%) |
| Escherichia coli (E. coli) species | 22 (34%) |
| Enteropathogenic E. coli | 13 (20%) |
| Enteroaggregative E. coli | 6 (9%) |
| Enterotoxigenic E. coli | 1 (2%) |
| Enteroinvasive E. coli | 1 (2%) |
| Shiga toxin-producing E. coli | 1 (2%) |
| E. Coli 0157:H7 | 0 (0%) |
| Campylobacter species | 4 (6%) |
| Salmonella | 0 (0%) |
| Vibrio species | 0 (0%) |
| Shigella species | 1 (2%) |
| Pleisomonas shigelloides | 1 (2%) |
| Yersinia enterocolitica | 0 (0%) |
| Clostridioides difficile | 26 (40%) |
| Parasites | 1 (2%) |
| Cryptosporidium | 0 (0%) |
| Cyclospora cayetanensis | 0 (0%) |
| Entamoeba histolytica | 1 (1%) |
| Giardia lamblia | 2 (3%) |
| Viruses | 10 (15%) |
| Norovirus (genogroups GI, GII) | 6 (9%) |
| Rotavirus A | 0 (0%) |
| Sapovirus (serotypes I, II, IV, V) | 4 (6) |
| Adenovirus | 0 (0%) |
| Astrovirus | 0 (0%) |
| Total | 65 (100%) |
aThere were 65 organisms identified among 42 unique individuals with infections.
Demographic and Clinical Characteristics
Baseline covariables (at time of PPI prescription) included age, gender, race/ethnicity (White, Hispanic, Black, other, and/or unknown), body mass index (BMI, categorized as <18.5 kg/m2, ≥18.5 kg/m2 to <30, or ≥30), Charlson comorbidity index (CCI, stratified as <2, ≥2 to <4, or ≥4), and IBD subtype (ulcerative colitis [UC], Crohn’s disease [CD], or indeterminate colitis). For time-varying covariables, we gathered IBD medications prescribed at the beginning of each risk period (systemic steroids, aminosalicylates, biologics, immunomodulators, and none), IBD severity (mild/remission: Harvey-Bradshaw Index Severity [HBIS] <7 or Partial Mayo Index Score [PMIS] <4; moderate: HBIS <16 or PMIS <6; and severe: HBIS >16 or PMIS >7), and antibiotics received during 90 days prior to the risk period start date (yes vs no).
Statistical Approach
For continuous variables, means and standard deviations were computed. Differences in means were calculated using Student t tests. Categorical variables were compared using χ2 tests. The incident rate ratio (IRR) was used to estimate the risk of enteric infection comparing the PPI-exposed to the PPI-unexposed periods. Unadjusted and adjusted IRRs were estimated using a Poisson regression model, conditioned on each individual patient (ie, so that each individual acted as a self-control). Time-varying covariables (IBD treatment type, IBD severity, and antibiotic exposure) were considered for the adjusted analyses. For visualization, cumulative incidence curves for patients in each risk period were estimated using the Kaplan-Meier method. Alpha of 0.05 was considered statistically significant, and all analyses were performed using STATA version 13.1.
Results
Study Population
We identified 221 IBD patients who met criteria for inclusion in this study, of which 49% had UC, 46% had CD, and 5% had indeterminate colitis (Table 1). The mean age was 41 (SD, 23) years, with a relatively even distribution of women (52%) and men (48%). Median PPI duration was 7 months (interquartile range, 4 to 11 months).
Table 1.
Time-fixed characteristics of IBD patients prescribed PPIs at the time of PPI initiation.
| Characteristics | N = 221 (%) |
|---|---|
| Age, mean (SD), years | 41 (23) |
| Gender, N (%) | |
| Female | 114 (52%) |
| Male | 107 (48%) |
| Race/Ethnicity, N (%) | |
| White | 92 (42%) |
| Black | 64 (29%) |
| Hispanic | 36 (16%) |
| Other | 13 (6%) |
| Unknown | 16 (7%) |
| Body mass index, mean (SD), kg/m2 | |
| <18.5 | 28 (13%) |
| >=18.5 to <30 | 159 (72%) |
| >=30 | 34 (15%) |
| Charlson comorbidity index, N (%) | |
| <2 | 110 (50%) |
| >=2 to <4 | 43 (19%) |
| >=4 | 68 (31%) |
| IBD subtype | |
| Ulcerative colitis | 108 (49%) |
| Crohn’s disease | 101 (46%) |
| Indeterminate colitis | 12 (5%) |
Time-varying Characteristics
Time-varying characteristics were compared between the pre-PPI, PPI exposed, and post-PPI risk periods (Table 2). There were no significant differences between the proportions of IBD medications used during each of the risk periods. There were differences in the rates of disease severity across the risk periods (P < .01); significantly more patients had moderate (24%) or severe (9%) disease during the PPI period compared with the pre-PPI or post-PPI periods. There were also differences in the rates of antibiotics received across the risk periods; significantly more patients received antibiotics during the pre-PPI (2%) and post-PPI (11%) periods compared with the PPI-exposed period (10%, P < .01).
