Abstract
Epstein-Barr virus (EBV) nuclear antigen leader protein (EBNA-LP) is a phosphoprotein suggested to play important roles in EBV-induced immortalization of B cells. One of the potential functions of EBNA-LP is a cooperative induction with EBNA-2 of viral and cellular gene expression, including that of the genes for viral latent membrane protein 1 (LMP-1) and cellular cyclin D2. We report here that the phosphorylation of EBNA-LP by cellular kinase(s) is critical to its ability to cooperate with EBNA-2 in up-regulating the expression of LMP-1 in a B-lymphoma cell line. Our conclusion is based on the following observations. (i) Mass-spectrometric analysis of purified EBNA-LP and mutational analyses of EBNA-LP revealed that the serine residue at position 35 in the W2 repeat domain is the major phosphorylation site of EBNA-LP in vivo. (ii) Substitutions of this site in each W2 repeat domain with alanine markedly reduced the ability of the protein to induce LMP-1 expression in combination with EBNA-2 in Akata cells. (iii) Replacement at the major phosphorylation sites with glutamic acids restored the wild-type phenotype. It is well established that this substitution mimics constitutive phosphorylation. These results indicated that the coactivator function of EBNA-LP is regulated by phosphorylation.
Epstein-Barr virus (EBV) is a ubiquitous human herpesvirus that is frequently associated with a variety of neoplastic diseases, including endemic Burkitt's lymphoma, nasopharyngeal carcinoma, Hodgkin's disease, various other lymphomas, and gastric carcinoma (17, 30). In vitro, EBV can efficiently immortalize human B cells, and the resultant lymphoblastoid cell lines express only a limited number of viral proteins (EBV nuclear antigen 1 [EBNA-1], 2, 3A, 3B, 3C, and leader protein [LP] and latent membrane protein 1 [LMP-1], 2A, and 2B) (17, 30), although the EBV genome encodes more than 80 (3). Among the viral proteins expressed in lymphoblastoid cell lines, we have been focusing on EBNA-LP. EBNA-LP consists of a multirepeat domain (W1W2) and a unique C-terminal domain (Y1Y2) (Fig. 1A) and is, therefore, expressed as a protein ladder, possibly as the result of heterogeneous polypeptides with different numbers of W1W2 repeats (7). Studies with recombinant EBNA-LP mutants revealed that EBNA-LP is not essential for EBV-induced B-cell immortalization, but the mutant viruses showed much less efficiency in the phenotype, indicating that EBNA-LP is a critical regulator of EBV-induced B-cell transformation (9, 21). Although the actual role of EBNA-LP in EBV-induced B-cell immortalization remains to be elucidated, several lines of evidence listed below suggest biological functions of EBNA-LP.
FIG. 1.
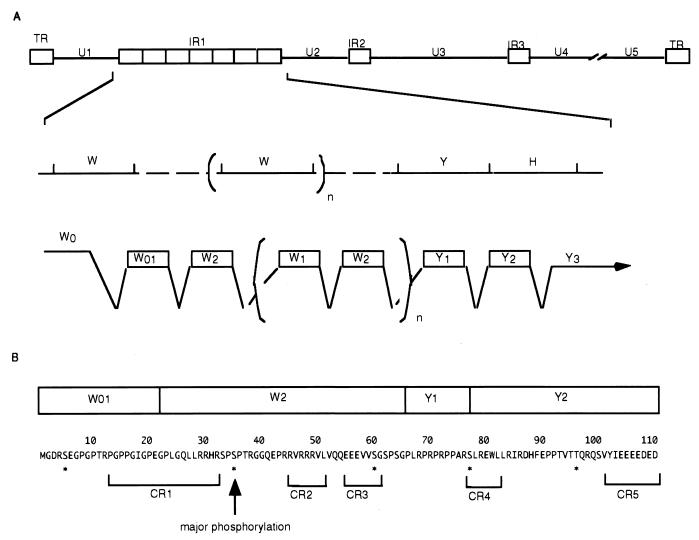
(A) Schematic diagram of the sequence of the EBV genome and location of the EBNA-LP gene. Line 1, linear representation of the EBV genome. The unique sequences are represented as unique 1 to 5 (U1 to U5). The terminal and internal repeats flanking the unique sequences are shown as open rectangles with their designations given above. Line 2, expanded section of the domain encoding the EBNA-LP gene. The exons of EBNA-LP open reading frames are derived from the BamHI W and Y fragments. Line 3, the structures of the EBNA-LP transcript and coding regions. (B) The predicted amino acid sequence of the EBNA-LP isoform containing one W repeat. The corresponding exon structures are indicated above the sequence. The conserved regions (CR1 to CR5) defined by Peng et al. (27) are indicated below the sequence. The conserved serines or threonines are indicated by asterisks. The major phosphorylation site identified in this study is shown by an arrow.
(i) EBNA-LP is primarily known as a coactivator of EBNA-2. It has been reported that EBNA-LP and EBNA-2 cooperatively stimulate the gene expression of cellular and viral proteins such as cyclin D2 (33) and LMP-1 (10, 24). Although the mechanism by which EBNA-LP functions as a coactivator of EBNA-2 is largely unknown, recent studies indicated that nuclear localization and/or nuclear matrix localization of EBNA-LP is required for the function (28, 42).
(ii) EBNA-LP interacts with various cellular proteins and structures. EBNA-LP was shown to bind to p53 and pRb in in vitro binding assays (37) and to be colocalized with an antigenically distinct form of pRb in a nuclear domain named ND10 (12, 38). However, EBNA-LP doesn't induce significant changes in the transcriptional activity of either p53 or E2F, at least not in transient-transfection assays (11). It was also reported that EBNA-LP and the 70-kDa family of heat shock proteins (hsp70s) are associated in vivo, colocalized in ND10, and translocated to the nucleolus under conditions of cellular stress, such as heat shock and high cell density (18, 22, 39). Recently we have shown that EBNA-LP is localized in the cytoplasm as well as the nucleus of EBV-infected cells and interacts with a cellular cytoplasmic protein, HAX-1 (14). HAX-1 has been suggested to be involved in B-cell signal transduction and apoptosis (36). Although the biological significance of the interaction between EBNA-LP and the cellular proteins is unclear at present, these results suggest that EBNA-LP is not only a coactivator of EBNA-2 but also a multifunctional protein that modulates various components of cellular machinery and that the functions of EBNA-LP in EBV-induced B-cell immortalization result from the sum of these interactions with viral and cellular proteins.
Phosphorylation of proteins by protein kinases is one of the most important strategies employed by eukaryotic cells to regulate cellular functions, such as transcription, translation, and protein degradation pathways (5, 41). Phosphorylation and dephosphorylation affect cellular and viral proteins in many ways, including their cellular localization, stability, interactions, and DNA binding activity (5, 41). Like the other EBV regulatory proteins, EBNA-LP is phosphorylated in EBV-infected cells (29, 31). The pattern of EBNA-LP phosphorylation is dependent on the cell cycle stage in that EBNA-LP is hyperphosphorylated in G2/M phase and hypophosphorylated in G1/S phase (19). Two-dimensional gel electrophoretic analysis revealed that EBNA-LP is phosphorylated at multiple sites, and p34cdc2 and casein kinase II mediate the phosphorylation of EBNA-LP in vitro (19). Taken together, these results suggest that the functions of EBNA-LP are regulated by phosphorylation.
