Abstract
Background
Depressive symptoms are common in knee osteoarthritis (OA), exacerbate knee pain severity and may influence outcomes of oral analgesic treatments. The aim was to assess whether oral analgesic effectiveness in knee OA varies by fluctuations in depressive symptoms.
Methods
The sample included Osteoarthritis Initiative (OAI) participants not treated with oral analgesics at enrolment (n = 1477), with radiographic disease at the first follow-up visit (defined as the index date). Oral analgesic treatment and depressive symptoms, assessed with the Center for Epidemiological Studies Depression [(CES-D) score ≥16] Scale, were measured over three annual visits. Knee pain severity was measured at visits adjacent to treatment and modifier using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) pain subscale (rescaled range = 0–100). Structural nested mean models (SNMMs) estimated causal mean differences in knee pain severity comparing treatment versus no treatment.
Results
The average causal effects of treated versus not treated for observations without depressive symptoms showed negligible differences in knee pain severity. However, causal mean differences in knee pain severity comparing treatment versus no treatment among observations with depressive symptoms increased over time from −0.10 [95% confidence interval (CI): −9.94, 9.74] to −16.67 (95% CI: −26.33, −7.01). Accordingly, the difference in average causal effects regarding oral analgesic treatment for knee pain severity between person-time with and without depressive symptoms was largest (−16.53; 95% CI: −26.75, −6.31) at the last time point. Cumulative treatment for 2 or 3 years did not yield larger causal mean differences.
Conclusions
Knee OA patients with persistent depressive symptoms and chronic pain may derive more analgesic treatment benefit than those without depressive symptoms and less pain.
Keywords: Knee osteoarthritis, depression, pain, pharmacoepidemiology
Key Messages.
Causal mean differences for oral analgesic treatment among person-time without depressive symptoms showed negligible reductions in knee pain severity, compared with observations without depressive symptoms and no treatment during the study period.
Average causal effects of oral analgesic treatment on knee pain severity among observations with depressive symptoms showed consistently larger symptomatic reductions, compared with person-time with depressive symptoms and no treatment.
Oral analgesic treatment may provide increasingly greater pain relief to knee osteoarthritis patients as the persistence of depressive symptoms and knee pain severity increases, compared with those without depressive symptoms and less pain.
Introduction
Knee osteoarthritis (OA), the most common arthritic condition worldwide, is a leading cause of physical disability.1 The condition is characterized by the ‘disease’, which includes localized inflammation and degradation of joint cartilage, and the ‘illness’ that manifests as pain and physical disability.2 Knee OA is considered a ‘serious disease’, with substantial unmet medical care needs and lack of treatments to inhibit progression.3 Medical management is focused on alleviating joint pain and improving physical function and quality of life. Treatments include education, exercise, weight loss, thermal modalities, psychosocial interventions, oral analgesics, intra-articular injections and joint replacement.4 However, pain management is complicated by psychiatric comorbidity, particularly depressive symptoms, which affect approximately 20% of persons with knee OA.5,6
There is incongruency between the ‘disease’ and ‘illness’, where deteriorations in joint structure do not always correlate with pain. Systematic reviews demonstrate that the proportion of knee OA patients with pain ranges from 15% to 81% among those with radiographic disease.7 Thus, other aetiological mechanisms may contribute to joint pain, such as depression, which is a barrier to medical management.8,9 Clinical phenotyping has identified a ‘depression’ subgroup characterized by depressive symptoms, greater muscle weakness, worse pain, more functional disability and slower gait speed.10,11 The associations between knee OA and depression are bidirectional, particularly pain and depressive symptoms, a well-documented aetiological relationship.12 Research indicates that pain in knee OA is determinative of depression onset and depressive symptom severity.13–15 Conversely, depressive symptoms in knee OA are associated with higher pain severity and worse pain trajectories.14,16–18 Knee pain severity is also increasingly high (i.e. dose-dependent) with greater depression persistence.19 Accordingly, depressive symptoms may be both mediator and modifier regarding the effects of common treatments and clinical progression of knee OA.
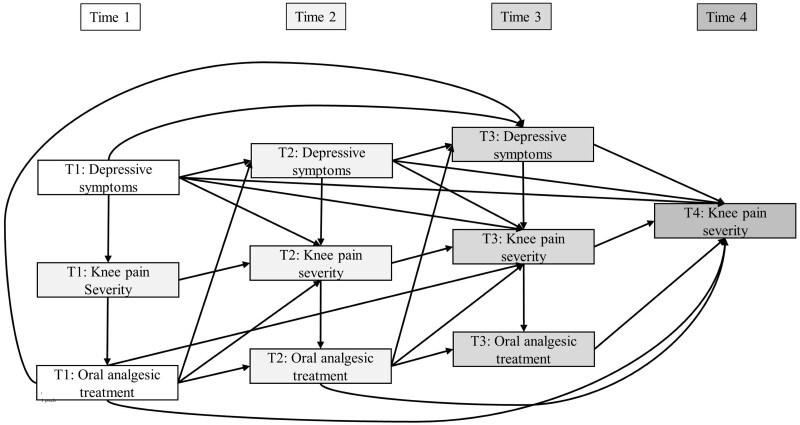
Knee OA patients seek medical care when pain becomes persistent, despite the use of over-the-counter treatments. Nonsteroidal anti-inflammatory drugs (NSAIDs) and opioid analgesics have greater effectiveness but more side effects than paracetamol.20,21 Research suggests that knee OA patients with depression receiving oral analgesics have twice the odds of inadequate pain relief than those without depression.22 Depression may increase knee pain severity, influence oral analgesic effectiveness and be a source of treatment effect heterogeneity (Figure 1). Treatment effect heterogeneity—often described as treatment effect moderation—represents a decrease or increase in intervention effectiveness within strata of a modifier—also referred to as moderators or effect modifiers.23 Depression is often an exclusion criterion in randomized controlled trials (RCTs) to increase the potential of treatment response and reduce adverse events and dropouts.24 As such, variation in pharmacological pain treatment effectiveness associated with depression in knee OA has not been assessed in RCTs. Moreover, no observational studies have evaluated whether depressive symptoms are a source of treatment effect heterogeneity regarding oral analgesics among persons with arthritis, to determine if effectiveness varies as depressive symptoms change over time.
