Abstract
The thermodynamic forces on electrons (delta Eh) and protons (delta p) across mitochondrial complexes I, III and IV were measured in isolated mitochondria respiring on succinate. The force ratio (delta Eh/delta p) across complex I close to equilibrium was found to be about 2. The equilibrium force ratio across complex I was measured during sulphite oxidation and was again close to 2. These results indicate that the proton/electron stoichiometry of complex I is 2, in conditions of high protonmotive force.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Shawi M. K., Brand M. D. Steady-state H+/O stoichiometry of liver mitochondria. Biochem J. 1981 Dec 15;200(3):539–546. doi: 10.1042/bj2000539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavis A. D. Upper and lower limits of the charge translocation stoichiometry of cytochrome c oxidase. J Biol Chem. 1987 May 5;262(13):6174–6181. [PubMed] [Google Scholar]
- Beavis A. D. Upper and lower limits of the charge translocation stoichiometry of mitochondrial electron transport. J Biol Chem. 1987 May 5;262(13):6165–6173. [PubMed] [Google Scholar]
- Berry E. A., Hinkle P. C. Measurement of the electrochemical proton gradient in submitochondrial particles. J Biol Chem. 1983 Feb 10;258(3):1474–1486. [PubMed] [Google Scholar]
- Brand M. D., Reynafarje B., Lehninger A. L. Re-evaluation of the H+/site ratio of mitochondrial electron transport with the oxygen pulse technique. J Biol Chem. 1976 Sep 25;251(18):5670–5679. [PubMed] [Google Scholar]
- Brown G. C., Brand M. D. Thermodynamic control of electron flux through mitochondrial cytochrome bc1 complex. Biochem J. 1985 Jan 15;225(2):399–405. doi: 10.1042/bj2250399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabrini L., Landi L., Pasquali P., Lenaz G. Effect of endogenous ubiquinone on the interaction of exogenous Ubiquinone-1 with the respiratory chain of bovine heart mitochondria. Arch Biochem Biophys. 1981 Apr 15;208(1):11–19. doi: 10.1016/0003-9861(81)90117-x. [DOI] [PubMed] [Google Scholar]