To demonstrate the significance of the phosphorylation of EBNA-LP, we mapped and mutagenized a major phosphorylation site in EBNA-LP. Here we report that (i) serine 35 in the W2 repeat domain is the major phosphorylation site in EBNA-LP in vivo, (ii) the substitution of alanine for serine 35 abolished the cooperative induction of LMP-1 with EBNA-2 in B cells, and (iii) the substitution of glutamic acid for serine 35, which is considered to mimic constitutive phosphorylation (20), restored the wild-type phenotype. These results strongly support the hypothesis that the phosphorylation of EBNA-LP is crucial to one of its functions in infected cells.
MATERIALS AND METHODS
Cells.
BJAB is an EBV-negative B-cell line. BOSC23 cells are derived from 293T cells (25). BOSC23 and COS-7 cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal calf serum (FCS). Akata cells are derived from a sporadic Burkitt's lymphoma (40). BJAB and Akata cells were grown in RPMI medium supplemented with 10% FCS.
Plasmids.
pEBVHis-EBNA-LP(F) was generated by cloning the EBNA-LP cDNA (a generous gift from E. Kieff) tagged with a FLAG epitope sequence at its C-terminal end into pEBVhis (Invitrogen) as described previously (42). Deletion mutants of pEBVHis-EBNA-LP(F) were constructed by cloning into pEBVHis the DNA fragments amplified by PCR using the appropriate primer pairs. pM-W2(H), pM-W1(H), and pM-Y1Y2(H) expressing the hemagglutinin (HA)-tagged EBNA-LP domain fused to the GAL4 DNA binding domain and the series of deletion mutants of pM-W2(H) shown in Fig. 4B and D, respectively, were generated as described previously (42). pM-W2-S33A(H) and pM-W2-S35A(H) were constructed using the GeneEditor in vitro site-directed mutagenesis system (Promega) with the oligonucleotide CGGACAGCTCCTAAGAAGGCACCGGGCGCCCAGTCCTACCAGAGGGGGCCAAG for pM-W2-S33A(H) or GCTCCTAAGAAGGCACCGGTCGCCCGCTCCTACCAGAGGGGGCCAAGAACCC for pM-W2-S35A(H) from a template of pM-W2(H) according to the manufacturer's instructions. To construct pME-EBNA-LPR1(F), a PCR fragment encoding the entire coding sequence of EBNA-LP containing only one W repeat was cloned into pBS-Flag-Stop [pBS-EBNA-LPR1(F)] (42), and then the EcoRI-NotI fragment of pBS-EBNA-LPR1(F) was inserted into the EcoRI and NotI sites of pME18S (a generous gift from K. Maruyama). pME-EBNA-LPR3(F) was constructed by insertion of the ApaI fragments of pZip-EBNA-LP (a generous gift from E. Kieff) into the ApaI site of pME-EBNA-LPR1(F). pME-EBNA-LPR3-SA(F) or pME-EBNA-LPR3-SE(F), expressing mutant EBNA-LP in which serines 35, 101, and 167 (relative to the initiation codon of EBNA-LP) were replaced with alanines or glutamic acids, respectively, were constructed using the GeneEditor in vitro site-directed mutagenesis system (Promega) with the oligonucleotide GCTCCTAAGAAGGCACCGGTCGCCCGCTCCTACCAGAGGGGGCCAAGAACCC for the alanine substitution or GC TCC TAAGAAGGCACCGG TCGCCCGAACC TACCAGAGGGGGCCAAGAACCC for the glutamic acid substitution from the template of pME-EBNA-LPR3 according to the manufacturer's instructions. pcDNA4/HisMax-EBNA-LPR3(F), pcDNA4/HisMax-EBNA-LPR3-SA(F) and pcDNA4/HisMax-EBNA-LPR3-SE(F) were generated by cloning the EcoRI-NotI fragment of pME-EBNA-LPR3(F), pME-EBNA-LPR3-SA(F), and pME-EBNA-LPR3-SE(F), respectively, into the pcDNA4/HisMax C vector (Invitrogen). pME-EBNA-2 was generated by inserting the EcoRI fragment of pSG-E2 (a generous gift from S. Fujiwara) into the EcoRI site of the pME18S vector.
FIG. 4.
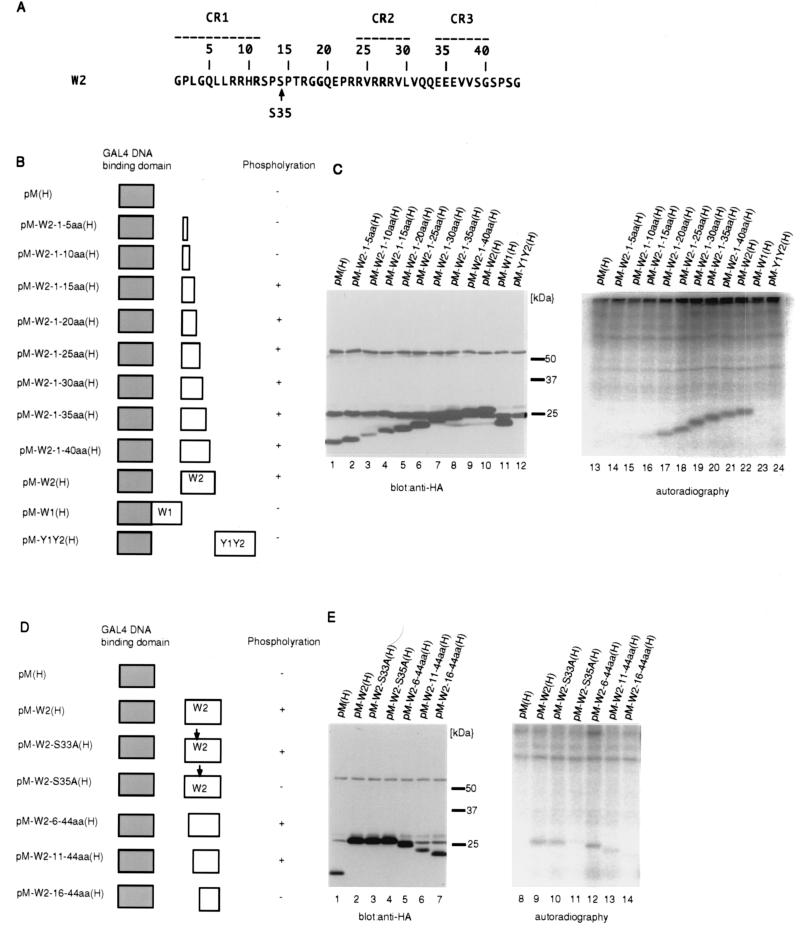
Identification of the major phosphorylation site in EBNA-LP. (A) The amino acid sequence of the W2 exon of EBNA-LP. The numbering of amino acids (relative to the first codon of the W2 domain) is shown above the sequence. Conserved regions defined by Peng et al. (27) are indicated as broken lines with their designation given above. The conserved serine that was found to be phosphorylated is indicated by an arrow. (B) Schematic representation of various deletion mutants of HA-tagged EBNA-LP domains fused to the GAL4 DNA binding domain. The levels of phosphorylation of the mutants are also shown. (C) BOSC23 cells were transiently transfected with the indicated expression vectors described in panel B, labeled with [32P]orthophosphate for 4 h, and then harvested, solubilized, immunoprecipitated with anti-GAL4 DNA binding domain monoclonal antibody (RK5C1), and electrophoretically separated in duplicate on two denaturing gels. Each gel was subjected to immunoblotting with anti-HA monoclonal antibody (3F10) (lanes 1 to 12) or autoradiography (lanes 13 to 24). (D) Schematic representation of various deletion or substitution mutants of the HA-tagged W2 domain fused to the GAL4 DNA binding domain. The levels of phosphorylation of the mutants are also shown. (E) Autoradiographic and photographic images of 32P-radiolabeled transfected BOSC23 cell lysates that were immunoprecipitated with anti-GAL4 DNA binding domain monoclonal antibody (RK5C1) and subjected to immunoblotting with anti-HA monoclonal antibody (3F10) (lanes 1 to 7) or autoradiography (lanes 8 to 14). Experiments were done exactly as described in the legend to panel C, except that BOSC23 cells were transfected with the indicated expression vectors described in panel D.