Figure 1.
Directed acyclic graph showing the study design and hypothesized causal relationships between depressive symptoms, knee pain severity and oral analgesic treatment. Effect moderation was constrained so that depressive symptoms could only modify the effectiveness of treatment measured concurrently at the same time points; T1: Time 1; T2: Time 2; T3: Time 3; T4: Time 4
Examining treatment effect heterogeneity is usually performed by assessing statistical interactions between interventions and potential modifiers. Traditional epidemiological methods were designed for scenarios with time-invariant interventions and treatment effect modifiers.23 Nonetheless, data are often longitudinal, where treatment and modifier status both change over time, and intervention effectiveness relates to cumulative exposure history.25 The original marginal structural model (MSM) implementation was developed to evaluate the cumulative impact of dynamic treatments but not designed to account for potential biases arising from time-varying effect moderation.26 By contrast, structural nested mean models (SNMMs) are appropriate for examining intervention effect heterogeneity when assessing the cumulative effects of dynamic treatments in terms of time-varying modifiers.27 Therefore, the objective was to evaluate if oral analgesic treatment effectiveness in knee OA varies as a function of person-time with and without depressive symptoms. It was hypothesized that persons with knee OA and depressive symptoms would show consistently lower longitudinal analgesic medication response compared with persons without depressive symptoms.
Methods
Study data and sample
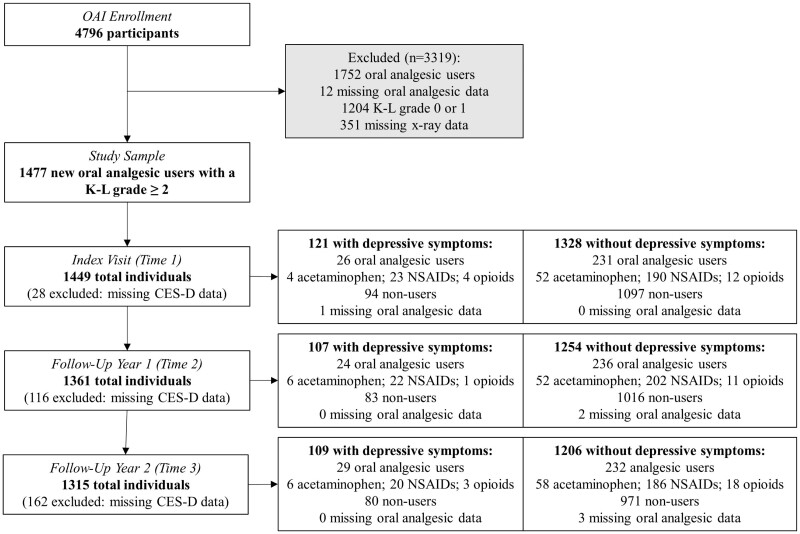
This study used publicly available Osteoarthritis Initiative (OAI) data collected from 2004 through 2010, which are accessible through the National Institutes of Health’s National Data Archive. The OAI is an observational cohort study of knee health which was designed to identify factors related to the onset and progression of symptomatic, radiographic knee OA; protocol details are available elsewhere [https://nda.nih.gov/oai/].28 Briefly, OAI participants provided written informed consent, and were 45 to 79 years old (n = 4796) and enrolled at four academic medical centres (University of Maryland/Johns Hopkins University; Memorial Hospital of Rhode Island; Ohio State University; University of Pittsburgh). Study visits occurred in prescheduled waves that were approximately 1 year apart. Data collected by trained research staff from the baseline visit and four annual follow-up assessments were used. These data comprise the original 4-year longitudinal study with all relevant measures available at every time point. Participants were restricted to those (n = 1477) with radiographic disease [Kellgren–Lawrence (K-L) grade ≥2 (range = 0–4)] and no oral analgesic treatment at baseline.29
Treatment: oral analgesic medications
Oral analgesic use in the previous 30 days was recorded by trained research staff using medication inventory forms. Prevalent users of oral analgesic treatments at enrolment (n = 1752) and those with missing treatment data (n = 12) were excluded. Any oral analgesic use was operationalized as a time-varying measure assessed at the first three time points starting from the assigned index date. Treatment was defined as use of prescription and non-prescription oral analgesics, including acetaminophen, NSAIDs and opioid medications. Pharmacotherapies were combined into a single measure to estimate pooled treatment effects and mitigate sample size issues related to assessing individual oral analgesics within strata of the modifier. Participants may have taken more than one treatment at each time point or switched oral analgesics between visits, which reflects real-world utilization patterns.
Effect modifier: depressive symptoms
Depressive symptoms were defined as a time-varying effect modifier assessed with the Center for Epidemiologic Studies Depression (CES-D) Scale at the same visits as oral analgesic treatment.30 The CES-D is a valid and reliable measure of depressive symptoms which assesses symptomology in the prior week; item response options range from 0 to 3, resulting in summary scores of 0 to 60, where increasing score corresponds to greater frequency and severity.30,31 The CES-D reference time-frame represents diagnostic criteria for major depression which requires core symptomology to be present for 2 weeks.30,32 Previous data show that OAI participants satisfying CES-D screening criteria (CES-D score ≥16) for major depression have perpetually worsening symptom severity.33 Consequently, modifier status was defined using the recommended CES-D screening threshold for major depression, and dichotomization yields easily interpretable estimates in the form of mean differences, which enhances the uptake and utility of results.34
Outcome: knee pain severity
The time-varying outcome was self-reported knee pain severity measured using the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) at visits adjacent to assessment of treatment and effect modifier at the second, third and fourth time points.35 The WOMAC is a valid, reliable and responsive questionnaire with three domains: stiffness (2 items), pain (5 items), and disability (17 items); items are scored on a 5-point Likert scale.35 The outcome was defined as participants’ WOMAC pain scores (rescaled to 0 to 100) for the index knee, and prior research indicates a minimally clinically significant difference to be approximately 9.7 units.36 Index knee (left or right) was selected using an algorithm with the following primacy: (i) greater structural disease severity; (ii) higher pain severity; (iii) right knee (presumed to be dominant).