- Cohen H. J., Betcher-Lange S., Kessler D. L., Rajagopalan K. V. Hepatic sulfite oxidase. Congruency in mitochondria of prosthetic groups and activity. J Biol Chem. 1972 Dec 10;247(23):7759–7766. [PubMed] [Google Scholar]
- Crane F. L. Hydroquinone dehydrogenases. Annu Rev Biochem. 1977;46:439–469. doi: 10.1146/annurev.bi.46.070177.002255. [DOI] [PubMed] [Google Scholar]
- Crompton M., Costi A., Hayat L. Evidence for the presence of a reversible Ca2+-dependent pore activated by oxidative stress in heart mitochondria. Biochem J. 1987 Aug 1;245(3):915–918. doi: 10.1042/bj2450915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F., Azzone G. F. Activation of site I redox-driven H+ pump by exogenous quinones in intact mitochondria. J Biol Chem. 1982 Apr 25;257(8):4106–4113. [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F., Lee C. P. Oxidation-reduction potentials of cytochromes in mitochondria. Biochemistry. 1970 Dec 22;9(26):5077–5082. doi: 10.1021/bi00828a006. [DOI] [PubMed] [Google Scholar]
- Freedman J. A., Lemasters J. J. Thermodynamics of reverse electron transfer across site 1: ATP/2e- is greater than one. Biochem Biophys Res Commun. 1984 Nov 30;125(1):8–13. doi: 10.1016/s0006-291x(84)80325-3. [DOI] [PubMed] [Google Scholar]
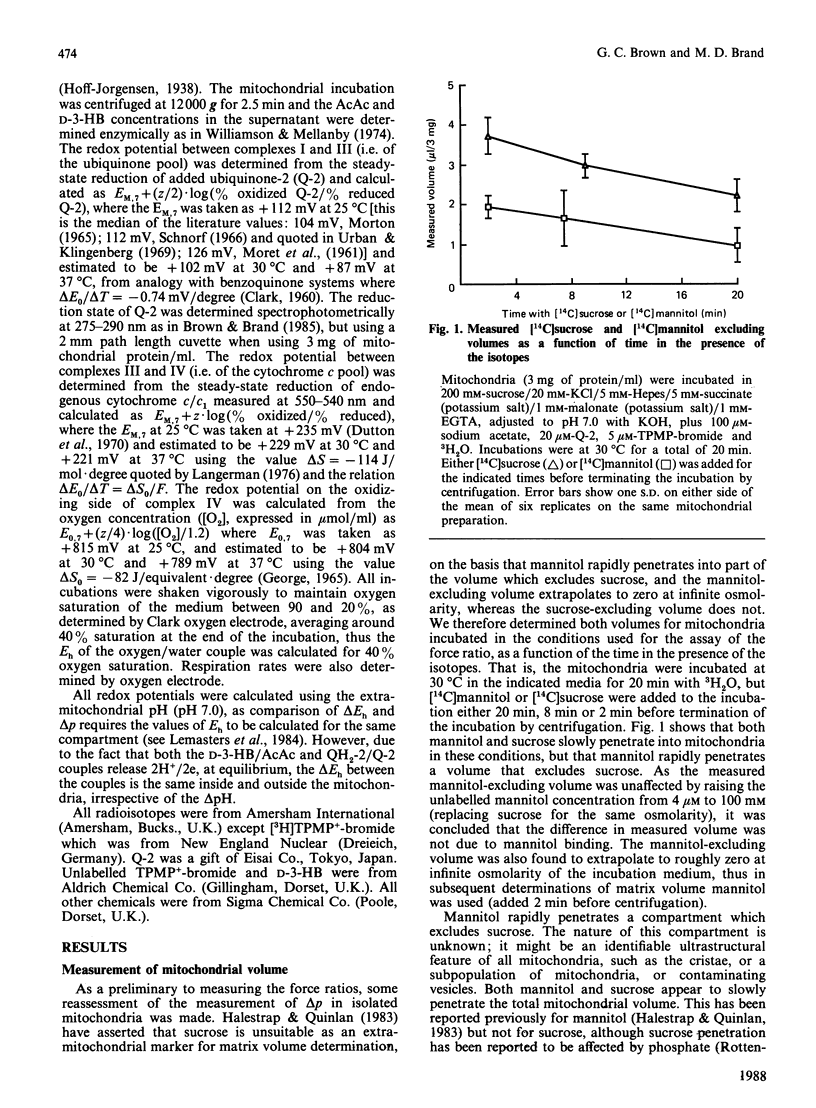
- Halestrap A. P., Quinlan P. T. The intramitochondrial volume measured using sucrose as an extramitochondrial marker overestimates the true matrix volume determined with mannitol. Biochem J. 1983 Aug 15;214(2):387–393. doi: 10.1042/bj2140387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson J. B., Nicholls D. G. Methods for the determination of membrane potential in bioenergetic systems. Methods Enzymol. 1986;127:557–577. doi: 10.1016/0076-6879(86)27044-5. [DOI] [PubMed] [Google Scholar]
- KREBS H. A., MELLANBY J., WILLIAMSON D. H. The equilibrium constant of the beta-hydroxybutyric-dehydrogenase system. Biochem J. 1962 Jan;82:96–98. doi: 10.1042/bj0820096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemasters J. J., Grunwald R., Emaus R. K. Thermodynamic limits to the ATP/site stoichiometries of oxidative phosphorylation by rat liver mitochondria. J Biol Chem. 1984 Mar 10;259(5):3058–3063. [PubMed] [Google Scholar]
