Abstract
Background
Gastroschisis is a congenital anomaly of the abdominal wall with an unknown aetiology. Recent trends in the prevalence of gastroschisis suggest that changing environmental or behavioural factors may contribute. We examined whether prenatal cannabis use disorder was associated with gastroschisis.
Methods
The Study of Outcomes of Mothers and Infants is a population-based cohort compiled of California birth records that have been linked to Department of Health Care Access and Information hospitalization, emergency department and ambulatory surgery records. We included 2007–19 singleton live births (n = 5 774 656). Cannabis use disorder was measured by diagnosis codes at any visit during pregnancy or at birth. Gastroschisis was measured by diagnosis or surgical repair procedure codes at birth or during the first year of life.
Results
The prevalence of cannabis use disorder was about 1%. The prevalence of gastroschisis was 0.14% and 0.06% among those with and without cannabis use disorder, respectively. There were positive associations between cannabis use disorder and gastroschisis when using a multivariable model [adjusted risk ratio (aRR) = 1.3, 95% confidence interval (CI) 1.0, 1.7) and a matched sample approach (aRR = 1.5, 95% CI 1.1, 2.1). The association varied by maternal age and was largest among people aged >34 years (aRR = 2.5, 95% CI 1.0, 5.8).
Conclusions
We confirm findings of a positive association between cannabis exposure and gastroschisis and add that it is strongest when maternal age is greater than 34 years. More investigation into whether the association is causal, and why the association varies by maternal age, is encouraged.
Keywords: Cannabis, gastroschisis, birth defect, sibling design
Key Messages.
We examined associations between prenatal cannabis use disorder and gastroschisis in a population-based cohort of >5 million births.
Prenatal cannabis use disorder was associated with a small increase in risk of gastroschisis in multivariable and matched sample models.
The association was modified by maternal age such that it was strongest among people aged >34 years.
Introduction
Gastroschisis is an abdominal wall defect causing fetal intestines to develop outside the abdominal cavity. The defect develops during the first trimester and is identified on prenatal ultrasound scans.1–3 Several hypotheses exist, though the pathogenesis and aetiology of gastroschisis are unknown.4–9
The strongest risk factor for gastroschisis is young maternal age.9–11 Individuals with maternal age <20 years have 5- to 10-fold higher odds of gastroschisis compared with individuals aged 25 years or older.11–13 Curiously, birth rates for females aged 15–19 in the USA dropped dramatically between 1990 and 2020, but there were no concomitant declines in gastroschisis prevalence.14,15 Rather, the US prevalence of gastroschisis increased from 1997 (2.9 per 1000 infants) to 2008 (6.4 per 1000 infants) before declining back to pre-2000 levels by 2018 (3.3 per 1000 infants).1,13 Considering these trends, gastroschisis risk cannot be explained only by young maternal age. Changes in environmental or behavioural exposures likely contribute.
One proposed causal exposure is prenatal cannabis use, which increased in prevalence among pregnant individuals between 2002 and 2014.16 Reece and Hulse recently cited 2.5- to 3-fold increases in gastroschisis prevalence and similar increases in cannabis use in California, deeming these parallel trends a ‘can[ary] in the Californian coal mine’.17 Similar ecological patterns were observed in Canada and Europe.18,19 Whereas it is plausible that cannabis use is contributing to trends in gastroschisis, causal inference is limited with ecological data.
Three cohort and three case-control studies with individual-level data estimated positive unadjusted associations between prenatal cannabis exposure and gastroschisis.20–25 Among these, only the case-control studies adjusted for sociodemographic characteristics and other prenatal drug exposures, reporting adjusted odds ratios of 1.2 [95% confidence interval (CI) 0.8, 1.7],25 3.0 (95% CI 1.3, 5.7)24 and 2.2 (95% CI 1.0, 4.8).21
Future research requires prospective cohort studies with adjustment for confounding variables. Leveraging population-based birth cohort data from California, we estimated the association between prenatal cannabis use disorder diagnosis and gastroschisis. Secondarily, we evaluated whether the association varied by maternal age.
Methods
The Study of Outcomes in Mothers and Infants (SOMI) is a population-based cohort study compiled from 2007 to 2019 California live birth records. Individual records were probabilistically linked to Department of Health Care Access and Information (HCAI) hospitalization, emergency department and ambulatory surgery records for the person giving birth and the infant. There were 6 934 814 live births between 2007 and 2019. Of these, we included singleton births, births with gestational age between 20 and 44 weeks, and births where linkage between birth records and HCAI was possible (n = 5 774 656) (Supplementary Figure S1, available as Supplementary data at IJE online). The SOMI study was approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California and the University of California San Diego Human Research Protections Program.
