Abstract
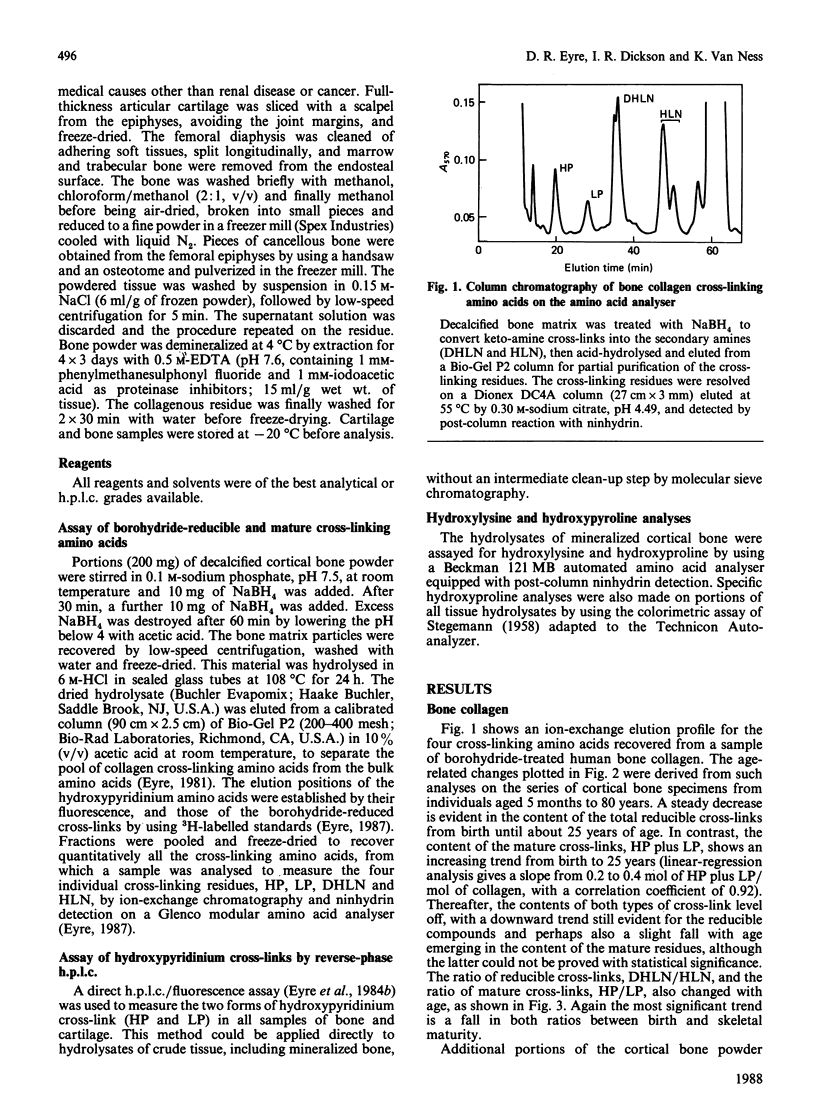
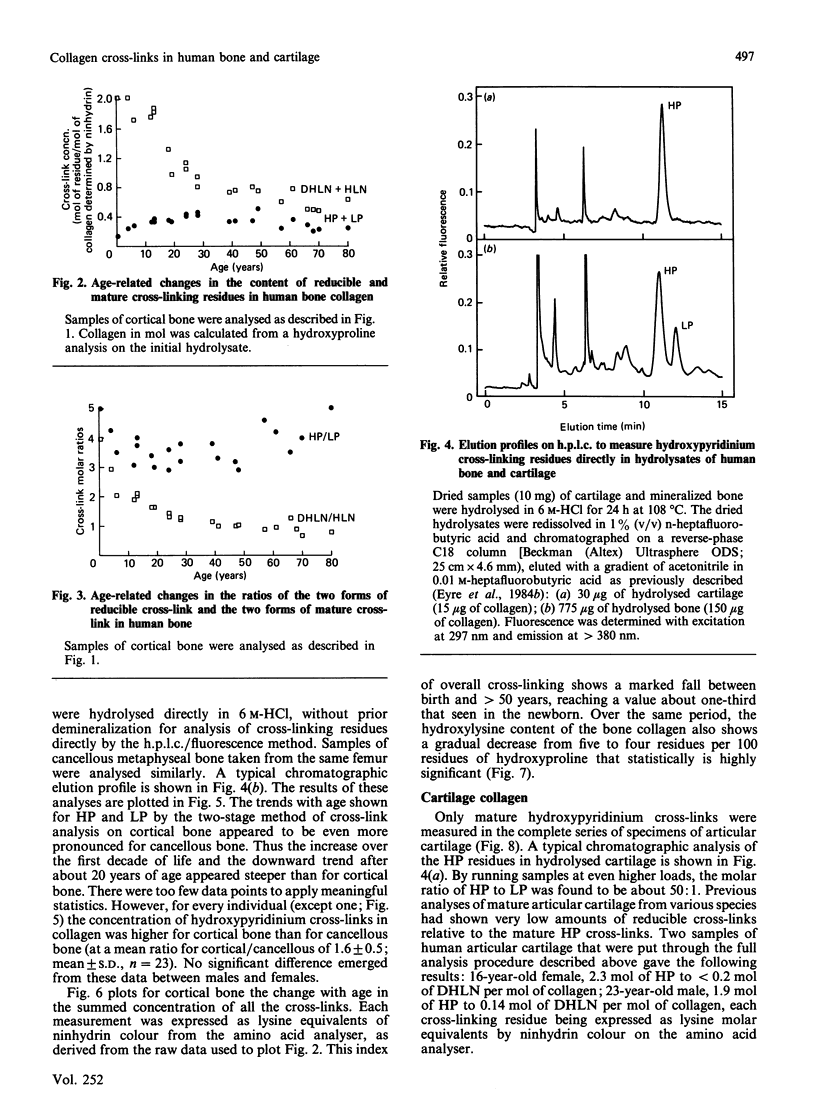
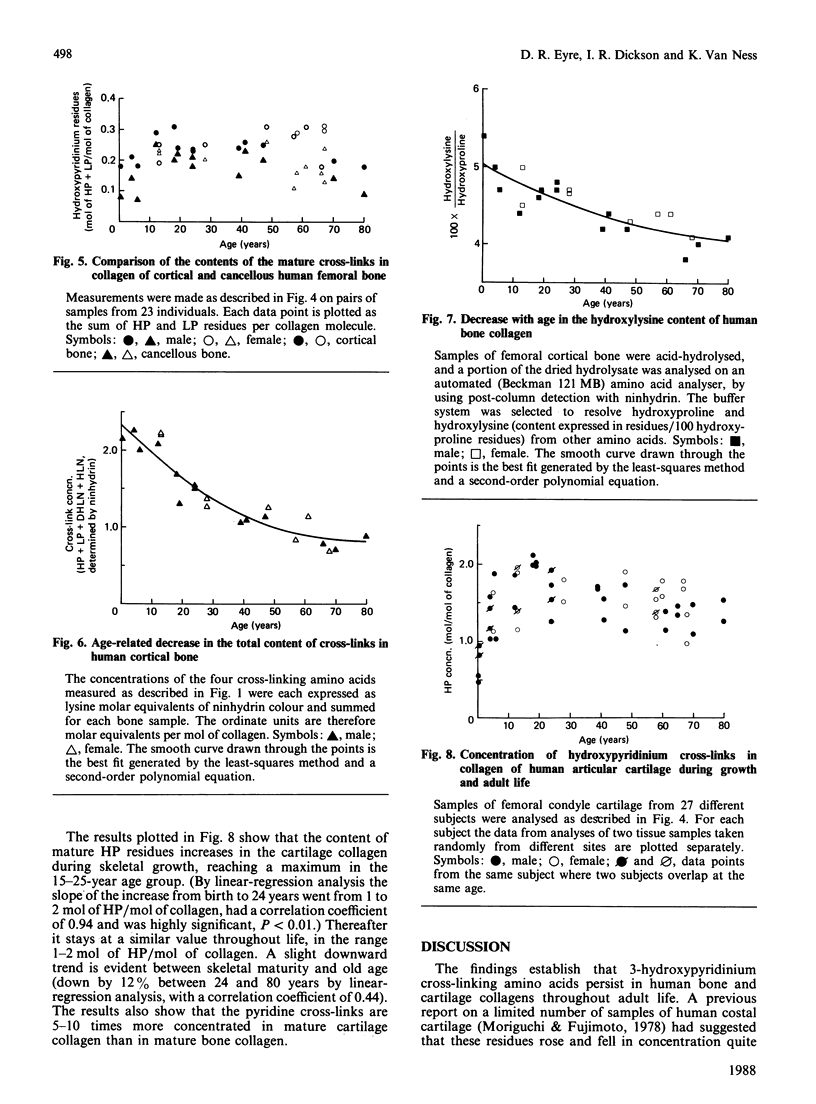
The concentration in collagen of hydroxypyridinium cross-linking amino acids was measured in samples of bone and cartilage from human subjects aged from 1 month to 80 years. Cortical and cancellous bone samples were dissected and analysed separately. In both bone and cartilage, the content of this mature form of cross-link reached a maximum by 10-15 years of age (the amount in cartilage being 5-10 times that in bone), then stayed essentially in the same range throughout adult life. In bone the ratio of the two chemical variants of the mature cross-link, hydroxylysylpyridinoline to lysylpyridinoline, was constant throughout adult life at 3.5:1, whereas in cartilage it was always greater than 10:1. The ratio of hydroxypyridinium cross-links to borohydride-reducible keto-amine cross-links also changed with age. The reducible cross-links in bone collagen decreased steeply in content between birth and 25 years, but persisted in significant amounts throughout adult life. Reducible cross-links had virtually disappeared from cartilage by 10-15 years of age, being replaced by hydroxypyridinium residues, their maturation