Transfections.
COS-7 or BOSC23 cells were seeded one day before transfection, and 60 to 80% confluent cells were transfected with various expression plasmids by calcium phosphate precipitation methods using the Profection mammalian transfection system (Promega). At 12 h posttransfection, the cells were washed and placed in fresh DMEM growth medium. At 48 h after transfection, the cells were harvested and used for further experiments. BJAB or Akata cells growing in log phase were washed twice with RPMI supplemented with 10% FCS. Then, 107 cells were suspended in 0.5 ml of RPMI containing 10% FCS medium and mixed with 25 μg of plasmid DNA. Cells were electroporated at 0.25 kV (BJAB cells) or 0.27 kV (Akata cells) and 960 μF and then suspended in 10 ml of RPMI containing 10% FCS and cultured at 37°C in a humidified 5% CO2 atmosphere. Transfected BJAB cells were selected in the presence of hygromycin B (500 μg/ml) for 2 weeks to generate stable transformants expressing EBNA-LP and its mutants. Transfected Akata cells were harvested at 48 h posttransfection by pelleting them down, washed with phosphate-buffered saline (PBS), lysed with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis sample buffer (62.5 mM Tris-HCl [pH 6.8], 2% SDS, 0.7 M beta-mercaptoethanol) at a concentration of 5 × 107 cells per ml, and subjected to electrophoresis on polyacrylamide gels containing SDS.
Metabolic labeling and immunoprecipitation.
At 48 h posttransfection, BOSC23 cells were washed with phosphate-free DMEM twice and cultured in phosphate-free DMEM supplemented with dialyzed 10% FCS for 1 h. Cells were then labeled with [32P]orthophosphate (Phosphorus32; Amersham Pharmacia Biotech) at a final concentration of 50 μCi/ml for 4 h. The labeled cells were washed with phosphate-buffered saline (PBS) once and lysed in lysis buffer (20 mM sodium phosphate [pH 7.0], 250 mM NaCl, 30 mM sodium pyrophosphate, 0.1% Nonidet P-40, 5 mM EDTA, 10 mM NaF, 0.1 mM Na3VO4, protease inhibitor cocktail [complete EDTA-free; Roche] [one tablet/50 ml], and 1 mM AEBSF [4-2-aminoethyl benzene sulfonyl fluoride; Boehringer Mannheim]). Immunoprecipitations were done as described previously (16). Briefly, supernatant fluids obtained after centrifugation of the lysates at 40,000 rpm for 30 min were reacted with the appropriate antibody for 1 h on ice. Protein G-Sepharose beads (Pharmacia) were then added and allowed to react for an additional 2 h at 4°C with rotation. The beads were washed five times with 1 ml of the lysis buffer and subjected to electrophoresis on denaturing gels.
Affinity purification and mass spectrometry of EBNA-LP.
Lysates of the BOSC23 cells that transiently express EBNA-LP were prepared as described above. To a 1/100 volume of the lysate, agarose beads coupled with anti-FLAG antibody (FLAG M2; Sigma) were added and allowed to react for 4 h at 4°C with rotation. The beads were washed five times with 1 ml of the lysis buffer, and the bounded proteins were eluted by addition of 10 volumes against a bed volume of 0.2 mg of FLAG peptide/ml in lysis buffer for 1 h at 4°C with rotation. The eluted aliquot was concentrated by centrifugation on a nanocep (PALL) column, mixed with 2× SDS-polyacrylamide gel electrophoresis sample buffer, and electrophoretically separated in a denaturing gel. The gel was then stained with Coomassie brilliant blue, and the band corresponding to EBNA-LP was cut out, transferred to a new tube, incubated with 10 μg of trypsin/ml in 0.1 M N-ethylmorpholine acetate, pH 8.0, overnight, and analyzed by a mass spectrometer, MALDI-TOF MS (TOF Spec2E; Micromass).
Phosphatase treatment.
The immunoprecipitates prepared as described above were washed twice and resuspended in NEB 2 buffer containing 1 mM AEBSF. The suspension was incubated with 50 U of calf intestinal alkaline phosphatase (New England Biolabs) at 37°C for 1 h. Then the immunoprecipitates were subjected to electrophoresis on a denaturing gel.
Immunoblotting.
The electrophoretically separated proteins transferred to nitrocellulose sheets were reacted with the appropriate antibodies as described elsewhere (15). Briefly, the nitrocellulose sheets were blocked with 5% skim milk in PBS containing 0.1% Tween 20 for 1 h or overnight, rinsed twice, washed once for 15 min and twice for 5 min, and reacted for 2 h with primary antibodies. The blots were then washed as before, reacted for 1 h with peroxidase-conjugated goat anti-mouse immunoglobulin G (IgG) (Santa Cruz Biotechnology), rinsed twice, washed once for 15 min and four times for 5 min each time in PBS containing 0.1% Tween 20 and developed using the ECL chemiluminesence reagent (Amersham-Pharmacia).
Indirect immunofluorescence.
Cells on coverslips were washed with PBS, fixed in 4% formaldehyde in PBS, and permeabilized with 0.25% Triton X-100 in PBS. The fixed cells were blocked for 1 h at 37°C in PBS containing 10% FCS, reacted for 1.5 h at 37°C with the appropriate primary antibody, rinsed three times with PBS, reacted for 1 h at 37°C with the appropriate secondary antibody, rinsed three times with PBS, and mounted in PBS containing 90% glycerol. Goat anti-mouse and anti-rabbit IgG conjugated to fluorescein isothiocyanate was used as a secondary antibody. For 4′,6′-diamidino-2-phenylindole (DAPI) staining, the fixed cells were reacted in 2 μg of DAPI/ml in PBS for 3 min at room temperature and then washed four times with PBS and mounted in PBS containing 90% glycerol. The coverslips were examined in a Nikon fluorescence microscope.
Cellular fractionation.
Nuclear and cytoplasmic fractions were obtained as described previously (13, 42). Briefly, BOSC23 cells transiently transfected with various EBNA-LP expression plasmids were harvested, washed with PBS, and resuspended in buffer A (10 mM HEPES [pH 7.4], 1.5 mM MgCl2, 10 mM NaCl, and 1 mM AEBSF [Boehringer Mannheim]). The cells were then lysed by addition of Nonidet P-40 to 0.1%, and the nuclei were pelleted by centrifugation at top speed in a microcentrifuge. The supernatant (cytoplasmic fraction) was transferred to a new tube. The pellet was washed twice with buffer A, resuspended with CSK buffer [10 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES) (pH 6.8), 300 mM sucrose, 100 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, 2 mM VRC (vanadyl riboside complex) (5 Prime-3 Prime), and 1 mM AEBSF (Boehringer Mannheim)], and subjected to nuclear matrix fractionation. Subnuclear fractionation was performed as described elsewhere (6, 34, 42) by sequential extraction with CSK buffer and extraction buffer (10 mM PIPES [pH 6.8], 250 mM ammonium sulfate, 300 mM sucrose, 3 mM MgCl2, 1 mM EGTA, 2 mM VRC, and 1 mM AEBSF) each for 4 min at 4°C, and then the fractions were incubated with digestion buffer (10 mM PIPES [pH 6.8], 300 mM sucrose, 50 mM NaCl, 3 mM MgCl2, 1 mM EGTA, 0.5% Triton X-100, 2 mM VRC, 1 mM AEBSF, and 300 U of RNase-free DNase I [Roche]/ml) for 50 min at 32°C. Nucleoplasm, histone H1, and chromatin fractions were soluble materials obtained after extraction with the CSK, extraction, and digestion buffers, respectively. The residual insoluble fractions after extraction with digestion buffer were used as the nuclear matrix fractions. The integrity of this procedure was described in our previous paper (42).