HCAI hospitalization records include procedure and diagnosis codes recorded using the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9) and the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10). We used ICD codes to identify individuals with cannabis use disorder diagnosed at any visit during pregnancy or during the birth episode. We also identified gastroschisis cases from ICD codes in HCAI files, coding individuals with either a diagnosis of gastroschisis or a procedure code for gastroschisis repair as a gastroschisis case. In Supplementary Table S1, available as Supplementary data at IJE online, we compared the prevalence of gastroschisis when using different criteria.26 Prior research comparing case ascertainment using diagnostic and procedure codes with second trimester ultrasound findings from the California Prenatal Screening Program reported more than 96% agreement in the identification of gastroschisis.11
Other variables measured in the birth record were maternal age (in years), self-reported race/ethnicity (non-Hispanic White, Hispanic Mexican, Other Hispanic, Black, American Indian or Alaska Native, Native Hawaiian or Pacific Islander, Asian, two or more races, other or unknown), payer for delivery (private insurance, public insurance, self-pay, other, unknown), maternal education (≤12 years, >12 years, unknown), pre-pregnancy body mass index (underweight (<18.5 kg/m2), normal (18.5–<25 kg/m2), overweight (25–<30 kg/m2), obese (30 or more kg/m2), unknown), and nulliparity. We assigned the National Center for Health Statistics urban-rural classification system categories (large central fringe, metro, medium metro or small metro, micropolitan or non-core, unknown) based on reported county of residence at delivery.27 Other measured variables included pre-pregnancy diabetes, pre-pregnancy hypertension, tobacco use in pregnancy, alcohol use disorder, non-cannabis substance use disorder, major depressive disorder, anxiety disorder and bipolar disorder. We coded these variables as present if there was an indication in either the birth record or linked HCAI files (Supplementary Table S2, available as Supplementary data at IJE online). We used a directed acyclic graph (DAG) to inform selection of confounding variables (Supplementary Figure S2, available as Supplementary data at IJE online).
Statistical analysis
We described characteristics of the person giving birth according to the presence of cannabis use disorder diagnosis and, in Supplementary Table S3 (available as Supplementary data at IJE online), according to gastroschisis diagnosis. We plotted the prevalence of cannabis use disorder and the prevalence of gastroschisis by year. Within years, we estimated proportions of gastroschisis cases with maternal age <18 years, maternal age 18–34 years and maternal age >34 years.
We used sequentially adjusted modified Poisson regression models to estimate risk ratios (RRs) and 95% confidence intervals (CIs) for the association between cannabis use disorder and gastroschisis.28 The first model was unadjusted, the second model adjusted for maternal age and race/ethnicity and the third model additionally adjusted for nulliparity, county urbanicity, payer for delivery, nicotine-related diagnosis, non-cannabis drug use, alcohol use disorder, maternal depression and maternal body mass index. Missingness was treated as a valid response option for confounding variables.
Next, we matched individuals with a cannabis use disorder 1:2 to people without cannabis use disorder on maternal age (exact in years), year of delivery (exact), race/ethnicity (exact) and body mass index (exact category). We used a multi-level modified Poisson regression model [strata = matched pair identification (ID)] to estimate RRs and 95% CIs. The first set of models included only the random effect for the matching ID and so should be interpreted as having adjustment for the matching variables. The second model added multivariable adjustment for all other confounding variables.
Sensitivity analyses
We evaluated potential biases due to outcome misclassification, exposure misclassification and unmeasured confounding. In the first sensitivity analysis, we repeated multivariable regression analyses with differing definitions of gastroschisis, using only the gastroschisis repair procedure code to define the outcome. We did this because, whereas the diagnostic coding of gastroschisis and other abdominal wall abnormalities changed during the study period, the procedure code for gastroschisis repair was consistent across all years (Supplementary Table S1, available as Supplementary data at IJE online).26 In the second sensitivity analysis, we used the R package ‘episensr’ to conduct a probabilistic misclassification analysis assuming both non-differential and differential misclassification of cannabis use disorder and assuming specificity was good (0.9).29 For the non-differential misclassification analysis, we varied the sensitivity of cannabis use disorder from 0.2 to 0.8. For the differential misclassification analysis, we varied the sensitivity of cannabis use disorder only among those without gastroschisis, leaving sensitivity in the gastroschisis group at 0.9, until we found the combination that fully negated our findings. Our rationale was that individuals without a major structural defect like gastroschisis may be less likely to be evaluated for cannabis use disorder, resulting in a low sensitivity in this group.