Table 2.
Time-varying characteristics of IBD patients who were prescribed PPIs (N = 221).
| Pre-PPI | PPI | Post-PPI | P a | |
|---|---|---|---|---|
| IBD medications | ||||
| Topical steroids | 16 (7%) | 17 (8%) | 15 (7%) | 0.94 |
| Systemic steroids | 18 (8%) | 29 (13%) | 18 (8%) | 0.13 |
| 5-ASAs | 74 (33%) | 76 (34%) | 71 (32%) | 0.88 |
| Biologics | 56 (25%) | 63 (29%) | 75 (34%) | 0.13 |
| Immunomodulators | 23 (10%) | 31 (14%) | 25 (11%) | 0.47 |
| None | 67 (30%) | 54 (24%) | 56 (25%) | 0.32 |
| IBD severity | <0.01 | |||
| Mild/Remission | 172 (78%) | 146 (66%) | 176 (80%) | |
| Moderate | 40 (18%) | 56 (24%) | 36 (16%) | |
| Severe | 9 (4%) | 19 (9%) | 9 (4%) | |
| Antibiotic exposure within 90 days prior to risk period start date | 4 (2%) | 21 (10%) | 24 (11%) | <0.01 |
aThe χ2 P value.
Enteric Infections
Sixty-five enteric organisms were identified across all risk periods. A total of 25 (11%) patients had a positive stool gastrointestinal polymerase chain reaction test or C. difficile test showing enteric infection during the PPI period, 9 (4%) in the pre-PPI period, and 8 (4%) in the post-PPI period (Figure 2). One patient had more than 1 infection in the pre-PPI period, 8 patients had more than 1 infection in the PPI period, and no patients had more than 1 infection in the post-PPI period. When infections were censored after the initial enteric infection during each risk period, the observed incidence rates were 2.5, 7.4, and 2.2 per 100 person years for the pre-PPI, PPI-exposed, and post-PPI periods, respectively. After we allowed for multiple infections within individuals, the observed incidence rates were 6.7, 24.1, and 5.4 per 100 person years in the respective risk periods.
Figure 2.
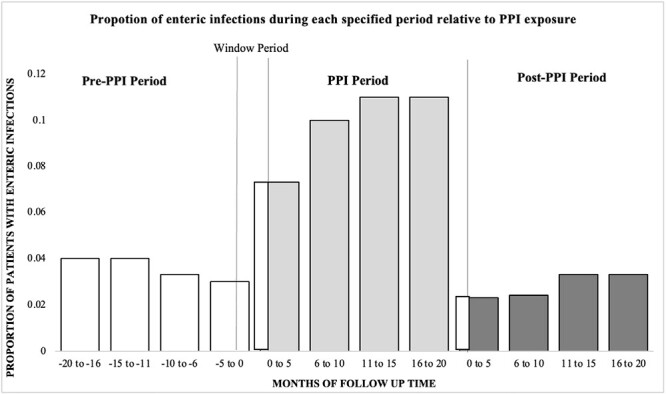
Proportion with enteric infections during prespecified risk periods.
Risk for Enteric Infections Associated With PPI Exposure
The crude incidence rate ratio for the PPI-exposed period compared with the pre-PPI period was 2.8 (95% confidence interval [CI], 1.3-6.0) and compared with the post-PPI period was 2.9 (95% CI, 1.3-6.4). These results were similar after adjusting for age, severity of IBD, and antibiotic use (Table 3). There was no significant difference in the rate of infection in the post-PPI period compared with the pre-PPI period (adjusted IRR 0.87; 95% CI, 0.33-2.28). We repeated our analyses after excluding those patients who had antibiotic exposure and found similar incidence rates of enteric infections during each of the 3 periods. We also found similar incident rate ratios when comparing the 3 risk periods to each other.
Table 3.