Antibodies.
Anti-FLAG mouse monoclonal antibody (M2; Sigma), anti-HA rat monoclonal antibody (3F10; Boehringer Mannheim), antipolyhistidine rabbit polyclonal antibody (H15; Santa Cruz Biotechnology), anti-GAL4 DNA binding domain mouse monoclonal antibody (RK5Cl; Santa Cruz Biotechnology), anti-EBNA2 mouse monoclonal antibody (PE2; DACO), and anti-EBNA-LP mouse monoclonal antibody (JF186; a generous gift from G. Klein) were used as the primary antibodies for immunoblotting or immunofluorescence assays.
RESULTS
EBNA-LP is phosphorylated in BJAB cells.
The objectives of the first set of experiments were to confirm that EBNA-LP stably expressed in B cells is in fact phosphorylated in vivo and to map the site(s) of phosphorylation. To this end, an expression vector of EBNA-LP [pEBVHis-EBNA-LPR4(F)] and a series of deletion mutants of the EBNA-LP Y domain [pEBVHis-EBNA-LPR4(F)d1, pEBVHis-EBNA-LPR4(F)d2, and pEBVHis-EBNA-LPR4(F)d3] were constructed as shown in Fig. 2A. BJAB cells were transfected with each expression plasmid and selected in the presence of hygromycin B for two weeks. The cells stably expressing EBNA-LPs tagged with both FLAG and polyhistidines were labeled with [32P]orthophosphate, and EBNA-LP immunoprecipitates obtained with anti-FLAG antibody (M2) or antipolyhistidine antibody (D-8) from the labeled cell lysate prepared as described in Materials and Methods were then solubilized, electrophoretically separated on denaturing gels, and subjected to autoradiography.
FIG. 2.
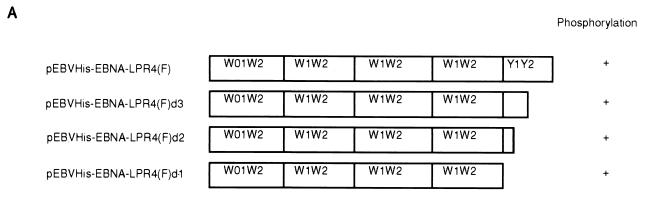
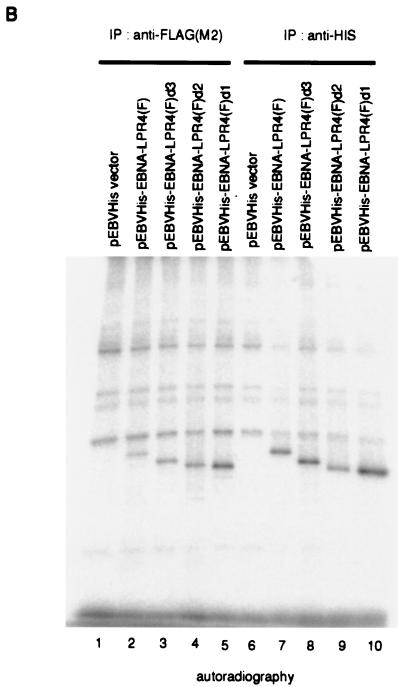
Stably expressed EBNA-LP is phosphorylated in BJAB cells. (A) Schematic representation of various deletion mutants of EBNA-LP. The levels of phosphorylation of the mutants are also shown. (B) Autoradiographic images of 32P-radiolabeled EBNA-LP and its mutants stably expressed in BJAB cells. BJAB cells were stably transfected with the empty vector (lanes 1 and 6), pEBVHis-EBNA-LPR4(F) (lanes 2 and 7), pEBVHis-EBNA-LPR4d3(F) (lanes 3 and 8), pEBVHis-EBNA-LPR4d2(F) (lanes 4 and 9), or pEBVHis-EBNA-LPR4d1(F) (lanes 5 and 10) and selected in the presence of hygromycin B. Each stable transformant was labeled with [32P]orthophosphate for 4 h and then harvested, solubilized, immunoprecipitated with anti-FLAG monoclonal antibody (M2) (lanes 1 to 5) or anti-His monoclonal antibody (D-8) (lanes 6 to 10), electrophoretically separated in a denaturing gel, and subjected to autoradiography.
The results were that wild-type EBNA-LP was labeled with 32P in BJAB cells, and all deletion mutants of the EBNA-LP Y domain were labeled with 32P as efficiently as the wild type. These results indicate that (i) stably expressed EBNA-LP is in fact phosphorylated in B cells, (ii) the phosphorylation doesn't require the expression of any viral genes other than that for EBNA-1, and (iii) the Y domain of EBNA-LP is dispensable for EBNA-LP phosphorylation.
Identification of the major phosphorylation site of EBNA-LP.
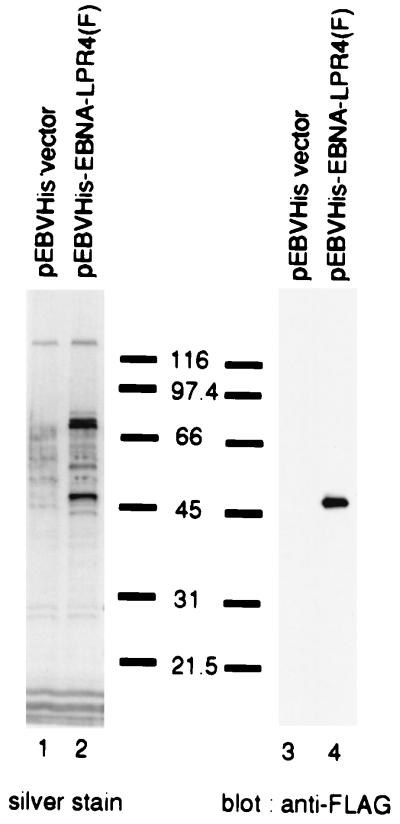
Next, we attempted to identify the phosphorylation site of EBNA-LP by mass spectrometry (MS). For purification of EBNA-LP, BOSC23 cells were transfected with pEBVHis-EBNA-LP(F), harvested, and lysed at 48 h posttransfection. Transiently expressed EBNA-LP was then affinity purified using the agarose beads coupled with anti-FLAG antibody (M2), eluted by FLAG peptide, separated in duplicate on denaturing gels, and subjected to silver staining or immunoblotting with anti-FLAG antibody (M2) (Fig. 3). Then the band corresponding to EBNA-LP was excised and subjected to mass-spectrometric analysis. Although various sizes of peptide fragments that covered with almost entire amino acid sequence of EBNA-LP were successfully identified with MS, the peptide fragment between amino acid codons 33 and 44 (SPSPTRGGQEPR) couldn't be detected in the expected mass size. In contrast, the signal of the phosphorylated form of the peptide fragment could be detected. The data suggested that the fragment of EBNA-LP between amino acid codons 33 and 44 (SPSPTRGGQEPR) is phosphorylated.
FIG. 3.