In the next analyses we calculated the e-value, which measures the magnitude of the association that an unmeasured confounder would need to have with both the exposure and the outcome to negate the findings.29 Our final analysis to address unmeasured confounding was a sibling comparison design. We identified a subset of births where the person giving birth in two separate pregnancies was the same and where cannabis use disorder was present during one birth but not the other. We included only first (noted as nulliparous) and second live births for each birthing person. We then used a multi-level modified Poisson regression model (strata = sibling pair) to estimate RRs. These models adjusted for all confounding variables except those that did not change between pregnancies, which were adjusted by design. Finally, due to concerns that our restriction to nulliparous and second births favoured a very young distribution of maternal age, we re-ran the sibling analysis including only individuals where the first birth occurred after age 25.
Results
Approximately 1% (n = 50 435) of records had a diagnosis of cannabis use disorder recorded. Compared with those without a cannabis use disorder diagnosis, those with a diagnosis were more likely to have younger maternal age and less than 12 years of education. They were more likely to be Non-Hispanic White, Black or American Indian/Alaska Native, publicly insured and living in more rural counties. Finally, those with a cannabis use disorder diagnosis were more likely to have used nicotine during pregnancy, have an alcohol use or other substance use disorder and have a diagnosis of major depressive disorder, anxiety disorder or bipolar disorder (Table 1).
Table 1.
Characteristics of the birthing person stratified by presence of a cannabis use disorder diagnosis, Study of Outcomes in Mothers and Infants, 2007–19
| Cannabis use disorder (n = 50 435) |
No cannabis use disorder (n = 5 724 221) |
|||
|---|---|---|---|---|
| Characteristic | n | % | n | % |
| Age | ||||
| <18 years | 1671 | 3.31 | 117 327 | 2.05 |
| 18–25 years | 22 012 | 43.64 | 1 357 081 | 23.71 |
| 25–30 years | 14 314 | 28.38 | 1 521 117 | 26.57 |
| 30–35 years | 8271 | 16.40 | 1 585 735 | 27.70 |
| >35 years | 4165 | 8.26 | 1 142 785 | 19.96 |
| Unknown | <5 | 176 | <0.01 | |
| Race/ethnicity | ||||
| Non-Hispanic White | 17 098 | 33.90 | 1 499 067 | 26.19 |
| Hispanic-Mexican | 10 662 | 21.14 | 2 139 504 | 37.38 |
| Other Hispanic | 5869 | 11.64 | 734 452 | 12.83 |
| Black | 10 725 | 21.26 | 278 235 | 4.86 |
| American Indian/Alaska Native | 662 | 1.31 | 18 362 | 0.32 |
| Hawaiian/Pacific Islander | 205 | 0.41 | 22 834 | 0.40 |
| Asian | 588 | 1.17 | 797 288 | 13.93 |
| Two or more races | 3097 | 6.14 | 116 349 | 2.03 |
| Other or unknown | 1529 | 3.03 | 118 130 | 2.06 |
| Payer for delivery | ||||
| Private | 11 397 | 22.60 | 2 724 587 | 47.60 |
| Public | 37 614 | 74.58 | 2 736 799 | 47.81 |
| Self-pay | 940 | 1.86 | 159 232 | 2.78 |
| Other | 479 | 0.95 | 103 373 | 1.81 |
| Missing | 5 | 0.01 | 230 | <0.01 |
| Urban–rural classification | ||||
| Large central | 23 885 | 47.36 | 3 562 192 | 62.23 |
| Fringe metropolitan | 7510 | 14.89 | 712 929 | 12.45 |
| Medium/small metropolitan | 16 086 | 31.89 | 1 331 628 | 23.26 |
| Micropolitan/non-core | 2761 | 5.47 | 95 787 | 1.67 |
| Unknown | 193 | 0.38 | 21 685 | 0.38 |
| Education | ||||
| LT or EQ to 12 years | 12 989 | 25.75 | 1 105 061 | 19.31 |
| >12 years | 34 634 | 68.67 | 4 382 717 | 76.56 |
| Unknown | 2812 | 5.58 | 236 443 | 4.13 |
| Pre-pregnancy health | ||||
| Diabetes | 956 | 1.90 | 65 563 | 1.15 |
| Hypertension | 2354 | 4.67 | 116 060 | 2.0 |
| Nulliparous | 21 421 | 42.47 | 2 216 704 | 38.72 |
| Mental health | ||||
| Major depressive disorder | 6986 | 13.85 | 130 212 | 2.27 |
| Anxiety disorder | 7300 | 14.47 | 165 004 | 2.88 |
| Bipolar disorder | 3748 | 7.43 | 29 151 | 0.51 |
| Substance use | ||||
| Non-cannabis drug-related diagnosisa | 12 177 | 24.14 | 48 925 | 0.85 |
| Alcohol-related diagnosis | 2760 | 5.47 | 12 927 | 0.23 |
| Tobacco use in pregnancy | 16 950 | 33.61 | 126 513 | 2.21 |
| Body mass index | ||||
| Underweight | 2955 | 5.86 | 238 452 | 4.17 |
| Normal | 21 134 | 41.90 | 2 580 751 | 45.08 |
| Overweight | 11 606 | 23.01 | 1 417 615 | 24.77 |
| Obese | 11 923 | 23.64 | 1 203 933 | 21.03 |
| Missing | 2817 | 5.59 | 283 470 | 4.95 |
Data from cells with less than five births are suppressed.