Potential confounders
Measures included as potential confounders were selected a priori based on literature review. Time-invariant variables assessed at enrolment were age (years), sex, race (White or non-White), marital status (married, widowed, divorced, separated, never married), educational attainment (high school, college graduate, postgraduate degree), employment status, health insurance, smoking (never, former, current), alcohol consumption (none, minimal, moderate), comorbidity and symptomatic knee OA status. Comorbid conditions were assessed using the Charlson comorbidity index.37 Symptomatic knee OA was defined by the presence of pain on most days for at least 1 month during the past year. Time-dependent confounders were measured at the same visits as treatment and effect modifier and included body mass index (BMI; kilograms per metres squared), intra-articular injections, knee injuries, physical performance, structural disease severity and lagged knee pain severity. Intra-articular injections included any self-reported use of hyaluronic acid and/or corticosteroids. Knee injuries were defined as a self-reported event resulting in a limited ability to walk for at least 2 days. Physical performance was assessed using walking speed (metres/second) from the average of two 20-m gait speed trials. Structural severity and lagged pain were measured using K-L grade and WOMAC pain subscale, respectively.29,35
Statistical analysis
There were 1477 participants in the original study sample with no current oral analgesic treatment at enrolment and radiographic knee OA at the index visit. Descriptive statistics for baseline characteristics were calculated stratifying participants by both depressive symptoms and oral analgesic treatment at the first time point. Knee pain severity was evaluated in a similar manner at three time points after the index date. Continuous variables were described using means and standard deviations, and frequencies and percentages were estimated for categorical covariates. Standardized mean differences (SMDs) were used to characterize treatment-covariate associations, and absolute values greater than or equal to 0.10 were considered evidence of imbalance.38 The effective analytical samples with complete data included 1337, 1221 and 1148 observations at the first, second and third time points, respectively.
SNMMs are an adaption of conventional regression modelling that use careful temporal ordering and re-anchoring of time regarding treatments, modifiers and outcomes and address methodological issues related to treatment effect heterogeneity in the longitudinal setting.27 First, dynamic treatments and modifiers that influence each other and affect outcomes lead to indirect effects of prior treatment that operate through future levels of the modifier.27 Second, naively conditioning upon modifiers affected by prior treatment over time can lead to bias by inducing associations between the latter and unobserved factors related to outcomes.27 These two methodological issues are over-control of intermediate pathways and collider-stratification biases and become more complex when controlling for time-dependent confounding. The inverse-probability-of-treatment-weighted regression-with-residuals (IPTW-RWR) SNMM approach overcomes these challenges and yields estimates that isolate the average causal effect of one additional treatment interval versus no further intervention conditional on prior treatment and modifier exposure history.27 Detailed information on the implementation, causal estimands and interpretations are provided (Supplementary File S1, available as Supplementary data at IJE online).
Analyses were performed using R statistical software (version 4.0.3), and the analytical code is provided (Supplementary File S2, available as Supplementary data at IJE online). Briefly, stabilized inverse probability weights were estimated using logistic regression models to account for measured time-invariant and time-varying confounders and missing observations. The effect modifier was also regressed on past treatment, modifier and treatment-modifier interaction history using generalized linear models and then residualized. Finally, a weighted outcome model was fit with indicator terms for treatment, residualized modifier, time and treatment-modifier-time interactions using the survey package in R, specifying participants as the clustering unit to account for intra-individual correlation of repeated measures outcome data. Post-estimation linear combinations estimated average causal effects as differences in pain (range = 0–100) with 95% confidence intervals (95% CI) comparing treatment versus no treatment at each follow-up time point. An unweighted outcome model without residual effect modifier variables was fit as a secondary analysis to assess associations without controlling for measured confounders and other potential biases.
Results
Participant characteristics
There were 1328, 1254 and 1206 observations without depressive symptoms, of which 231 (18%), 236 (19%) and 232 (19%) had oral analgesic treatment at the first, second and third time points, respectively (Figure 2). At the first three time points, there were 121, 107 and 109 observations with depressive symptoms which included 26 (21%), 24 (22%) and 29 (27%) with oral analgesic treatment, respectively. The most used treatment among observations with and without depressive symptoms were NSAIDs, followed by acetaminophen, and opioid analgesics were the least used. Pain was consistently higher at Times 2, 3 and 4 among person-time with, than without, oral analgesic treatment and depressive symptoms (Table 1).
Figure 2.
Original study sample flow diagram. Participants may take more than one oral analgesic at each time point; effective complete-case analysis samples for each visit are lower than these frequencies. CES-D, Center for Epidemiological Studies Depression Scale; K-L grade, Kellgren–Lawrence Grade; NSAIDs, non-steroidal anti-inflammatory drugs; OAI, Osteoarthritis Initiative
Table 1.
Knee pain severity during follow-up between those treated and not treated with oral analgesics among participants with and without depressive symptoms
| No depressive symptoms, mean (SD) |
Depressive symptoms, mean (SD) |
||||||
|---|---|---|---|---|---|---|---|
| Treatment | Pain assessment | Not treated | Treated | SMD | Not treated | Treated | SMD |
| T1 | T2 | 10.25 (13.68) | 16.01 (18.14) | 0.359 | 17.26 (19.08) | 22.39 (19.53) | 0.266 |
| T2 | T3 | 9.25 (13.27) | 14.38 (15.71) | 0.352 | 16.35 (18.42) | 20.00 (17.25) | 0.205 |
| T3 | T4 | 9.43 (13.19) | 16.23 (16.95) | 0.448 | 18.85 (22.17) | 23.39 (20.91) | 0.211 |
SMD, standardized mean difference; SD, standard deviation; T1, Time 1; T2, Time 2; T3, Time 3; T4, Time 4.