Photographic images of a silver-stained denaturing gel (left panel) and an immunoblot (right panel) of electrophoretically separated cell proteins bound to anti-FLAG monoclonal antibody (M2)-conjugated agarose beads. BOSC23 cells were transiently transfected with the empty vector (lanes 1 and 3) or pEBVHis-EBNA-LPR4(F) (lanes 2 or 4), harvested, solubilized, and reacted with anti-FLAG monoclonal antibody (M2)-conjugated agarose beads. The beads were pelleted, rinsed extensively, and eluted with the FLAG peptide. The eluted fractions were then electrophoretically separated in duplicate on two denaturing gels. Each gel was subjected to silver staining (left panel) or immunoblotting with anti-FLAG monoclonal antibody (M2) (right panel). The band corresponding to EBNA-LP was cut out, digested with trypsin, and subjected to mass-spectrometric analysis as described in the text.
To confirm that EBNA-LP is phosphorylated in the region between codons 33 and 44 (relative to the initiation codon of EBNA-LP), we constructed a series of deletion mutants of HA-tagged EBNA-LP fused to the GAL4 DNA binding domain (Fig. 4B and D) and tested whether these mutants transiently expressed in BOSC23 cells were phosphorylated. Because molecules with only one W repeat domain were difficult to express, presumedly due to instability, we expressed the EBNA-LP mutant fused to the GAL4 DNA binding domain in order to make sure of its nuclear localization (32) and increase the stability of the protein, as reported previously (42). BOSC23 cells were transfected with each mutant and labeled with [32P]orthophosphate. Immunoprecipitates obtained with the anti-GAL4 DNA binding domain (RK5C1) from the labeled cell lysates as described in Materials and Methods were then solubilized, electrophoretically separated in duplicate on denaturing gels, and subjected to immunoblotting with anti-HA antibody (3F10) or autoradiography (Fig. 4C and D). The results were as follows.
(i) Among the EBNA-LP mutants containing W1, W2, and Y1Y2 domains, only the mutant containing the W2 domain was labeled (Fig. 4C, lanes 10 to 12 and 22 to 24). We should point out that the mutant containing the Y1Y2 domain was not expressed as efficiently as those containing the W1 or W2 domain. It therefore remains unknown whether the Y1Y2 domain is phosphorylated. These results indicated that the W2 domain contains the phosphorylation site(s).
(ii) With the aid of 3′ (Fig. 4C, lanes 1 to 10 and 13 to 22) and 5′ (Fig. 4E, lanes 5 to 7 and 12 to 14) sequential deletion mutants of the W2 domain, the region of the domain to be phosphorylated was mapped to amino acids RSPSP between residues 11 and 15 (relative to the first codon of the W2 domain), which corresponds to positions 32 to 36 (relative to the initiation codon of EBNA-LP) (Fig. 1B and 4A). These results were consistent with those obtained by MS analysis.
(iii) In the mapped region, there are two potential phosphorylation sites, serines 33 and 35 (relative to the initiation codon of EBNA-LP). We therefore mutagenized the GAL4-W2 construct by replacing each serine in the region with alanine as described in Materials and Methods and tested whether these mutants are phosphorylated. Here we refer to the gene products of pM-W2(H), pM-W2-S33A(H), and pM-W2-S35A(H) as GAL4-W2, GAL4-W2-S33A, and GAL4-W2-S35A, respectively. The results were that the GAL4-W2-S33A mutant was phosphorylated as efficiently as GAL4-W2 (Fig. 4E, lanes 3 and 10); while the level of phosphorylation of GAL4-W2-S35A was severely reduced (Fig. 4E, lanes 4 and 11).
We concluded from these results that serine 35 is the major phosphorylation site of EBNA-LP in vivo. We should note that a weak signal of phosphorylation in autoradiography of the GAL4-W2-S35A mutant was detected (Fig. 4E, lanes 11 and 14), suggesting that some other phosphorylation site(s) exists within the W2 domain.
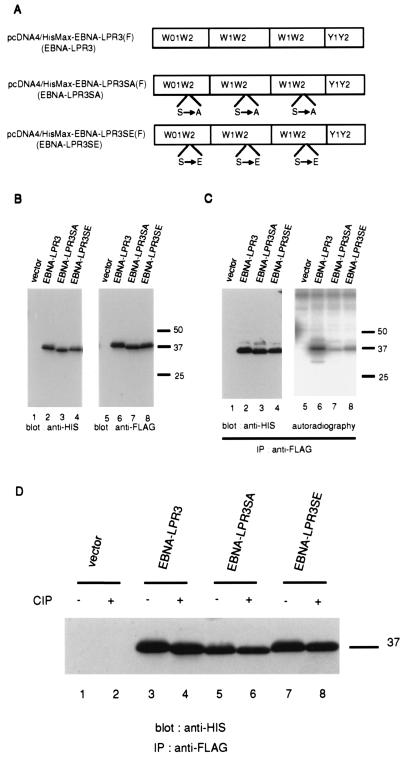
Construction of EBNA-LP mutants containing multiple W repeat domains in which the mapped phosphorylation site in each W2 domain has a substitution of alanine or glutamic acid.
Nitsche et al. reported that more than two copies of the W repeat are required for EBNA-LP to express one of its biological phenotypes, the up-regulation of LMP1 with EBNA-2 (24). To investigate the importance of phosphorylation of EBNA-LP for its function, we mutagenized EBNA-LP cDNA containing three repeats of the W domain in the expression vector [pcDNA4/HisMax-EBNA-LPR3(F)] by introducing an alanine [pcDNA4/HisMax-EBNA-LPR3SA(F)] or glutamic acid [pcDNA4/HisMax-EBNA-LPR3SE(F)] at the phosphorylation site in each W repeat domain (Fig. 5A). We refer to the gene products of pcDNA4/HisMax-EBNA-LPR3(F), pcDNA4/HisMax-EBNA-LPR3SA(F), and pcDNA4/ HisMax-EBNA-LPR3SE(F) as EBNA-LPR3, EBNA-LPR3SA, and EBNA-LPR3SE, respectively. The results were as follows.
FIG. 5.
(A) Schematic representation of FLAG epitope-tagged wild-type EBNA-LP and EBNA-LP mutants with serine-alanine and serine-glutamic acid substitutions at the identified phosphorylation site. (B) Photographic images of immunoblots of electrophoretically separated lysates of BOSC23 cells transiently transfected with the empty vector and the expression vectors shown in panel A, harvested, solubilized, electrophoretically separated in denaturing gels, and subjected to immunoblotting with anti-His polyclonal antibody (H15) (left panel) or anti-FLAG monoclonal antibody (M2) (right panel). Molecular weights, given in thousands, are shown on the right. (C) Autoradiogram (left panel) and photograph of an immunoblot (right panel) of 32P-labeled proteins immunoprecipitated with anti-FLAG monoclonal antibody from lysates of BOSC23 cells transiently transfected with the indicated expression vectors. Experiments were done exactly as described in the legend to Fig. 4C, except that BOSC23 cells were transfected with the indicated expression vectors and the cell lysates were immunoprecipitated with anti-FLAG monoclonal antibody (M2). Molecular weights, given in thousands, are shown on the right. (D) Photographic image of an immunoblot of proteins immunoprecipitated with the anti-FLAG monoclonal antibody (M2) from lysates of transfected BOSC23 cells, treated with alkaline phosphatase as described in Materials and Methods, electrophoretically separated in a denaturing gel, transferred to a nitrocellulose sheet, and reacted with anti-His polyclonal antibody (H15). BOSC23 cells were transfected with the indicated expression vectors, harvested, and subjected to immunoblotting with anti-HIS polyclonal antibody (H15). A molecular weight marker, given in thousands, is shown on the right.