LT: less than; EQ: equals.
Non-cannabis drug-related diagnoses included opioid-related disorders; sedative-, hypnotic-, or anxiolytic-related disorders; cocaine-related disorders; other stimulant-related disorders; hallucinogen-related disorders; inhalant-related disorders; and other psychoactive substance-related disorders (International Classification of Disease codes presented in Supplementary Table S2, available as Supplementary data at IJE online).
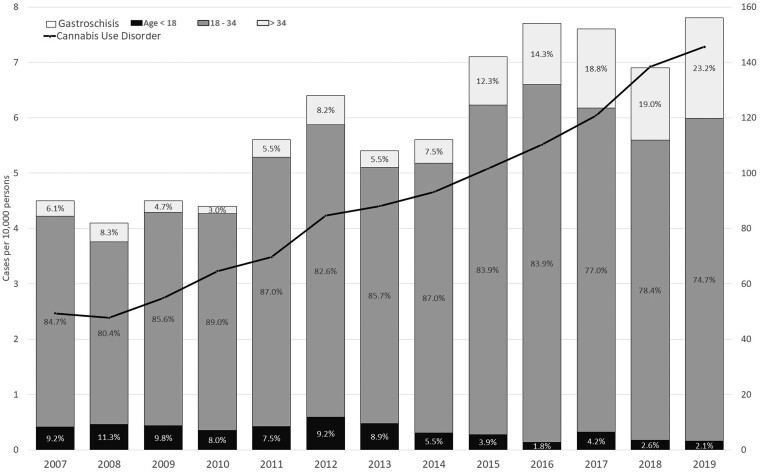
The prevalence of cannabis use disorder increased from 49.3 to 145.6 per 10 000 births between 2007 and 2019. The prevalence of gastroschisis increased from 4.5 to 7.8 per 10 000 births. The proportion of gastroschisis cases born to people with young maternal age declined over time. In 2007, 9.2% of gastroschisis cases had maternal age <18 and 6.1% of cases had maternal age >34. In 2019, these percentages were 2.1% and 23.2%, respectively (Figure 1).
Figure 1.
Trends in the prevalence of cannabis use disorder (right axis, line) and gastroschisis (left axis, bars) in California, 2007–19. The percentage inside each bar graph is the proportion of all gastroschisis cases in that year born to people with the ages highlighted. For example in 2007, 9.2% of the gastroschisis cases had maternal age <18, 84.7% of gastroschisis cases had maternal age 18–34 and 6.1% of gastroschisis cases had maternal age >34. Left axis: bar chart, prevalence of gastroschisis per 10 000 persons. Right axis: line chart, prevalence of cannabis use disorder per 10 000 persons
Among individuals with no cannabis use disorder diagnosis, the prevalence of gastroschisis was 6 per 10 000 births, or 0.06%. Among births with a cannabis use disorder diagnosis, the prevalence of gastroschisis was 14 per 10 000 births, or 2.5 (95% CI 2.0, 3.1) times higher. After adjustment for maternal age and race/ethnicity, the aRR was attenuated to 1.8 (95% CI 1.4, 2.3), and after full adjustment was 1.3 (95% CI 1.0, 1.7) (Table 2). Results were similar in the matched cohort [aRR = 1.5 (95% CI 1.1, 2.1)] (Table 2).
Table 2.
Associations between cannabis use disorder and gastroschisis, Study of Outcomes in Mothers and Infants, 2007–19
| Gastroschisis |
RR (95% CI) | aRR a (95% CI) | aRR b (95% CI) | ||
|---|---|---|---|---|---|
| Cannabis use disorder | n | % | |||
| Full sample | |||||
| Yes (n = 50 435) | 72 | 0.14 | 2.5 (2.0–3.1) | 1.8 (1.4–2.3) | 1.3 (1.0–1.7) |
| No (n = 5 724 221) | 3317 | 0.06 | 1.0 (ref) | 1.0 (ref) | 1.0 (ref) |
| Matched samplec | |||||
| Yes (n = 50 358) | 72 | 0.14 | 1.5 (1.1–2.0) | 1.5 (1.1–2.1) | |
| No (n = 100 716) | 97 | 0.10 | 1.0 (ref) | n/a | 1.0 (ref) |
aRR, adjusted risk ratio; CI, confidence interval; RR, risk ratio; .ref, reference value; n/a, not available.