At the index visit, among observations without depressive symptoms, oral analgesic treatment was related to being non-White and having more pain, higher BMI, receiving injections, history of knee injury, slower gait speed, greater disease severity and more comorbidity (Table 2). Observations with depressive symptoms and oral analgesic treatment at the index visit were associated with being younger, female, non-White, not married, less educated, covered by health insurance, smoking and alcohol consumption. Treatment-covariate associations showed similar trends for clinical characteristics among baseline observations with symptoms of depression compared with those without depressive symptoms; however, SMDs were generally larger in the former than the latter.
Table 2.
Descriptive characteristics at the index visit between those treated and not treated with oral analgesics among participants with and without depressive symptoms
| Variables | No depressive symptoms (n = 1328) |
Depressive symptoms (n = 121) |
||||
|---|---|---|---|---|---|---|
| Not treated (n = 1097) | Treated (n = 231) | SMD | Not treated (n = 94) | Treated (n = 26) | SMD | |
| Age, years | 62.63 (8.95) | 63.12 (9.05) | 0.054 | 61.36 (9.27) | 58.04 (8.46) | 0.374 |
| Female sex | 570 (52.0%) | 126 (54.5%) | 0.052 | 55 (58.5%) | 17 (65.4%) | 0.142 |
| Non-White | 189 (17.2%) | 49 (21.2%) | 0.101 | 24 (25.5%) | 10 (38.5%) | 0.280 |
| Marital status | 0.075 | 0.287 | ||||
| Married | 762 (69.8%) | 163 (70.6%) | 53 (56.4%) | 11 (44.0%) | ||
| Widowed | 85 (7.8%) | 20 (8.7%) | 11 (11.7%) | 3 (12.0%) | ||
| Divorced | 135 (12.4%) | 29 (12.6%) | 19 (20.2%) | 6 (24.0%) | ||
| Separated | 13 (1.2%) | 3 (1.3%) | 2 (2.1%) | 1 (4.0%) | ||
| Never | 96 (8.8%) | 16 (6.9%) | 9 (9.6%) | 4 (16.0%) | ||
| Education | 0.093 | 0.264 | ||||
| No college degree | 372 (34.1%) | 89 (38.5%) | 48 (51.1%) | 15 (60.0%) | ||
| College degree | 362 (33.2%) | 72 (31.2%) | 25 (26.6%) | 4 (16.0%) | ||
| Graduate degree | 358 (32.8%) | 70 (30.3%) | 21 (22.3%) | 6 (24.0%) | ||
| Employed | 656 (59.9%) | 143 (61.9%) | 0.042 | 60 (63.8%) | 16 (64.0%) | 0.004 |
| Insured | 1074 (98.4%) | 226 (97.8%) | 0.038 | 88 (93.6%) | 24 (96.0%) | 0.108 |
| Smoking | 0.024 | 0.315 | ||||
| Never | 590 (54.6%) | 127 (55.2%) | 53 (57.0%) | 12 (48.0%) | ||
| Current | 48 (4.4%) | 11 (4.8%) | 6 (6.5%) | 4 (16.0%) | ||
| Former | 443 (41.0%) | 92 (40.0%) | 34 (36.6%) | 9 (36.0%) | ||
| Alcohol use | 0.059 | 0.293 | ||||
| None | 180 (16.5%) | 43 (18.6%) | 26 (27.7%) | 4 (16.0%) | ||
| Minimal | 815 (74.7%) | 167 (72.3%) | 60 (63.8%) | 18 (72.0%) | ||
| Moderate | 96 (8.8%) | 21 (9.1%) | 8 (8.5%) | 3 (12.0%) | ||
| Charlson Comorbidity Index | 0.34 (0.82) | 0.43 (0.76) | 0.118 | 0.45 (0.88) | 0.62 (0.97) | 0.187 |
| Symptomatic | 485 (44.3%) | 123 (53.2%) | 0.181 | 52 (55.3%) | 17 (65.4%) | 0.207 |
| BMI, kg/m2 | 28.70 (4.53) | 29.73 (4.66) | 0.226 | 29.48 (4.48) | 31.62 (4.64) | 0.470 |
| Knee injections | 23 (2.1%) | 19 (8.2%) | 0.280 | 5 (5.3%) | 3 (11.5%) | 0.225 |
| Knee injuries | 38 (3.5%) | 15 (6.5%) | 0.140 | 2 (2.1%) | 6 (23.1%) | 0.665 |
| Gait speed, m/s | 1.36 (0.20) | 1.31 (0.20) | 0.273 | 1.26 (0.22) | 1.19 (0.26) | 0.258 |
| WOMAC pain | 10.59 (14.00) | 18.41 (19.26) | 0.465 | 16.65 (19.98) | 26.98 (23.70) | 0.471 |
| K-L grade | 0.251 | 0.320 | ||||
| 2 | 621 (56.6%) | 104 (45.0%) | 56 (59.6%) | 13 (50.0%) | ||
| 3 | 365 (33.3%) | 90 (39.0%) | 30 (31.9%) | 8 (30.8%) | ||
| 4 | 111 (10.1%) | 37 (16.0%) | 8 (8.5%) | 5 (19.2%) | ||
Figures are n (%) or mean (SD).
BMI, body mass index; K-L grade, Kellgren–Lawrence grade; SMD, standardized mean difference; SD, standard deviation; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index.
Time-specific estimates
The average causal effects (Table 3) of being treated versus not treated at Time 1 on knee pain severity at Time 2 among observations within subgroups of the modifier were −0.10 (95% CI: −9.94, 9.74) and −0.77 (95% CI: −3.24, 1.71) for those with and without depressive symptoms, respectively. After 3 years, the average causal effect of treatment versus no treatment at Time 3 on knee pain severity at Time 4 changed little among observations without depressive symptoms (1.00; 95% CI: −1.22, 3.21). By contrast, the average causal effects of being treated versus not treated at Times 2 and 3 on knee pain severity at Times 3 and 4 increased to -6.01 (95% CI: −16.34, 4.32) and −16.67 (95% CI: −26.33, −7.01) among observations with depressive symptoms, respectively. Thus, differences in average causal effects for treated versus not treated between person-time with and without depressive symptoms increased in magnitude: −1.10 (95% CI: −11.19, 9.00) at Time 2, −5.24 (95% CI: −15.88, 5.39) at Time 3 and −16.53 (95% CI: −26.75, −6.31) at Time 4. Results from a naïve outcome model with bias showed similar time trends for treatment-associated differences in knee pain severity between observations with and without depressive symptoms (Table 4).