Incidence rate ratios (95% confidence intervals) of enteric infection comparing the specified time periods (N = 221)
| Comparison of Risk Period (number of events, incidence rate) | Unadjusted IRR | Adjusted IRRa |
|---|---|---|
| PPI (25, 7.4) vs pre-PPI (9, 2.5) risk period | 2.8 (1.3-6.0) | 2.8 (1.3-6.0) |
| PPI (25, 7.4) vs post-PPI (8, 2.2) risk period | 3.1 (1.4-6.9) | 2.9 (1.3-6.4) |
| Post-PPI (8, 2.2) vs pre-PPI (9, 2.5) risk period | 0.90 (0.30-2.3) | 0.87 (0.33-2.28) |
aAdjusted for age, severity of IBD, and antibiotic use. Incidence rates shown as rates per 100 person years.
The cumulative incidence of enteric infection in the cohort is shown in Figure 3, with a significantly lower proportion of patients remaining free of enteric infection in the PPI-exposed period compared with the other periods (log-rank P < .01). The adjusted absolute excess risk for enteric infection associated with PPIs among those with IBD was 4.9 events per 100 person years or 17.4 events per 100 person years when multiple infections were allowed for each individual. The number needed to harm for 1 year of treatment with PPIs was 20, with censoring after 1 infection or 6 if multiple infections were allowed for each individual.
Figure 3.
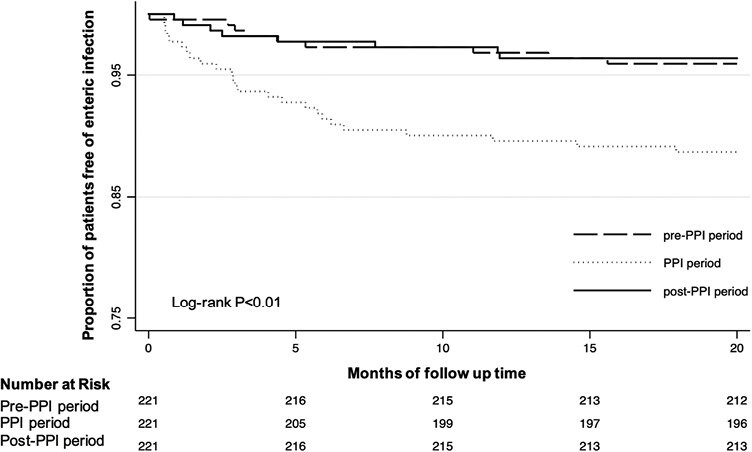
Cumulative proportion of enteric infection in 221 patients with IBD, stratified by specified risk periods.
Enteric Infections: Organism Types
Sixty-five unique organisms were identified, of which bacterial species were most prevalent (83%), followed by viruses (15%), and parasites (2%). The most common organisms were C. difficile (40%) and Escherichia coli (E. coli) species (Table 4).
Discussion
Proton pump inhibitors are one of the most widely used medication classes in the United States, and their use has increased over time. Although they appear reasonably safe when used appropriately, they are often prescribed inappropriately. For example, PPIs are often prescribed for abdominal pain or initiated during hospitalization for stress ulcer prophylaxis and then inappropriately continued at hospital discharge.24 Patients with IBD, who often have gastrointestinal symptoms and who may have multiple providers spanning the in- and outpatient settings, may be at elevated risk for inappropriate prescribing of PPIs. This study evaluated the incidence rates of enteric infections in IBD patients who were PPI users using a self-controlled case series study design. After adjusting for time-varying factors, patients had a nearly 3-fold increased risk for enteric infection during the period of PPI exposure compared with the periods before or after PPI exposure. One of the virtues of the study design is that it allows direct calculation of the absolute risks associated with PPIs. The absolute excess risk for enteric infection associated with PPIs was 4.9 events per 100 person years for a number needed to harm of 20. Most of these infections were C. difficile or traveler’s diarrhea type E. coli species.
Prior studies have examined acid suppression medications in patients with IBD, but few of these studies have focused specifically on enteric infections. One case-control study using the Veteran Affairs Database found that PPIs were associated with an increased risk of hospitalization or surgery in patients with IBD.25 Another study using a claims database found that PPIs were associated with a medication change in those with UC.26 A meta-analysis of 5 randomized trials of IBD patients treated with infliximab found that patients on PPI were less likely to achieve remission compared with patients not taking PPI.27 Our study is one of the first to specify the risk of enteric infections in patients with IBD who are also PPI users.