- MORET V., PINAMONTI S., FORNASARI E. Polarographic study on the redox potential of ubiquinones. Biochim Biophys Acta. 1961 Dec 9;54:381–383. doi: 10.1016/0006-3002(61)90388-2. [DOI] [PubMed] [Google Scholar]
- Moyle J., Mitchell P. The proton-translocating nicotinamide-adenine dinucleotide (phosphate) transhydrogenase of rat liver mitochondria. Biochem J. 1973 Mar;132(3):571–585. doi: 10.1042/bj1320571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M. P., Brand M. D. Variable stoichiometry of proton pumping by the mitochondrial respiratory chain. Nature. 1987 Sep 10;329(6135):170–172. doi: 10.1038/329170a0. [DOI] [PubMed] [Google Scholar]
- Murphy M. P., Brown G. C., Brand M. D. Thermodynamic limits to the stoichiometry of H+ pumping by mitochondrial cytochrome oxidase. FEBS Lett. 1985 Jul 22;187(1):16–20. doi: 10.1016/0014-5793(85)81204-7. [DOI] [PubMed] [Google Scholar]
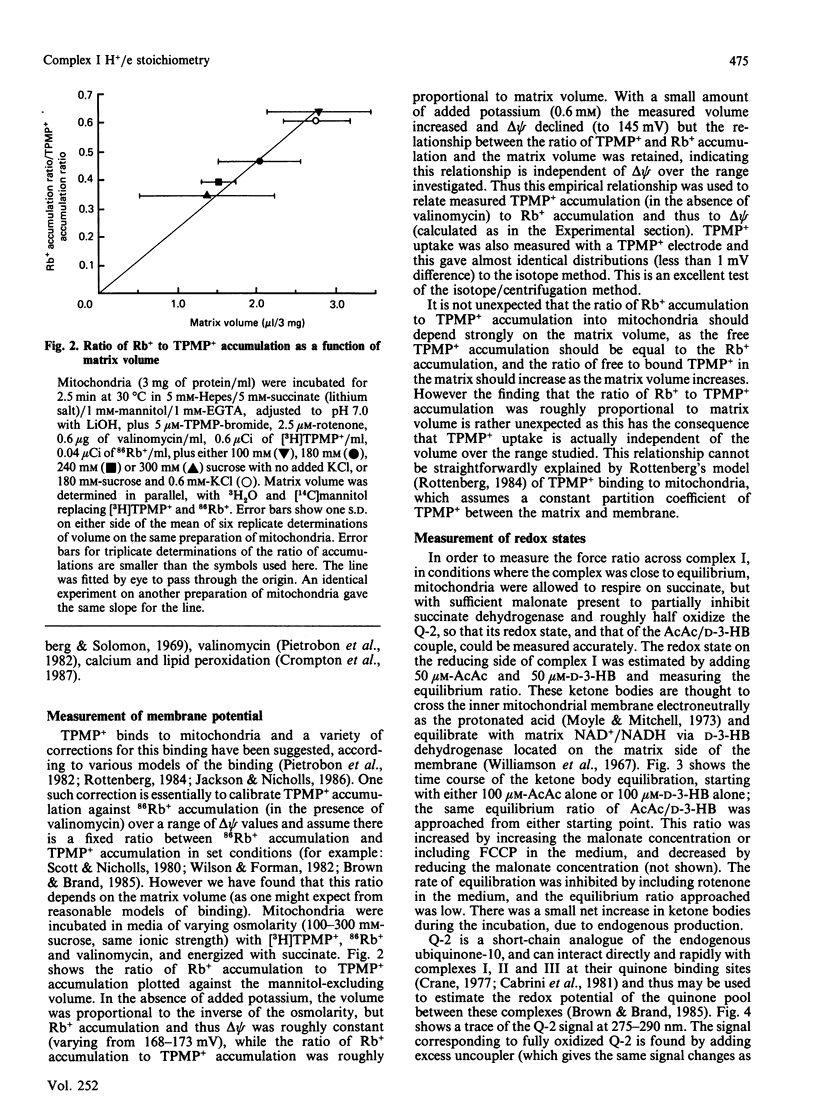
- Pietrobon D., Zoratti M., Azzone G. F., Stucki J. W., Walz D. Non-equilibrium thermodynamic assessment of redox-driven H+ pumps in mitochondria. Eur J Biochem. 1982 Oct;127(3):483–494. doi: 10.1111/j.1432-1033.1982.tb06897.x. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Gutman M. Control of the rate of reverse electron transport in submitochondrial particles by the free energy. Biochemistry. 1977 Jul 12;16(14):3220–3227. doi: 10.1021/bi00633a028. [DOI] [PubMed] [Google Scholar]
- Rottenberg H. Membrane potential and surface potential in mitochondria: uptake and binding of lipophilic cations. J Membr Biol. 1984;81(2):127–138. doi: 10.1007/BF01868977. [DOI] [PubMed] [Google Scholar]
- Rottenberg H., Solomon A. K. The osmotic nature of the ion-induced swelling of rat-liver mitochondria. Biochim Biophys Acta. 1969 Oct 14;193(1):48–57. doi: 10.1016/0005-2736(69)90057-1. [DOI] [PubMed] [Google Scholar]
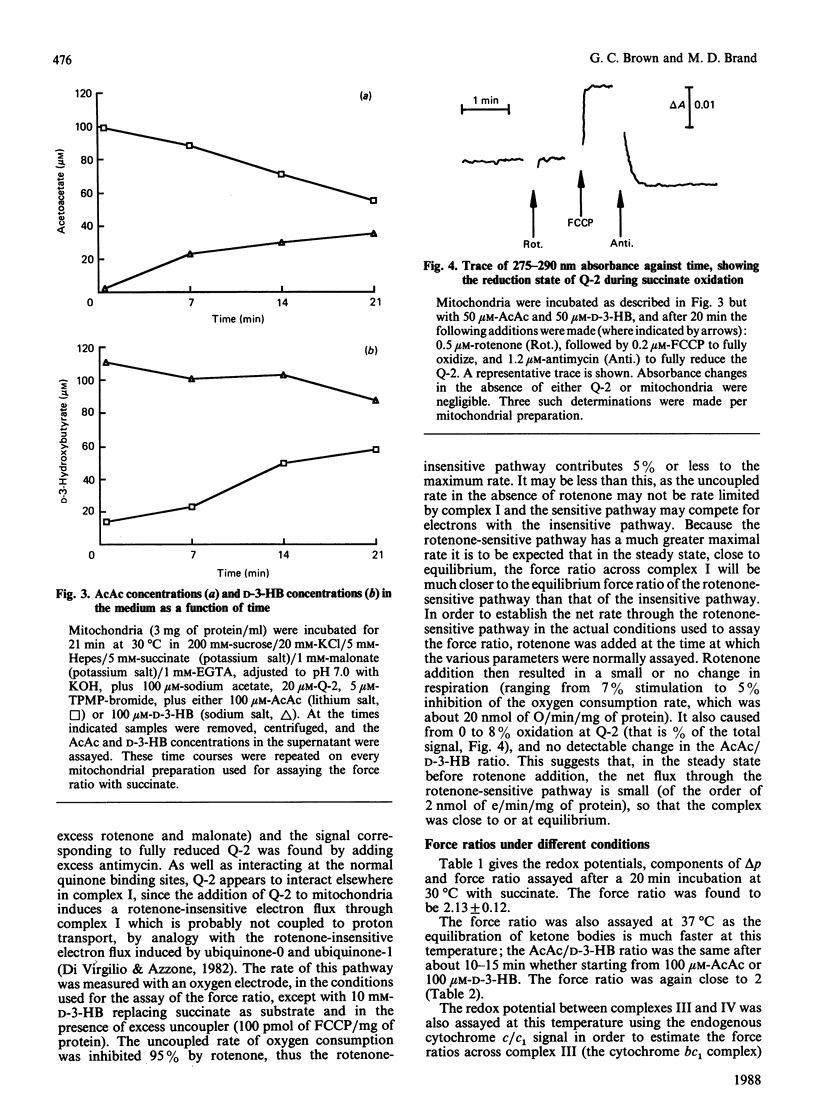