Table 3.
Average causal effects of oral analgesic treatment on knee pain severity in participants with and without depressive symptoms from an IPTW-RWR SNMM
| Treatment | Pain assessment | Depressive symptoms, µ (95% CI) | No depressive symptoms, µ (95% CI) | Difference, µ (95% CI) |
|---|---|---|---|---|
| T1 | T2 | –0.10 (–9.94, 9.74) | 1.00 (–1.22, 3.21) | –1.10 (–11.19, 9.00) |
| T2 | T3 | –6.01 (–16.34, 4.32) | –0.77 (–3.24, 1.71) | –5.24 (–15.88, 5.39) |
| T3 | T4 | –16.67 (–26.33, –7.01) | –0.14 (–3.33, 3.06) | –16.53 (–26.75, –6.31) |
| T1–T2 | T3 | –5.38 (–20.62, 9.85) | –2.14 (–5.10, 0.83) | –3.25 (–18.62, 12.13) |
| T1–T3 | T4 | –2.76 (–24.11, 18.60) | –0.75 (–6.61, 5.11) | –2.01 (–24.08, 20.06) |
µ, causal mean difference; CI, confidence interval; IPTW-RWR SNMM, inverse-probability-of-treatment-weighted regression-with-residuals structural nested mean model; T1, Time 1; T2, Time 2; T3, Time 3; T4, Time 4; T1–T2, Time 1–Time 2; T1–T3, Time 1–Time 3.
Table 4.
Average causal effects of oral analgesic treatment on knee pain severity in participants with and without depressive symptoms estimated from a naïve outcome model with bias
| Treatment | Pain assessment | Depressive symptoms, µ (95% CI) | No depressive symptoms, µ (95% CI) | Difference, µ (95% CI) |
|---|---|---|---|---|
| T1 | T2 | 5.12 (–4.43, 14.68) | 5.83 (3.29, 8.37) | –0.70 (–10.59, 9.18) |
| T2 | T3 | 1.22 (–7.24, 9.69) | 4.03 (1.58, 6.48) | –2.81 (–11.62, 6.00) |
| T3 | T4 | –6.55 (–16.65, 3.55) | 5.00 (2.35, 7.64) | –11.54 (–21.90, –1.19) |
| T1–T2 | T3 | 5.84 (–7.95, 19.63) | 7.48 (4.55, 10.41) | –1.63 (–15.48, 12.22) |
| T1–Y3 | T4 | 11.4 (–6.15, 28.95) | 12.38 (9.01, 15.75) | –0.98 (–18.63, 16.67) |
µ, causal mean difference; CI, confidence interval; T1, Time 1; T2, Time 2; T3, Time 3; T4, Time 4; T1–T2, Time 1–Time 2; T1–T3, Time 1–Time 3.
Cumulative estimates
The average causal effects for 2 years and 3 years of treatment versus no treatment were associated with small differences in knee pain severity at Times 3 and 4 among observations without depressive symptoms; estimates were −2.14 (95% CI: −5.10, 0.83) and −0.75 (95% CI: −6.61, 5.11), respectively. Among person-time with depressive symptoms, average causal effects comparing treatment versus no treatment for 2 and 3 years were slightly larger in magnitude than those without depressive symptoms, and knee pain severity was lower at Time 3 (−5.38; 95% CI: −20.62, 9.85) and Time 4 (−2.76; 95% CI: −24.11, 18.60). Differences in average causal effects for treated versus not treated between observations with and without depressive symptoms were similar in magnitude for 2 years (−3.25; 95% CI: −18.62, 12.13) and 3 years (−2.01; 95% CI: −24.08, 20.06) of treatment. The naïve outcome model with potential bias yielded differences in treatment-related associations between person-time with and without depressive symptoms, analogous to primary findings.
Discussion
The current study represents the first epidemiological assessment focused on examining how depression influences variability in oral analgesic treatment effectiveness among individuals with knee OA. Results showed that average causal effects for treatment versus no treatment among person-time without symptoms of depression were associated with negligible differences in knee pain severity. By contrast, observations with depressive symptoms and oral analgesic treatment had average causal effects that showed increasingly lower knee pain severity compared with person-time without treatment. Cumulative treatment for 2 or 3 years did not yield incrementally larger average causal effects, but did show consistently lower knee pain severity among person-time with and without depressive symptoms. Findings suggest that there are scenarios where persons with co-occurring knee OA and depressive symptoms could derive clinically meaningful benefit from oral analgesic treatment.
Unlike prior clinical research,39–41 results from this study showed no substantive treatment-related reductions in knee pain severity among person-time without depressive symptoms, although meta-analysis of RCTs has estimated the pooled treatment effect of analgesic pharmacotherapies to be 0.46 standard deviations.40 Knee OA patients often reduce medication dosage because of poor tolerability and take as little as needed to produce a ‘satisfactory’ state wherein they can function, and long-term oral analgesic treatment is associated with decreased effectiveness.25 Participants in the OAI without symptoms of depression have different characteristics and behaviours compared with patients who are enrolled into RCTs.42,43 These differences may explain the small magnitude and mixed directionality of the average causal effects for treated versus not treated observations without depressive symptoms. Prior research implies that self-reported knee pain severity changes little and is characterized by persistence rather than worsening symptoms: a contention supported by descriptive data from the current study.17 Thus, person-time without depressive symptoms comprise knee pain trajectories that have lower severity and less measurement variability.17 In contrast to these observations, person-time with depressive symptoms includes knee pain trajectories with higher severity that, in part, could be affected by recall bias and measurement response-shift related to depression.17,44 Future research with high-dimensional data is needed to assess the reproducibility and generalizability of results from this study.