There is now considerable data related to PPIs and enteric infection in the general (ie, non-IBD) population. Although there is some heterogeneity in this data, the effect size for PPIs and enteric infection has generally been the strongest among the many hypothesized PPI adverse effects that have been studied.5 A number of studies with varying methodologies including meta-analyses, cohort studies, and large database studies have found that PPI therapy was associated with incident and recurrent gastrointestinal infection, with an effect size ranging anywhere from 1.5- to 3-fold.28–30 Interestingly, Brophy et al used a similar study design to ours to assess risk for Campylobacter and Salmonella infections but concluded that there was no risk associated with PPIs.31 We found that IBD patients had a nearly 3-fold increased risk for enteric infections that was associated with PPIs, which is consistent with most prior studies in the non-IBD population,
Proton pump inhibitor use may facilitate intestinal infection by causing achlorhydria, which leads to decreased killing of ingested organisms (eg, Campylobacter or Salmonella). For C. difficile, ingested spores are likely resistant to gastric acid regardless of PPIs, but PPIs may alter the colonic microbiota to decrease the prevalence of commensal Clostridia that normally compete in the same niche as C. difficile.32 Compared with the general population, patients with IBD diagnosed with enteric infection have more pronounced dysbiosis and are more likely to acquire CDI.33 The combination of CDI and IBD is associated with risk for poor outcomes.34–37 Several studies have shown increased rates of hospitalization, longer lengths of stay, higher recurrence rates, increased risk for surgery or colectomy, and higher mortality rates in patients with IBD and CDI.35,38–40 Similarly, IBD outcomes are affected by infection with other bacteria including Salmonella, Campylobacter jejuni, or the E. coli species.40,41 For many reasons, enteric infections are particularly worrisome in IBD patients.
This study has several strengths. Importantly, it utilized a self-controlled case series design in which cases were their own controls. This design eliminates confounding related to baseline patient characteristics that may differ between users and nonusers and produces results that can be interpreted to yield estimates of attributable risk and number needed to harm. The self-controlled case series method allows estimation of absolute risks and relative risks. These absolute excess risks should be understood as the estimated increased risks faced by individuals who occasionally use PPIs, when the periods on PPIs are compared with the periods off PPIs within those individuals. Additionally, although the study was relatively small, it utilized granular patient data including data on the key time-varying covariables including antibiotic exposure, IBD treatment, and IBD disease severity. This study also has limitations. It was observational and retrospective. The primary exposure of PPI use was ascertained based on prescription data from the electronic medical records rather than directly from pharmacy data. Given limitations of the data, we were unable to comment on any dose or duration relationships between PPI and the outcome of interest. We did not collect patient samples and therefore cannot report on genetic, microbiome-related, or immunologic data, all of which may impact the PPI-enteric infection relationship. In addition, there may be biological or other differences between patients who use PPIs consistently and for extremely long durations (eg, patients with Barrett’s esophagus) and those who use PPIs intermittently. These study results should only be generalized to intermittent PPI users.
Conclusion
In summary, this self-controlled case series study found that IBD patients had an increased risk of enteric infection during the period of exposure to PPI compared with the periods immediately before or after PPI exposure. The absolute excess risk associated with PPIs was 4.9 per 100 person years for an annualized number needed to harm of 20. Overall, these data suggest that there is a real but modest risk for intestinal infection associated with PPIs in IBD patients. Enteric infections can exacerbate IBD, and inappropriate PPIs should be aggressively discontinued in those with IBD. In those with IBD who are appropriately receiving PPIs (eg, for Barrett’s esophagus), these results can help guide a thoughtful risk-benefit assessment.
Acknowledgments
This study was approved by the Columbia University Irving Medical Center Institutional Review Board.
Contributor Information
Sanskriti Varma, Division of Gastroenterology, Massachusetts General Hospital, Boston, MA, USA.
Stephen J Trudeau, Columbia Vagelos College of Physicians and Surgeons, New York, NY, USA.
Jianhua Li, Biomedical Informatics, Columbia University Irving Medical Center, New York, NY, USA.
Daniel E Freedberg, Division of Digestive and Liver Diseases, Department of Medicine, Columbia University Irving Medical Center, New York, NY, USA.
Conflicts of Interest
D.F. was funded in part by a Department of Defense Clinical Trial Award (PR181960) and in part by a Columbia University Irving Medical Center Irving Scholars Award.