- Scholes T. A., Hinkle P. C. Energetics of ATP-driven reverse electron transfer from cytochrome c to fumarate and from succinate to NAD in submitochondrial particles. Biochemistry. 1984 Jul 3;23(14):3341–3345. doi: 10.1021/bi00309a035. [DOI] [PubMed] [Google Scholar]
- Scott I. D., Nicholls D. G. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980 Jan 15;186(1):21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban P. F., Klingenberg M. On the redox potentials of ubiquinone and cytochrome b in the respiratory chain. Eur J Biochem. 1969 Jul;9(4):519–525. doi: 10.1111/j.1432-1033.1969.tb00640.x. [DOI] [PubMed] [Google Scholar]
- Vercesi A., Reynafarje B., Lehninger A. L. Stoichiometry of H+ ejection and Ca2+ uptake coupled to electron transport in rat heart mitochondria. J Biol Chem. 1978 Sep 25;253(18):6379–6385. [PubMed] [Google Scholar]
- Wikstrom M. K. Proton pump coupled to cytochrome c oxidase in mitochondria. Nature. 1977 Mar 17;266(5599):271–273. doi: 10.1038/266271a0. [DOI] [PubMed] [Google Scholar]
- Wikström M. Two protons are pumped from the mitochondrial matrix per electron transferred between NADH and ubiquinone. FEBS Lett. 1984 Apr 24;169(2):300–304. doi: 10.1016/0014-5793(84)80338-5. [DOI] [PubMed] [Google Scholar]
- Williamson D. H., Lund P., Krebs H. A. The redox state of free nicotinamide-adenine dinucleotide in the cytoplasm and mitochondria of rat liver. Biochem J. 1967 May;103(2):514–527. doi: 10.1042/bj1030514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson D. F., Forman N. G. Mitochondrial transmembrane pH and electrical gradients: evaluation of their energy relationships with respiratory rate and adenosine 5'-triphosphate synthesis. Biochemistry. 1982 Mar 16;21(6):1438–1444. doi: 10.1021/bi00535a051. [DOI] [PubMed] [Google Scholar]
- de Jonge P. C., Westerhoff H. V. THe proton-per-electron stoicheiometry of 'site 1' of oxidative phosphorylation at high protonmotive force is close to 1.5. Biochem J. 1982 May 15;204(2):515–523. doi: 10.1042/bj2040515. [DOI] [PMC free article] [PubMed] [Google Scholar]