(i) Immunoblot analysis of BOSC23 cells transfected with each expression vector showed that the wild-type and mutant EBNA-LPs were expressed at similar levels, while the EBNA-LPR3SA mutant migrated faster than EBNA-LPR3 and EBNA-LPR3SE (Fig. 5B), presumably due to a different state of phosphorylation.
(ii) As predicted, the mutant EBNA-LPs were labeled in vivo with [32P]orthophosphate much less efficiently than the wild-type EBNA-LP (Fig. 5C). Consistent with the results shown in Fig. 4E, the mutant EBNA-LPs were slightly labeled. Furthermore, phosphatase treatment of EBNA-LPR3, EBNA-LPR3SA, and EBNA-LPR3SE affected the electrophoretic mobility (Fig. 5D), supporting the possibility that another phosphorylation site exists in EBNA-LP.
EBNA-LPR3SA and EBNA-LPR3SE mutants show the same pattern of subcellular localization as the wild-type EBNA-LP.
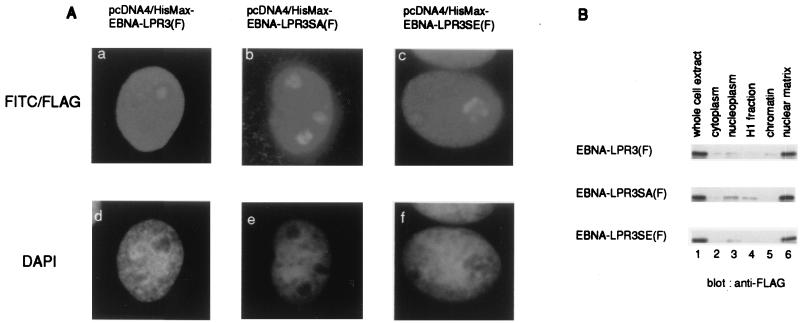
It has been reported that EBNA-LP containing more than one copy of the W1W2 repeat is localized predominantly in the nucleus (14, 28, 29) and is associated with the nuclear matrix (29, 42). To examine whether phosphorylation of EBNA-LP affects the subcellular localization of the protein, we examined the localization of EBNA-LPR3, EBNA-LPR3SA, and EBNA-LPR3SE by immunofluorescence assay and cell fractionation analysis using BOSC23 cells transfected with each expression vector. As shown in Fig. 6, there was no difference in the patterns of localization among the wild-type and mutant EBNA-LPs.
FIG. 6.
Subcellular localization of EBNA-LP mutants. (A) COS-7 cells were transiently transfected with the expression vectors described in Fig. 4A and subjected to an indirect immunofluorescence assay as described in Materials and Methods. The expressions of EBNA-LPs were visualized with anti-FLAG monoclonal antibody (M2) and fluorescein isothiocyanate conjugated anti-mouse IgG antibody (a to c). DNA was visualized with DAPI (d to f). (B) BOSC23 cells were transiently transfected with pME-EBNA-LPR3(F) (upper panel), pME-EBNA-LPR3SA(F) (middle panel), and pME-EBNA-LPR3SE(F) (lower panel) and fractionated as described in Materials and Methods. Each fraction (lane 1, whole extract; lane 2, cytoplasmic fraction; lane 3, nucleoplasmic fraction; lane 4, H1 fraction; lane 5, chromatin fraction; lane 6, nuclear matrix fraction) was solubilized, separated in denaturing gels, and subjected to immunoblotting with anti-FLAG monoclonal antibody (M2).
Phosphorylation of EBNA-LP is critical for its cooperative induction of LMP-1 with EBNA-2 in B cells.
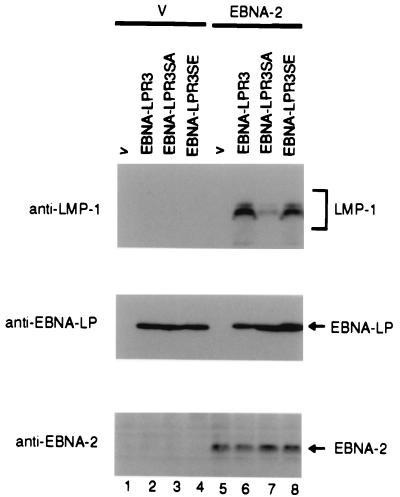
As described above, EBNA-LP functions as a coactivator for EBNA-2 and induces the expression of LMP-1 in B cells (10, 24). To examine whether the phosphorylation of EBNA-LP is required for its coactivator function, Akata cells were transfected with the EBNA-LPR3, EBNA-LPR3SA, or EBNA-LPR3SE expression vector along with the EBNA-2 expression vector, harvested at 48 h posttransfection, solubilized, electrophoretically separated in a denaturing gel, and subjected to immunoblotting with anti-LMP-1 monoclonal antibody (S-12), anti-EBNA-LP monoclonal antibody (JF186), and anti-EBNA-2 monoclonal antibody (PE2).
As reported previously (24), the expression of LMP-1 was markedly induced in Akata cells when both the wild-type EBNA-LP and EBNA-2 were coexpressed (Fig. 7, lane 6). In contrast, EBNA-LPR3SA only weakly induced the expression of LMP-1 in combination with EBNA-2 in Akata cells, the extent of the induction being much less than that with the wild-type EBNA-LP and EBNA-2 (Fig. 7, lane 7). Furthermore, the wild-type phenotype was restored when the EBNA-LPR3SE mutant and EBNA-2 were coexpressed (Fig. 7, lane 8). It is well established that substitution of an amino acid for glutamic acid mimics constitutive phosphorylation (20). The expression of EBNA-LP and of EBNA-2 was constant (Fig. 7, middle panel and lower panel).
FIG. 7.
Photographic images of immunoblots of electrophoretically separated Akata cells transiently transfected with various EBNA-LP expression vectors in combination with pME18S (V) (lanes 1 to 4) or pME-EBNA-2 (EBNA-2) (lanes 5 to 8). At 48 h posttransfection, cells were harvested, solubilized, and subjected to immunoblotting with anti-LMP1 monoclonal antibody (S-12) (upper panel), anti-EBNA-LP monoclonal antibody (JF186) (middle panel), or anti-EBNA-2 monoclonal antibody (PE2) (lower panel).
These results indicate that phosphorylation of EBNA-LP at serines 35, 107, and 167 is crucial for its transactivation of LMP-1 expression with EBNA-2 in B cells and that this activity of EBNA-LP is regulated by phosphorylation.
DISCUSSION
Phosphorylation of proteins by protein kinase is a major strategy employed by eukaryotic cells to regulate various cellular functions (5, 41). Viruses adapt to the cellular environment and use a similar strategy to regulate the replicative process. The key finding reported here is that the coactivator function of EBNA-LP, an important regulator for EBV-induced B-cell immortalization, is regulated by phosphorylation by cellular protein kinase(s) in EBV-infected B cells. The salient features of our results are as follows.
(i) Peptide mapping of the purified EBNA-LP by MS and mutational analyses identified the serine codon at position 35 in the W2 repeat domain as the major site of phosphorylation of EBNA-LP by cellular kinase(s). These results are consistent with the published observation that EBNA-LP is phosphorylated on serine residues only (19). Furthermore, this phosphorylation site is well conserved among a subset of primate gammaherpesviruses, suggesting that the phosphorylation at serine 35 has a significant role in EBNA-LP function.