References
- 1. McDonald EG, Milligan J, Frenette C, Lee TC. Continuous proton pump inhibitor therapy and the associated risk of recurrent Clostridium difficile infection. JAMA Intern Med. 2015;175(5):784-791. [DOI] [PubMed] [Google Scholar]
- 2. Kelly OB, Dillane C, Patchett SE, Harewood GC, Murray FE. The inappropriate prescription of oral proton pump inhibitors in the hospital setting: a prospective cross-sectional study. Dig Dis Sci. 2015;60(8):2280-2286. [DOI] [PubMed] [Google Scholar]
- 3. Leonard J, Marshall JK, Moayyedi P. Systematic review of the risk of enteric infection in patients taking acid suppression. Am J Gastroenterol. 2007;102(9):2047-56; quiz 2057. [DOI] [PubMed] [Google Scholar]
- 4. Janarthanan S, Ditah I, Adler DG, Ehrinpreis MN. Clostridium difficile-associated diarrhea and proton pump inhibitor therapy: a meta-analysis. Am J Gastroenterol. 2012;107(7):1001-1010. [DOI] [PubMed] [Google Scholar]
- 5. Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706-715. [DOI] [PubMed] [Google Scholar]
- 6. Minalyan A, Gabrielyan L, Scott D, Jacobs J, Pisegna JR. The gastric and intestinal microbiome: role of proton pump inhibitors. Curr Gastroenterol Rep. 2017;19(8):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goyal H, Katner H. Proton pump inhibitors and Clostridium difficile infection. Off J Am Coll Gastroenterol | ACG. 2017;112(5):805. [DOI] [PubMed] [Google Scholar]
- 8. Inghammar M, Svanstrom H, Voldstedlund M, et al. Proton-pump inhibitor use and the risk of community-associated clostridium difficile infection. Clin Infect Dis. 2021;72(12):e1084-e1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Trifan A, Stanciu C, Girleanu I, et al. Proton pump inhibitors therapy and risk of Clostridium difficile infection: systematic review and meta-analysis. World J Gastroenterol. 2017;23(35):6500-6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology. 2015;149(4):883-5.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146(6):1489-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Longman RS, Littman DR. The functional impact of the intestinal microbiome on mucosal immunity and systemic autoimmunity. Curr Opin Rheumatol. 2015;27(4):381-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature. 2012;491(7422):119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10(4):845-864. [DOI] [PubMed] [Google Scholar]
- 15. Dubinsky M, Braun J. Diagnostic and prognostic microbial biomarkers in inflammatory bowel diseases. Gastroenterology. 2015;149(5):1265-1274.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35-48. [DOI] [PubMed] [Google Scholar]
- 17. Hallas J, Pottegard A. Use of self-controlled designs in pharmacoepidemiology. J Intern Med. 2014;275(6):581-589. [DOI] [PubMed] [Google Scholar]
- 18. Petersen I, Douglas I, Whitaker H. Self controlled case series methods: an alternative to standard epidemiological study designs. BMJ. 2016;354(12):i4515. [DOI] [PubMed] [Google Scholar]
- 19. Hensgens MP, Goorhuis A, Dekkers OM, Kuijper EJ. Time interval of increased risk for Clostridium difficile infection after exposure to antibiotics. J Antimicrob Chemother. 2012;67(3):742-748. [DOI] [PubMed] [Google Scholar]
- 20. Dial S, Kezouh A, Dascal A, Barkun A, Suissa S. Patterns of antibiotic use and risk of hospital admission because of Clostridium difficile infection. CMAJ. 2008;179(8):767-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Piralla A, Lunghi G, Ardissino G, et al. FilmArray GI panel performance for the diagnosis of acute gastroenteritis or hemorragic diarrhea. BMC Microbiol. 2017;17(1):111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buss SN, Leber A, Chapin K, et al. Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol. 2015;53(3):915-925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Larson AM, Fung AM, Fang FC. Evaluation of tcdB real-time PCR in a three-step diagnostic algorithm for detection of toxigenic Clostridium difficile. J Clin Microbiol. 2010;48(1):124-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blackett JW, Chen L, Li J, Wright JD, Freedberg DE. Inappropriateness of proton pump inhibitors after hospital discharge is associated with thirty-day hospital readmission. Dig Dis Sci. 2022;67(3):817-825. [DOI] [PubMed] [Google Scholar]