Whereas prior evidence suggests that depression in knee OA is associated with inadequate pain relief among those receiving oral analgesics,22 results from the current study indicate that such treatment is associated with incrementally larger decreases in knee pain severity among observations with depressive symptoms. Moreover, the difference in average causal effects compared with observations without depressive symptoms at the final time point was clinically meaningful.36 Similarly, cumulative estimates for 2 and 3 years of treatment indicated lower knee pain severity across time, but the causal mean differences were not larger than 9.7 WOMAC units. Knee OA heterogeneity causes treatment variability, where effectiveness of therapies differs between clinical phenotypes.43 The only consistently identified knee OA subgroup is individuals with depressive symptoms,10,11 and OAI participants satisfying CES-D screening criteria for major depression have worsening depressive symptoms over time.33 Knee pain severity among person-time with depressive symptoms may be more closely related to affective symptomology than structural joint pathology.45 For example, research has shown that the onset, intensity and duration of knee pain is associated with the presence and severity of depressive symptoms.9,46 Given that greater duration of depressive symptoms is associated with dose-dependent effects among individuals with radiographic knee OA,19 the relationship between depression and knee pain severity may become more amenable to intervention using analgesic treatments, as the persistence of both continues over time. Despite being a patient subgroup often excluded in RCTs, results highlight the potential benefits of oral analgesic treatment in the ‘depression’ clinical phenotype, particularly patients with a history of chronic depressive symptoms and knee pain severity.
Findings must be interpreted in relation to their limitations. First, sample sizes regarding person-time with depressive symptoms limited measurement precision, but reflect the appropriate level of uncertainty related to the prevalence and natural epidemiology of depression in the general knee OA population. Frequencies of treated and untreated observations with depressive symptoms were also comparable to a single-site RCT focused on depression treatment among knee OA patients.47 Second, no information was available on medication adherence between time points. Depression in chronic diseases is associated with poorer medication adherence, leading to decreased effectiveness, and would bias results conservatively toward the null.48 Moreover, depressive symptoms defined with the CES-D are not equitable to major depression using diagnostic criteria, and there was potential for within-participant variability regarding severity of symptomatology between assessments.30 Finally, confounding by unmeasured factors could potentially bias results, as well as violations of other assumptions implicit to estimating moderated causal effects.27 Nonetheless, there would need to be (i) an unmeasured confounder (e.g. concomitant treatment) strongly associated with both treatment and outcome, or (ii) many weak unmeasured confounders to substantially bias findings.49
There are also multiple study strengths, which include a clear causal framework and temporal ordering between exposure, modifier and outcome. The OAI cohort is an extensively documented, high-quality, open-access resource with comprehensive longitudinal measures of radiographic disease severity, knee OA symptoms and a myriad of sociodemographic and clinical characteristics. This data richness permitted robust adjustment, both directly and indirectly, for an array of potential confounders that included concomitant treatments individuals may have received. Additionally, an augmented version of the IPTW-RWR SNMM approach was adapted to repeated measures outcome data and appropriately adjusted for time-dependent confounders, over-control of intermediate pathways and collider-stratification biases, and missing observations. Last, differences in oral analgesic treatment effectiveness were similar for estimates with and without potential biases, suggesting a high degree of internal validity, and the magnitude of treatment effect moderation increased after controlling for potential confounders and addressing other methodological issues.
Conclusion
Collectively, findings highlight the potential effectiveness of oral analgesic treatment as an intervention to alleviate joint pain exacerbated by depressive symptoms, and demonstrate the utility of SNMMs to evaluate time-varying effect moderation of causal treatment effects within prospective cohorts. However, estimated causal effects indicated that pain relief among person-time with radiographic knee OA and symptoms of depression only manifested and progressively became meaningfully greater than observations without depressive symptoms, over the entire study period. This result diverges from the contention that depression is related to inadequate pain relief among treated knee OA patients, and exhibits the relevance of using SNMMs to investigate how the effectiveness of standard interventions differs between subgroups typically excluded from RCTs. Nevertheless, it remains unclear whether the benefits of oral analgesic treatment among OAI participants with versus without depressive symptoms would be observed in experimental studies. Additional observational research using modern epidemiological methods (e.g. SNMMs) is needed to evaluate the effectiveness of real-world interventions, among generalizable patient samples, with representative treatment utilization patterns, to build upon and replicate the current study and develop new protocols combining different medical care modalities tailored to the specific clinical phenotypes in which they are applied.
Ethics approval
Study procedures were followed in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with Helsinki Declaration of 1975, as revised in 2000. Institutional review boards at participating universities (University of Maryland/Johns Hopkins University; Memorial Hospital of Rhode Island; Ohio State University; University of Pittsburgh) and the OAI coordinating centre (University of California, San Francisco) approved the study.
Supplementary Material
Acknowledgements
The OAI is a public-private partnership comprised of five contracts (N01-AR-2–2258; N01-AR-2–2259; N01-AR-2–2260; N01-AR-2–2261; N01-AR-2–2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript was prepared using an OAI public use dataset and does not necessarily reflect the opinions or views of the OAI investigators, the NIH or the private funding partners.
Contributor Information
Alan M Rathbun, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA; Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA.
Michelle D Shardell, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA.
Joseph J Gallo, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Alice S Ryan, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA; Geriatric Research Education and Clinical Center, VA Maryland Health Care System, Baltimore, MD, USA.
Elizabeth A Stuart, Department of Mental Health, Johns Hopkins Bloomberg School of Public Health, Baltimore, MD, USA.
Megan S Schuler, RAND Corporation, Arlington, VA, USA.
Yu Dong, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA.
Brock Beamer, Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA; Geriatric Research Education and Clinical Center, VA Maryland Health Care System, Baltimore, MD, USA.
Rhea Mehta, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA.