Risk ratio adjusted for maternal age in years and race/ethnicity.
Risk ratio adjusted for maternal age in years, nulliparity, race/ethnicity, county urbanicity, payer, tobacco use, non-cannabis drug use, alcohol use disorder, maternal depression and maternal body mass index.
Matched cohort includes individual matching 1:2 (exposed to cannabis use disorder: unexposed to cannabis use disorder) on race (exact), birth year (exact to rounded year), maternal age (exact) and body mass index (exact category: underweight, normal, overweight, obese). Exposed births that were not matched to two unexposed births were excluded from the matched analyses.
After stratifying by maternal age, the baseline incidence of gastroschisis was much higher at lower maternal ages. Among individuals with no cannabis use disorder, the prevalence of gastroschisis was 0.17% among individuals aged <18, 0.06% among individuals aged 18–34 and 0.03% among individuals aged >34. In fully adjusted models, there was no association between cannabis use disorder and gastroschisis in the youngest group (aRR = 0.9, 95% CI 0.3, 3.0), a small association among individuals aged 18–34 (aRR = 1.3, 95% CI 1.0, 1.7) and a larger association among individuals older than 34 years (aRR = 2.4, 95% CI 1.0, 5.8) (Table 3).
Table 3.
Associations between cannabis use disorder and gastroschisis stratified by maternal age, Study of Outcomes in Mothers and Infants, 2007–19
| n | n (%) with gastroschisis | RR | aRR | |
|---|---|---|---|---|
| Maternal age <18 years | ||||
| Cannabis use disorder | 1671 | 3 (0.18) | 1.0 (0.3–3.2) | 0.9 (0.3–3.0) |
| No cannabis use disorder | 117 327 | 203 (0.17) | ref | ref |
| Maternal age 18–34 years | ||||
| Cannabis use disorder | 44 597 | 63 (0.14) | 2.3 (1.8–3.0) | 1.3 (1.0–1.7) |
| No cannabis use disorder | 4 463 933 | 2742 (0.06) | ref | ref |
| Maternal age >34 years | ||||
| Cannabis use disorder | 4165 | 6 (0.14) | 4.4 (2.0–9.9) | 2.4 (1.0–5.8) |
| No cannabis use disorder | 1 142 785 | 372 (0.03) | ref | ref |
Multivariable model includes adjustment for race/ethnicity, county urbanicity, payer, tobacco use, other drug use, alcohol use disorder, maternal depression, maternal body mass index and nulliparity.
RR, risk ratio; aRR, adjusted risk ratio; ref, reference value.
In the first sensitivity analysis, re-running our main analyses with a different operationalization of the outcome did not change the findings (Supplementary Table S4, available as Supplementary data at IJE online). In the second sensitivity analysis, we found that to fully attenuate our observed RR to the null, there would need to be differential misclassification where the sensitivity in the no gastroschisis group was 0.5. Third, to assess potential unmeasured confounding, we estimated an e-value of 1.9, suggesting that an unmeasured confounder would need to have at least a 2-fold association with both the exposure and outcome to attenuate the reported adjusted estimate to the null value.29
Finally, in the sibling cohort, we identified 943 506 pairs of siblings, of whom 5524 had cannabis use disorder documented in the first birth only and 6501 had cannabis use disorder documented in the second birth only. Analysis of sibling pairs discrepant on exposure yielded an adjusted RR of 1.0 (95% CI 0.5, 1.9). After restricting the sibling analysis to birthing people where the first birth occurred at ages >25 years, the age-adjusted RR was 3.1 (95% CI 0.3, 29.4) (Supplementary Table S5, available as Supplementary data at IJE online).
Discussion
In this study, we estimated associations between cannabis use disorder and gastroschisis in a population-based cohort of births in California. The prevalence of both cannabis use disorder and gastroschisis increased between 2007 and 2019. There were small positive associations between cannabis use disorder and gastroschisis in adjusted models. These associations varied by maternal age, with stronger associations observed among individuals aged more than 34 years..