- 25. Shah R, Richardson P, Yu H, Kramer J, Hou JK. Gastric acid suppression is associated with an increased risk of adverse outcomes in inflammatory bowel disease. Digestion. 2017;95(3):188-193. [DOI] [PubMed] [Google Scholar]
- 26. Juillerat P, Schneeweiss S, Cook EF, Ananthakrishnan AN, Mogun H, Korzenik JR. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther. 2012;36(3):239-247. [DOI] [PubMed] [Google Scholar]
- 27. Lu TX, Dapas M, Lin E, Peters T, Sakuraba A. The influence of proton pump inhibitor therapy on the outcome of infliximab therapy in inflammatory bowel disease: a patient-level meta-analysis of randomised controlled studies. Gut. 2021;70(11):2076-2084. [DOI] [PubMed] [Google Scholar]
- 28. Deshpande A, Pant C, Pasupuleti V, et al. Association between proton pump inhibitor therapy and Clostridium difficile infection in a meta-analysis. Clin Gastroenterol Hepatol. 2012;10(3):225-233. [DOI] [PubMed] [Google Scholar]
- 29. Kwok CS, Arthur AK, Anibueze CI, Singh S, Cavallazzi R, Loke YK. Risk of Clostridium difficile infection with acid suppressing drugs and antibiotics: meta-analysis. Am J Gastroenterol. 2012;107(7):1011-1019. [DOI] [PubMed] [Google Scholar]
- 30. Xia B, Yang M, Nguyen LH, et al. Regular use of proton pump inhibitor and the risk of inflammatory bowel disease: pooled analysis of 3 prospective cohorts. Gastroenterology. 2021;161(6):1842-1852.e10. [DOI] [PubMed] [Google Scholar]
- 31. Brophy S, Jones KH, Rahman MA, et al. Incidence of Campylobacter and Salmonella infections following first prescription for PPI: a cohort study using routine data. Am J Gastroenterol. 2013;108(7):1094-1100. [DOI] [PubMed] [Google Scholar]
- 32. Freedberg DE, Salmasian H, Friedman C, Abrams JA. Proton pump inhibitors and risk for recurrent Clostridium difficile infection among inpatients. Am J Gastroenterol. 2013;108(11):1794-1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Axelrad JE, Cadwell KH, Colombel JF, Shah SC. The role of gastrointestinal pathogens in inflammatory bowel disease: a systematic review. Therap Adv Gastroenterol. 2021;14(31):17562848211004493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varma S, Faye AS, Kannan A, et al. Patients with more severe IBD Get Clostridioides difficile rather than Clostridioides difficile increasing the severity of IBD. Dig Dis Sci. 2021;66(9):3113-3123. [DOI] [PubMed] [Google Scholar]
- 35. Issa M, Vijayapal A, Graham MB, et al. Impact of Clostridium difficile on inflammatory bowel disease. Clin Gastroenterol Hepatol. 2007;5(3):345-351. [DOI] [PubMed] [Google Scholar]
- 36. Berg AM, Kelly CP, Farraye FA. Clostridium difficile infection in the inflammatory bowel disease patient. Inflamm Bowel Dis. 2013;19(1):194-204. [DOI] [PubMed] [Google Scholar]
- 37. Khanna S, Pardi DS. IBD: poor outcomes after Clostridium difficile infection in IBD. Nat Rev Gastroenterol Hepatol. 2012;9(6):307-308. [DOI] [PubMed] [Google Scholar]
- 38. Nguyen GC, Kaplan GG, Harris ML, Brant SR. A national survey of the prevalence and impact of Clostridium difficile infection among hospitalized inflammatory bowel disease patients. Am J Gastroenterol. 2008;103(6):1443-1450. [DOI] [PubMed] [Google Scholar]
- 39. Jen MH, Saxena S, Bottle A, Aylin P, Pollok RC. Increased health burden associated with Clostridium difficile diarrhoea in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33(12):1322-1331. [DOI] [PubMed] [Google Scholar]
- 40. Axelrad JE, Joelson A, Nobel YR, et al. Enteric infection in relapse of inflammatory bowel disease: the utility of stool microbial PCR testing. Inflamm Bowel Dis. 2017;23(6):1034-1039. [DOI] [PubMed] [Google Scholar]
- 41. Gradel KO, Nielsen HL, Schonheyder HC, Ejlertsen T, Kristensen B, Nielsen H. Increased short- and long-term risk of inflammatory bowel disease after salmonella or campylobacter gastroenteritis. Gastroenterology. 2009;137(2):495-501. [DOI] [PubMed] [Google Scholar]