Jason E Peer, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD, USA; Mental Health Clinical Care Center, VA Maryland Health Care System, Baltimore, MD, USA.
Marc C Hochberg, Department of Epidemiology and Public Health, University of Maryland School of Medicine, Baltimore, MD, USA; Department of Medicine, University of Maryland School of Medicine, Baltimore, MD, USA; Medical Clinical Care Center, VA Maryland Health Care System, Baltimore, MD, USA.
Data Availability
The data that support the findings of this study are available upon request. These data were derived from the resources available in the public domain [https://nda.nih.gov/oai/].
Supplementary data
Supplementary data are available at IJE online.
Author contributions
A.M.R. participated in study design and data acquisition, conducted statistical analyses, interpreted results and drafted and wrote the manuscript. M.D.S., E.A.S. and M.S.S. participated in study design and statistical analyses, interpreted results and critically revised the manuscript for important intellectual content. R.M and M.C.H. participated in study design and data acquisition, interpreted results and critically revised the manuscript for important intellectual content. J.J.G., A.S.R., Y.D., B.B. and J.E.P. interpreted results and critically revised the manuscript for important intellectual content. A.M.R. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors read and approved the final version of the manuscript for publication.
Funding
This work was supported by the Rheumatology Research Foundation’s Scientist Development Award and grants from the National Institute on Aging [K01 AG064041].
Conflict of interest
M.C.H. is the president of Rheumcon and receives consulting fees from Bone Therapeutics, Bristol-Myers Squibb, Eli Lilly, Galapagos, IBSA Insititut Biotechniq SA, Novartis Pharma AG, Pfizer, Samumed LLC, Theralogix LLC and Kolon TissueGene. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage 2013;21:1145–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lane NE, Brandt K, Hawker G et al. OARSI-FDA initiative: defining the disease state of osteoarthritis. Osteoarthritis Cartilage 2011;19:478–82. [DOI] [PubMed] [Google Scholar]
- 3. Hawker GA. Osteoarthritis is a serious disease. Clin Exp Rheumatol 2019;37(Suppl 120):3–6. [PubMed] [Google Scholar]
- 4. Kolasinski SL, Neogi T, Hochberg MC et al. 2019 American College of Rheumatology/Arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol 2020;72:220–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bair MJ, Matthias MS, Nyland KA et al. Barriers and facilitators to chronic pain self‐management: a qualitative study of primary care patients with comorbid musculoskeletal pain and depression. Pain Med 2009;10:1280–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Stubbs B, Aluko Y, Myint PK, Smith TO. Prevalence of depressive symptoms and anxiety in osteoarthritis: a systematic review and meta-analysis. Age Ageing 2016;45:228–35. [DOI] [PubMed] [Google Scholar]
- 7. Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord 2008;9:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Axford J, Heron C, Ross F, Victor CR. Management of knee osteoarthritis in primary care: pain and depression are the major obstacles. J Psychosom Res 2008;64:461–67. [DOI] [PubMed] [Google Scholar]
- 9. Furlough K, Miner H, Crijns TJ, Jayakumar P, Ring D, Koenig K. What factors are associated with perceived disease onset in patients with hip and knee osteoarthritis? J Orthop 2021;26:88–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Knoop J, van der Leeden M, Thorstensson CA et al. Identification of phenotypes with different clinical outcomes in knee osteoarthritis: data from the Osteoarthritis Initiative. Arthritis Care Res (Hoboken) 2011;63:1535–42. [DOI] [PubMed] [Google Scholar]
- 11. Van der Esch M, Knoop J, van der Leeden M et al. Clinical phenotypes in patients with knee osteoarthritis: a study in the Amsterdam osteoarthritis cohort. Osteoarthritis Cartilage 2015;23:544–49. [DOI] [PubMed] [Google Scholar]
- 12. Goesling J, Clauw DJ, Hassett AL. Pain and depression: an integrative review of neurobiological and psychological factors. Curr Psychiatry Rep 2013;15:421. [DOI] [PubMed] [Google Scholar]
- 13. Hawker GA, Gignac MAM, Badley E et al. A longitudinal study to explain the pain‐depression link in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1382–90. [DOI] [PubMed] [Google Scholar]
- 14. Kroenke K, Wu J, Bair MJ, Krebs EE, Damush TM, Tu W. Reciprocal relationship between pain and depression: a 12-month longitudinal analysis in primary care. J Pain 2011;12:964–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rathbun AM, Shardell MD, Ryan AS et al. Association between disease progression and depression onset in persons with radiographic knee osteoarthritis. Rheumatology (Oxford) 2020;59:3390–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Riddle DL, Kong X, Fitzgerald GK. Psychological health impact on 2-year changes in pain and function in persons with knee pain: data from the Osteoarthritis Initiative. Osteoarthritis Cartilage 2011;19:1095–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Collins JE, Katz JN, Dervan EE, Losina E. Trajectories and risk profiles of pain in persons with radiographic, symptomatic knee osteoarthritis: data from the osteoarthritis initiative. Osteoarthritis Cartilage 2014;22:622–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rathbun AM, Schuler MS, Stuart EA et al. Depression subtypes in individuals with or at risk for symptomatic knee osteoarthritis. Arthritis Care Res (Hoboken) 2020;72:669–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rathbun AM, Stuart EA, Shardell M, Yau MS, Baumgarten M, Hochberg MC. Dynamic effects of depressive symptoms on osteoarthritis knee pain. Arthritis Care Res (Hoboken) 2018;70:80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kingsbury SR, Hensor EM, Walsh CA, Hochberg MC, Conaghan PG. How do people with knee osteoarthritis use osteoarthritis pain medications and does this change over time? Data from the Osteoarthritis Initiative. Arthritis Res Ther 2013;15:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Langer RD. The role of medications in successful aging. Climacteric 2021;24:505–12. [DOI] [PubMed] [Google Scholar]