Anderson et al. previously reported a rise in California’s prevalence of gastroschisis from 1.5 cases per 10 000 births in 1995 to 5.3 cases per 10 000 births in 2012.10 We observed similar prevalence estimates for 2007 to 2012 and added that the estimates continued to increase through 2019. This rising prevalence of cannabis use disorder may reflect true increases in the occurrence of cannabis use disorder. It may also reflect shifting norms surrounding acceptance of cannabis use, leading to increased disclosure, screening and diagnosis in medical settings.
We reported that those with a cannabis use disorder diagnosis had a small increased risk in gastroschisis after adjustment for confounding variables and after exact matching on maternal age, race, birth year and body mass index. Our estimates were smaller than the adjusted odds ratios of 3.0 (95% CI 1.3, 6.8) reported by Torfs et al.24 and 2.2 (95% CI 1.0, 4.8) reported by Lam and Torfs.21 However, we note that the former did not adjust for maternal smoking, and neither adjusted for alcohol, which are confounding variables. Estimates without adjustment for these variables may be biased away from the null.
The magnitude of estimates from the multivariable and matched sample models were slightly larger than the estimate published by van Gelder et al., who reported an adjusted odds ratio of 1.2 (95% CI 0.8, 1.7) using data from the National Birth Defects Prevention case-control study.25 Of the prior studies, van Gelder et al. had the most comprehensive set of confounding variables, including sociodemographic variables, body mass index, maternal reported periconceptional smoking and drinking and folic acid supplementation.25 Notably, our study exposure, cannabis use disorder measured by diagnosis codes, is not directly comparable to the self-reported periconceptual cannabis use exposure examined by van Gelder et al.25 Our exposed group included individuals who were identified as having life problems related to their cannabis use and likely used cannabis more frequently than other cannabis users. If there is a causal effect of cannabis on gastroschisis, it is possible that having an exposed sample using cannabis with greater frequency or consequence is contributing to a larger effect estimate than what would be observed if evaluating normative cannabis use.
The stratification by maternal age was motivated by prior studies showing that the effects of cigarette smoking on gastroschisis were much larger at older maternal ages.12,30 Our results show the same phenomenon for cannabis use disorder. We reported no associations between cannabis use disorder and gastroschisis among births with maternal age <18 years and a doubling of gastroschisis risk with cannabis use disorder among births with maternal age >34 years. It is also noteworthy that when examining trends in gastroschisis prevalence over time, the proportion of cases born to people >34 years increased over the study period. It is possible that older birthing persons are more likely to have pre-pregnancy vascular diseases which leave them more vulnerable to the effects of vasoconstrictive exposures, one of the proposed risk factors for gastroschisis.30 This hypothesis could be investigated further by examining effect modification by vasoconstrictive exposures such as hypertension, cocaine use, or some therapeutic drugs. It is also possible that older maternal age is a marker for longer duration of cannabis exposure. More investigation of mechanisms explaining potentially different aetiological factors at different maternal ages is needed.
Last, we extended our interrogation of the association between cannabis use and gastroschisis with use of a sibling comparison design, by leveraging data from a subset of people with nulliparous and second births that were discrepant on exposure to cannabis use disorder. Estimates from this approach should be interpreted as adjusted for any shared confounders that are specific to the birthing person (e.g. genetic factors, common household factors) in addition to the measured confounders included in regression models. The RR estimated from the sibling comparison sample was 1.0, suggesting that the positive associations observed using previous approaches may have been inflated due to unmeasured confounding. However, this estimate should be viewed considering limitations. First, limiting the sibling sample to birthing persons’ nulliparous and second births shifted the age distribution of the sample to younger individuals. In post hoc analyses, we restricted our sibling sample to individuals where maternal age was older than 25 and observed a much larger RR (3.0), paralleling findings from the effect modification analyses. Another limitation is that discrepancies in cannabis use disorder across two pregnancies may have reflected differences in recording of the diagnosis and not differences in exposure to cannabis. Also, change of paternity between pregnancies, which has been linked to gastroschisis risk, was not considered.8
Other limitations should be considered. First, administrative data may have poor measurement of confounding variables, leaving unmeasured and residual confounding likely. Second, use of diagnosis codes in hospitalization records to identify cannabis use disorder most likely resulted in under-ascertainment of the exposure. Third, our sample was restricted to live births. If cannabis exposure in pregnancy caused either spontaneous abortion or stillbirth, our restriction to live births would have induced selection bias towards the null. Similarly, we lacked data on elective termination. Gastroschisis is readily prenatally diagnosed, and likelihood of termination may vary by socioeconomic and demographic factors. Our estimates may be biased by this non-random selection. The trend data could also be influenced by trends in termination of gastroschisis cases.