(ii) Substitution at the major phosphorylation site in each W2 repeat domain with alanine resulted in a substantial diminishment of the ability to transactivate with EBNA-2 the expression of LMP-1 in EBV-infected B cells. Similar results were reported quite recently by Peng et al., who intended to focus on the serine 35 residue because it is evolutionarily conserved (27). Substitution at the major phosphorylation sites with glutamic acids, which is known to mimic constitutive phosphorylation, restored the wild-type phenotype, further supporting our conclusion that phosphorylation at serine 35 in the W2 repeat domain is critical for the cooperative function with EBNA-2. These results also eliminate the slim possibility that replacement of the serine residues with other amino acids led by chance to a significant conformational change of the protein and that the reduced cooperative activity is due to a structural change of the protein.
In summary, these results support our conclusion that phosphorylation of EBNA-LP is critical for one of its functions. The relevant issues are as follows.
(i) Further elucidation of the biological significance of the phosphorylation of EBNA-LP at the conserved serine in each W2 repeat domain requires identification of the cellular protein kinase(s) responsible for the phosphorylation. Kitay and Rowe reported that EBNA-LP is phosphorylated in vitro by cdc-2 kinase and casein kinase II (19, 26). A consensus phosphorylation site for cdc-2 kinase ([S/T]PX[KR]) completely matches the flanking region of the identified phosphorylation site (SPTR) (23). Furthermore, the major phosphorylation region of EBNA-LP also fills the requirement of the consensus phosphorylation site of calmodulin-dependent kinase II, cGMP-dependent kinase, and protein kinase C (26). It should also be noted that EBV encodes a serine-threonine protein kinase (4; K. Kato, K. Hirai, and Y. Kawaguchi, unpublished observation). Further study to investigate whether these protein kinases are in fact involved in the phosphorylation of EBNA-LP in infected cells is needed and is currently under way in this laboratory.
(ii) The EBNA-LPR3SA mutant was weakly labeled with [32P]orthophosphate in vivo (Fig. 5C). This result indicates that EBNA-LP has additional phosphorylation site(s). Consistently, Petti et al. and Kitay and Rowe predicted a potential phosphorylation site in the Y domain of EBNA-LP. We also provided evidence that there is an additional phosphorylation site(s) in the W2 domain, based on the observation that the GAL4-W2SA mutant is weakly labeled with [32P]orthophosphate in vivo. It is conceivable that the function of EBNA-LP is regulated by multiple phosphorylation events involving cellular kinases. Furthermore, we should note that the signal of EBNA-LPR3SE(F) is much stronger than that of EBNA-LPR3SA(F) (Fig. 5C, lane 8). These results raised the possibility that the residual phosphorylation of EBNA-LP is coupled with that at serine 35.
(iii) The functions of various coactivators are known to be regulated by phosphorylation. For instance, CBP interacts with various sequence-specific transcription factors and stimulates their transcription by acetylating histones in the vicinity of the recognition sequences (8, 35). It has been reported that CBP is phosphorylated by cell cycle-dependent kinases and p44 mitogen-activated protein kinase/ERK1 and that the phosphorylation stimulates the histone acetyl transferase activity of CBP in vitro (1, 2). BOB.1/OBF.1 is also a transcriptional coactivator which interacts with the Oct1 and Oct2 transcription factors. BOB.1/OBF.1 is phosphorylated at serine 184 in vivo, and this modification is required for its coactivator function (43). Thus phosphorylation of coactivators is frequently employed to regulate their activities. These results further support our conclusion that phosphorylation of EBNA-LP by cellular kinase(s) is important to expression of the functional activity of the protein.
ACKNOWLEDGMENTS
We thank E. Kieff for the EBNA-LP cDNA and anti-LMP-1 mouse monoclonal antibody, G. Klein for the mouse monoclonal antibody to EBNA-LP, S. Fujiwara for pSG-E2, K. Maruyama for pME18S, and D. Baltimore for the BOSC23 cells. We thank S. Mitani, C. Hatanaka, and M. Mori for technical assistance.
This study was supported in part by grants for scientific research (Y.K. and K.H.) from the Ministry of Education, Science, Sports and Culture of Japan. Y.K. was supported by a grant from the Inamori Foundation.
Footnotes
This article is dedicated to the memory of Kanji Hirai.
REFERENCES
- 1.Ait-Si-Ali S, Carlisi D, Ramirez S, Upegui-Gonzalez L C, Duquet A, Robin P, Rudkin B, Harel-Bellan A, Trouche D. Phosphorylation by p44 MAP Kinase/ERK1 stimulates CBP histone acetyl transferase activity in vitro. Biochem Biophys Res Commun. 1999;262:157–162. doi: 10.1006/bbrc.1999.1132. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali S, Ramirez S, Barre F X, Dkhissi F, Magnaghi-Jaulin L, Girault J A, Robin P, Knibiehler M, Pritchard L L, Ducommun B, Trouche D, Harel-Bellan A. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. doi: 10.1038/24190. [DOI] [PubMed] [Google Scholar]
- 3.Baer R, Bankier A T, Biggin M D, Deininger P L, Farrel P J, Tuffnell P S, Barrel B G. DNA sequence and expression of B95–8 Epstein-Barr virus genome. Nature. 1984;310:207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- 4.Chen M R, Chang S J, Huang H, Chen J Y. A protein kinase activity associated with Epstein-Barr virus BGLF4 phosphorylates the viral early antigen EA-D in vitro. J Virol. 2000;74:3093–3104. doi: 10.1128/jvi.74.7.3093-3104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edelman A M, Blumenthal D K, Krebs E G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- 6.Fey E G, Wan K M, Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1984;98:1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finke J, Rowe M, Kallin B, Ernberg I, Rosen A, Dillner J, Klein G. Monoclonal and polyclonal antibodies against Epstein-Barr virus nuclear antigen 5 (EBNA-5) detect multiple protein species in Burkitt's lymphoma and lymphoblastoid cell lines. J Virol. 1987;61:3870–3878. doi: 10.1128/jvi.61.12.3870-3878.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grunstein M. Histone acetylation in chromatin structure and transcription. Nature. 1997;389:349–352. doi: 10.1038/38664. [DOI] [PubMed] [Google Scholar]
- 9.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein-Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]
- 10.Harada S, Kieff E. Epstein-Barr virus nuclear protein LP stimulates EBNA-2 acidic domain-mediated transcriptional activation. J Virol. 1997;71:6611–6618. doi: 10.1128/jvi.71.9.6611-6618.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Inman G J, Farrel P J. Epstein-Barr virus EBNA-LP and transcriptional regulation properties of pRB, p107 and p53 in transfection assays. J Gen Virol. 1995;76:2141–2149. doi: 10.1099/0022-1317-76-9-2141. [DOI] [PubMed] [Google Scholar]
- 12.Jiang W Q, Szekely L, Wendel-Hansen V, Ringertz N, Klein G, Rosen A. Co-localization of the retinoblastoma protein and the Epstein-Barr virus-encoded nuclear antigen EBNA-5. Exp Cell Res. 1991;197:314–318. doi: 10.1016/0014-4827(91)90438-z. [DOI] [PubMed] [Google Scholar]