- 22. Conaghan PG, Peloso PM, Everett SV et al. Inadequate pain relief and large functional loss among patients with knee osteoarthritis: evidence from a prospective multinational longitudinal study of osteoarthritis real-world therapies. Rheumatology (Oxford) 2015;54:270–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Robins JM, Hernan MA, Rotnitzky A. Effect modification by time-varying covariates. Am J Epidemiol 2007;166:994–1002. discussion 1003–1004. [DOI] [PubMed] [Google Scholar]
- 24. Du Vaure CB, Dechartres A, Battin C, Ravaud P, Boutron I. Exclusion of patients with concomitant chronic conditions in ongoing randomised controlled trials targeting 10 common chronic conditions and registered at ClinicalTrials. gov: a systematic review of registration details. BMJ Open 2016;6:e012265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gregori D, Giacovelli G, Minto C et al. Association of pharmacological treatments with long-term pain control in patients with knee osteoarthritis: a systematic review and meta-analysis. JAMA 2018;320:2564–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Robins JM, Hernán MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology 2000;11:550–60. [DOI] [PubMed] [Google Scholar]
- 27. Wodtke GT, Almirall D. Estimating moderated causal effects with time-varying treatments and time-varying moderators: structural nested mean models and regression with residuals. Sociol Methodol 2017;47:212–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lester G. Clinical research in OA–the NIH osteoarthritis initiative. J Musculoskelet Neuronal Interact 2008;8:313–14. [PubMed] [Google Scholar]
- 29. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis 1957;16:494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Radloff LS. The CES-D scale a self-report depression scale for research in the general population. App Psychol Meas 1977;1:385–401. [Google Scholar]
- 31. Smarr KL, Keefer AL. Measures of depression and depressive symptoms: Beck Depression Inventory‐II (BDI‐II), Center for Epidemiologic Studies Depression Scale (CES‐D), Geriatric Depression Scale (GDS), Hospital Anxiety and Depression Scale (HADS), and Patient Health Questionnaire‐9 (PHQ‐9). Arthritis Care Res (Hoboken) 2011;63(Suppl 11):S454–66. [DOI] [PubMed] [Google Scholar]
- 32. Shankman SA, Funkhouser CJ, Klein DN, Davila J, Lerner D, Hee D. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM‐5 (SCID). Int J Methods Psychiatr Res 2018;27:e1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. White DK, Neogi T, Zhang Y, Niu J, Katz PP. The association of slow gait speed with trajectories of worsening depressive symptoms in knee osteoarthritis: An observational study. Arthritis Care Res (Hoboken) 2017;69:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. VanderWeele TJ, Robins JM. Four types of effect modification: a classification based on directed acyclic graphs. Epidemiology 2007;18:561–68. [DOI] [PubMed] [Google Scholar]
- 35. Bellamy N. Validation study of WOMAC: a health status instrument for measuring clinically-important patient-relevant outcomes following total hip or knee arthroplasty in osteoarthritis. J Orthop Rheumatol 1988;1:95–108. [PubMed] [Google Scholar]
- 36. Ehrich EW, Davies GM, Watson DJ, Bolognese JA, Seidenberg BC, Bellamy N. Minimal perceptible clinical improvement with the Western Ontario and McMaster Universities osteoarthritis index questionnaire and global assessments in patients with osteoarthritis. J Rheumatol 2000;27:2635–41. [PubMed] [Google Scholar]
- 37. Charlson M, Szatrowski TP, Peterson J, Gold J. Validation of a combined comorbidity index. J Clin Epidemiol 1994;47:1245–51. [DOI] [PubMed] [Google Scholar]
- 38. Imai K, King G, Stuart EA. Misunderstandings between experimentalists and observationalists about causal inference. J R Stat Soc Series A Stat Soc 2008;171:481–502. [Google Scholar]
- 39. Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med 2015;162:46–54. [DOI] [PubMed] [Google Scholar]
- 40. Henriksen M, Hansen JB, Klokker L, Bliddal H, Christensen R. Comparable effects of exercise and analgesics for pain secondary to knee osteoarthritis: a meta-analysis of trials included in Cochrane systematic reviews. J Comp Eff Res 2016;5:417–31. [DOI] [PubMed] [Google Scholar]
- 41. da Costa BR, Pereira TV, Saadat P et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ 2021;375:n2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nevitt MC, Felson DT, Lester G. The Osteoarthritis Initiative. Protocol for the Cohort Study. National Institute of Arthritis, Musculoskeletal and Skin Diseases. V 1.1 6.21.06. https://oai.epi-ucsf.org/datarelease/About.asp (13 October 2023, date last accessed).
- 43. Felson DT. Identifying different osteoarthritis phenotypes through epidemiology. Osteoarthritis Cartilage 2010;18:601–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Previtali D, Boffa A, Di Martino A, Deabate L, Delcogliano M, Filardo G. Recall bias affects pain assessment in knee osteoarthritis: a pilot study. Cartilage 2022;13:50–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kopp B, Furlough K, Goldberg T, Ring D, Koenig K. Factors associated with pain intensity and magnitude of limitations among people with hip and knee arthritis. J Orthop 2021;25:295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fonseca-Rodrigues D, Rodrigues A, Martins T et al. Correlation between pain severity and levels of anxiety and depression in osteoarthritis patients: a systematic review and meta-analysis. Rheumatology (Oxford) 2021;61:53–75. [DOI] [PubMed] [Google Scholar]
- 47. O'Moore KA, Newby JM, Andrews G et al. Internet cognitive behaviour therapy for depression in older adults with knee osteoarthritis: a randomized controlled trial. Arthritis Care Res (Hoboken) 2018;70:61–70. [DOI] [PubMed] [Google Scholar]
- 48. Grenard JL, Munjas BA, Adams JL et al. Depression and medication adherence in the treatment of chronic diseases in the United States: a meta-analysis. J Gen Intern Med 2011;26:1175–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Groenwold RHH, Sterne JAC, Lawlor DA, Moons KGM, Hoes AW, Tilling K. Sensitivity analysis for the effects of multiple unmeasured confounders. Ann Epidemiol 2016;26:605–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available upon request. These data were derived from the resources available in the public domain [https://nda.nih.gov/oai/].