Despite limitations, we provide the most comprehensive investigation of the association between disordered cannabis use and gastroschisis to date. We used data from a large population-based sample, considered multiple approaches to adjust for confounding bias and conducted sensitivity analyses to account for confounding and information biases. Considering all analyses together, we conclude that cannabis use disorder and gastroschisis are correlated at the ecological and individual levels. The question of whether these associations are causal remains. We found that after adjustment for measured confounding variables, a small positive association remained which was strongest in individuals over age 34. These findings were reinforced by the sibling design. We encourage replication to see whether these findings persist across different settings.
Ethics approval
The Study of Outcomes in Mothers and Infants study was approved by the Committee for the Protection of Human Subjects within the Health and Human Services Agency of the State of California and the University of California San Diego Human Research Protections Program.
Supplementary Material
Contributor Information
Erin Delker, Department of Pediatrics, University of California San Diego, San Diego, CA, USA.
Rebecca J Baer, Department of Pediatrics, University of California San Diego, San Diego, CA, USA; California Preterm Birth Initiative, University of California San Francisco, San Francisco, CA, USA.
Ann E Kelly, Department of Pediatrics, University of California San Diego, San Diego, CA, USA.
Christina Chambers, Department of Pediatrics, University of California San Diego, San Diego, CA, USA.
Gretchen Bandoli, Department of Pediatrics, University of California San Diego, San Diego, CA, USA.
Data availability
The data underlying this article cannot be shared under the current Institutional Review Board agreement. We direct researchers to the California Department of Public Health Center for Health Statistics and Information, and the California Department of Health Care Access and Information for information on requesting and accessing California state data.
Supplementary data
Supplementary data are available at IJE online.
Author contributions
E.D. and G.B. conceived the study methods and design. R.J.B. and C.C. were responsible for data access and advised on the data analytical plan. C.C. and A.E.K. provided subject matter and clinical expertise. All authors were involved in the interpretation of the data. E.D. drafted the initial manuscript, which was reviewed and revised by all authors. All authors read and approved the final manuscript.
Funding
The study was supported by the Study of Mothers and Infants at the University of California San Diego. G.B. is funded by an National Institutes of Health. National Institute on Alcohol Abuse and Alcoholism Award (K01 AA027811).
Conflict of interest
None declared.
References
- 1. Bhat V, Moront M, Bhandari V. Gastroschisis: a state-of-the-art review. Children (Basel) 2020;7:E302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Saada J, Oury JF, Vuillard E et al. Gastroschisis. Clin Obstet Gynecol 2005;48:964–72. [DOI] [PubMed] [Google Scholar]
- 3. Lepigeon K, Van Mieghem T, Vasseur Maurer S, Giannoni E, Baud D. Gastroschisis—what should be told to parents? Prenat Diagn 2014;34:316–26. [DOI] [PubMed] [Google Scholar]
- 4. Beaudoin S. Insights into the etiology and embryology of gastroschisis. Semin Pediatr Surg 2018;27:283–88. [DOI] [PubMed] [Google Scholar]
- 5. Folkerth RD, Habbe DM, Boyd TK et al. ; Prenatal Alcohol, SIDS, and Stillbirth (PASS) Research Network. Gastroschisis, destructive brain lesions, and placental infarction in the second trimester suggest a vascular pathogenesis. Pediatr Dev Pathol 2013;16:391–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hoyme HE, Higginbottom MC, Jones KL. The vascular pathogenesis of gastroschisis: intrauterine interruption of the omphalomesenteric artery. J Pediatr 1981;98:228–31. [DOI] [PubMed] [Google Scholar]
- 7. Jones KL, Weiss LA, Hagey LR, Gonzalez V, Benirschke K, Chambers CD. Altered lipid metabolism in gastroschisis: a novel hypothesis. Am J Med Genet A 2013;161A:1860–65. [DOI] [PubMed] [Google Scholar]
- 8. Chambers CD, Chen BH, Kalla K, Jernigan L, Jones KL. Novel risk factor in gastroschisis: change of paternity. Am J Med Genet A 2007;143A:653–59. [DOI] [PubMed] [Google Scholar]