- 13.Kawaguchi Y, Bruni R, Roizman B. Interaction of herpes simplex virus 1 α regulatory protein ICP0 with elongation factor 1δ: ICP0 affects translational machinery. J Virol. 1997;71:1019–1024. doi: 10.1128/jvi.71.2.1019-1024.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawaguchi Y, Nakajima K, Igarashi M, Morita T, Tanaka M, Suzuki M, Yokoyama A, Matsuda G, Kato K, Kanamori M, Hirai K. Interaction of Epstein-Barr virus nulear antigen leader protein (EBNA-LP) with HS1-associated protein X-1: implication of cytoplasmic function of EBNA-LP. J Virol. 2000;74:10104–10111. doi: 10.1128/jvi.74.21.10104-10111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawaguchi Y, Van Sant C, Roizman B. Herpes simplex virus type 1 α regulatory protein ICP0 interacts with and stabilizes the cell cycle regulator cyclin D3. J Virol. 1997;71:7328–7336. doi: 10.1128/jvi.71.10.7328-7336.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kawaguchi Y, Van Sant C, Roizman B. Eukaryotic elongation factor 1δ is hyperphosphorylated by the protein kinase encoded by the UL13 gene of herpes simplex virus 1. J Virol. 1998;72:1731–1736. doi: 10.1128/jvi.72.3.1731-1736.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2343–2396. [Google Scholar]
- 18.Kitay M K, Rowe D T. Protein-protein interactions between Epstein-Barr virus nuclear antigen-LP and cellular gene products: binding of 70-kilodalton heat shock proteins. Virology. 1996;220:91–99. doi: 10.1006/viro.1996.0289. [DOI] [PubMed] [Google Scholar]
- 19.Kitay M K, Rowe D T. Cell cycle stage-specific phosphorylation of the Epstein-Barr virus immortalization protein EBNA-LP. J Virol. 1996;70:7885–7893. doi: 10.1128/jvi.70.11.7885-7893.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J S, Collins K M, Brown A L, Lee C H, Chung J H. hCds1-mediated phosphorylation of BRCA1 regulates the DNA damage response. Nature. 2000;404:201–204. doi: 10.1038/35004614. [DOI] [PubMed] [Google Scholar]
- 21.Mannick J B, Cohen J I, Birkenbach M, Marchini A, Kieff E. The Epstein-Barr virus nuclear protein encoded by the leader of the EBNA RNAs is important in B-lymphocyte transformation. J Virol. 1991;65:6826–6837. doi: 10.1128/jvi.65.12.6826-6837.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mannick J B, Tong X, Hemnes A, Kieff E. The Epstein-Barr virus nuclear antigen leader protein associates with hsp72/hsc73. J Virol. 1995;69:8169–8172. doi: 10.1128/jvi.69.12.8169-8172.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marin O, Meggio F, Draetta G, Pinna L A. The consensus sequences for cdc2 kinase and for casein kinase-2 are mutually incompatible. A study with peptides derived from the beta-subunit of casein kinase-2. FEBS Lett. 1992;301:111–114. doi: 10.1016/0014-5793(92)80221-2. [DOI] [PubMed] [Google Scholar]
- 24.Nitsche F, Bell A, Rickinson A. Epstein-Barr virus leader protein enhances EBNA-2-mediated transactivation of latent membrane protein 1 expression: a role for the W1W2 repeat domain. J Virol. 1997;71:6619–6628. doi: 10.1128/jvi.71.9.6619-6628.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pear W S, Nolan G P, Scott M L, Baltimore D. Production of high-titer helper-free retroviruses by transient transfection. Proc Natl Acad Sci USA. 1993;90:8392–8396. doi: 10.1073/pnas.90.18.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearson R B, Kemp B E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- 27.Peng R, Gordadze A V, Fuentes Panana E M, Wang F, Zong J, Hayward G S, Tan J, Ling P D. Sequence and functional analysis of EBNA-LP and EBNA2 proteins from nonhuman primate lymphocryptoviruses. J Virol. 2000;74:379–389. doi: 10.1128/jvi.74.1.379-389.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peng R, Tan J, Ling P D. Conserved regions in the Epstein-Barr virus leader protein define distinct domains required for nuclear localization and transcriptional cooperation with EBNA2. J Virol. 2000;74:9953–9963. doi: 10.1128/jvi.74.21.9953-9963.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petti L, Sample C, Kieff E. Subnuclear localization and phosphorylation of Epstein-Barr virus latent infection nuclear proteins. Virology. 1990;176:563–574. doi: 10.1016/0042-6822(90)90027-o. [DOI] [PubMed] [Google Scholar]
- 30.Rickinson A B, Kieff E. Epstein-Barr virus and its replication. In: Fields B N, Knipe D M, Howley P, Chanock R M, Hirsch M S, Melnick J L, Monath T P, Roizman B, editors. Virology. 3rd ed. New York, N.Y: Raven Press; 1996. pp. 2397–2446. [Google Scholar]
- 31.Sauter M, Boos H, Hirsch F, Mueller-Lantzsch N. Characterization of a latent protein encoded by the large internal repeats and the BamHI Y fragment of the Epstein-Barr virus (EBV) genome. Virology. 1988;166:586–590. doi: 10.1016/0042-6822(88)90530-2. [DOI] [PubMed] [Google Scholar]
- 32.Silver P A, Keegan L P, Ptashine M. Amino terminus of the yeast GAL4 gene product is sufficient for nuclear localization. Proc Natl Acad Sci USA. 1984;81:5951–5955. doi: 10.1073/pnas.81.19.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Spector D S, Goldman R D, Leinwand L A. Cells: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1998. The nuclear matrix: preparation for microscopy and biochemical analysis; pp. 44.1–44.8. [Google Scholar]
- 35.Struhl K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998;12:599–606. doi: 10.1101/gad.12.5.599. [DOI] [PubMed] [Google Scholar]
- 36.Suzuki Y, Demoliere C, Kitamura D, Takeshita H, Deushle U, Watanabe T. HAX-1, a novel intracellular protein, localized on mitochondria, directly associates with HS1, a substrate of Src family tyrosine kinases. J Immunol. 1997;158:2736–2744. [PubMed] [Google Scholar]
- 37.Szekely L, Selivanova G, Magnusson K P, Klein G, Wiman K G. EBNA-5, an Epstein-Barr virus-encoded nuclear antigen, binds to the retinoblastoma and p53 proteins. Proc Natl Acad Sci USA. 1993;90:5455–5459. doi: 10.1073/pnas.90.12.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Szekely L, Pokrovskaja K, Jiang W Q, de The H, Ringertz N, Klein G. The Epstein-Barr virus-encoded nuclear antigen EBNA-5 accumulates in PML-containing bodies. J Virol. 1996;70:2562–2568. doi: 10.1128/jvi.70.4.2562-2568.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szekely L, Jiang W Q, Pokrovskaja K, Wiman K G, Klein G, Ringertz N. Reversible nucleolar translocation of Epstein-Barr virus-encoded EBNA-5 and hsp70 proteins after exposure to heat shock or cell density congestion. J Gen Virol. 1955;76:2423–2432. doi: 10.1099/0022-1317-76-10-2423. [DOI] [PubMed] [Google Scholar]
- 40.Takada K, Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989;63:445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Whitmarsh A J, Davis R J. Regulation of transcription factor function by phosphorylation. Cell Mol Life Sci. 2000;57:1172–1183. doi: 10.1007/PL00000757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yokoyama A, Kawaguchi Y, Kitabayashi I, Ohki M, Hirai K. The conserved domain CR2 of Epstein-Barr virus nuclear antigen leader protein is responsible not only for nuclear matrix association but also for nuclear localization. Virology. 2001;279:401–413. doi: 10.1006/viro.2000.0715. [DOI] [PubMed] [Google Scholar]
- 43.Zwilling S, Dieckmann A, Pfisterer P, Angel P, Wirth T. Inducible expression and phosphorylation of coactivator BOB.1/OBF.1 in T cells. Science. 1997;277:221–225. doi: 10.1126/science.277.5323.221. [DOI] [PubMed] [Google Scholar]