- 9. Chuaire Noack L. New clues to understand gastroschisis. Embryology, pathogenesis and epidemiology. Colomb Med (Cali) 2021;52:e4004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Anderson JE, Galganski LA, Cheng Y et al. Epidemiology of gastroschisis: a population-based study in California from 1995 to 2012. J Pediatr Surg 2018;53:2399–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baer RJ, Chambers CD, Jones KL et al. Maternal factors associated with the occurrence of gastroschisis. Am J Med Genet A 2015;167:1534–41. [DOI] [PubMed] [Google Scholar]
- 12. Feldkamp ML, Alder SC, Carey JC. A case control population-based study investigating smoking as a risk factor for gastroschisis in Utah, 1997-2005. Birth Defects Res A Clin Mol Teratol 2008;82:768–75. [DOI] [PubMed] [Google Scholar]
- 13. Clark RH, Sousa J, Laughon MM, Tolia VN. Gastroschisis prevalence substantially decreased from 2009 through 2018 after a 3-fold increase from 1997 to 2008. J Pediatr Surg 2020;55:2640–41. [DOI] [PubMed] [Google Scholar]
- 14.Martin JA, Hamilton BE, Osterman MJK et al. Births: Final data for 2015. National Vital Statistics Report, Vol. 66. Hyattsville, MD: National Center for Health Statistics, 2017. [PubMed]
- 15.Osterman MJK, Hamilton BE, Martin JA, Driscoll AK, Valenzuela CP. Births: Final data for 2020. National Vital Statistics Reports, Vol. 70. Hyattsville, MD: National Center for Health Statistics. 2022. 10.15620/cdc:112078. [DOI]
- 16. Brown QL, Sarvet AL, Shmulewitz D, Martins SS, Wall MM, Hasin DS. Trends in Marijuana use among pregnant and nonpregnant reproductive-aged women, 2002-2014. JAMA 2017;317:207–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Reece AS, Hulse GK. Gastroschisis and autism-dual canaries in the Californian coalmine. JAMA Surg 2019;154:366–67. [DOI] [PubMed] [Google Scholar]
- 18. Reece AS, Hulse GK. Canadian cannabis consumption and patterns of congenital anomalies: an ecological geospatial analysis. J Addict Med 2020;14:e195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Reece AS, Hulse GK. European epidemiological patterns of cannabis- and substance-related body wall congenital anomalies: geospatiotemporal and causal inferential study. IJERPH 2022;19:9027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bourque DK, Meng L, Dougan S et al. Gastroschisis in Ontario, Canada: 2012–2018. Birth Defects Res 2021;113:1044–51. [DOI] [PubMed] [Google Scholar]
- 21. Lam PK, Torfs CP. Interaction between maternal smoking and malnutrition in infant risk of gastroschisis. Birth Defects Res A Clin Mol Teratol 2006;76:182–86. [DOI] [PubMed] [Google Scholar]
- 22. Forrester MB, Merz RD. Risk of selected birth defects with prenatal illicit drug use, Hawaii, 1986-2002. J Toxicol Environ Health A 2007;70:7–18. [DOI] [PubMed] [Google Scholar]
- 23. Skarsgard ED, Meaney C, Bassil K et al. ; Canadian Pediatric Surgery Network (CAPSNet). Maternal risk factors for gastroschisis in Canada: Risk Factors for Gastroschisis in Canada. Birth Defects Res A Clin Mol Teratol 2015;103:111–18. [DOI] [PubMed] [Google Scholar]
- 24. Torfs CP, Velie EM, Oechsli FW, Bateson TF, Curry CJ. A population-based study of gastroschisis: demographic, pregnancy, and lifestyle risk factors. Teratology 1994;50:44–53. [DOI] [PubMed] [Google Scholar]
- 25. van Gelder MMHJ, Donders ART, Devine O, Roeleveld N, Reefhuis J; National Birth Defects Prevention Study. Using bayesian models to assess the effects of under-reporting of cannabis use on the association with birth defects, national birth defects prevention study, 1997-2005. Paediatr Perinat Epidemiol 2014;28:424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Williams CA, Hauser KW, Correia JA, Frias JL. Ascertainment of gastroschisis using the ICD-9-CM surgical procedure code. Birth Defects Res A Clin Mol Teratol 2005;73:646–48. [DOI] [PubMed] [Google Scholar]
- 27. Ingram DD, Franco SJ. 2013. NCHS urban–rural classification scheme for counties. National Center for Health Statistics. Vital Health Stat 2014;2. [PubMed] [Google Scholar]
- 28. Zou G. A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–706. [DOI] [PubMed] [Google Scholar]
- 29. Haine D. Package ‘episensr.’ 2023. https://cran.r-project.org/web/packages/episensr/episensr.pdf (16 February 2024, date last accessed).
- 30. Werler MM, Mitchell AA, Moore CA, Honein MA; National Birth Defects Prevention Study. Is there epidemiologic evidence to support vascular disruption as a pathogenesis of gastroschisis? Am J Med Genet A 2009;149A:1399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article cannot be shared under the current Institutional Review Board agreement. We direct researchers to the California Department of Public Health Center for Health Statistics and Information, and the California Department of Health Care Access and Information for information on requesting and accessing California state data.