products. Cancellous and cortical bone collagens showed similar trends with age in their content of mature cross-links, though for each subject the concentration in cancellous bone was always lower than in cortical bone, presumably reflecting the higher turnover rate and hence the more immature state of cancellous bone.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey A. J., Ranta M. H., Nicholls A. C., Partridge S. M., Elsden D. F. Isolation of alpha-amino adipic acid from mature dermal collagen and elastin. Evidence for an oxidative pathway in the maturation of collagen and elastin. Biochem Biophys Res Commun. 1977 Oct 24;78(4):1403–1410. doi: 10.1016/0006-291x(77)91448-6. [DOI] [PubMed] [Google Scholar]
- Bailey A. J., Shimokomaki M. S. Age related changes in the reducible cross-links of collagen. FEBS Lett. 1971 Aug 1;16(2):86–88. doi: 10.1016/0014-5793(71)80338-1. [DOI] [PubMed] [Google Scholar]
- Barnard K., Light N. D., Sims T. J., Bailey A. J. Chemistry of the collagen cross-links. Origin and partial characterization of a putative mature cross-link of collagen. Biochem J. 1987 Jun 1;244(2):303–309. doi: 10.1042/bj2440303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonar L. C., Lees S., Mook H. A. Neutron diffraction studies of collagen in fully mineralized bone. J Mol Biol. 1985 Jan 20;181(2):265–270. doi: 10.1016/0022-2836(85)90090-7. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Koob T. J., Van Ness K. P. Quantitation of hydroxypyridinium crosslinks in collagen by high-performance liquid chromatography. Anal Biochem. 1984 Mar;137(2):380–388. doi: 10.1016/0003-2697(84)90101-5. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Oguchi H. The hydroxypyridinium crosslinks of skeletal collagens: their measurement, properties and a proposed pathway of formation. Biochem Biophys Res Commun. 1980 Jan 29;92(2):403–410. doi: 10.1016/0006-291x(80)90347-2. [DOI] [PubMed] [Google Scholar]
- Eyre D. R., Paz M. A., Gallop P. M. Cross-linking in collagen and elastin. Annu Rev Biochem. 1984;53:717–748. doi: 10.1146/annurev.bi.53.070184.003441. [DOI] [PubMed] [Google Scholar]
- Eyre D. Collagen cross-linking amino acids. Methods Enzymol. 1987;144:115–139. doi: 10.1016/0076-6879(87)44176-1. [DOI] [PubMed] [Google Scholar]
- Housley T., Tanzer M. L., Henson E., Gallop P. M. Collagen crosslinking: isolation of hydroxyaldol-histidine, a naturally-occurring crosslink. Biochem Biophys Res Commun. 1975 Nov 17;67(2):824–830. doi: 10.1016/0006-291x(75)90887-6. [DOI] [PubMed] [Google Scholar]
- Mechanic G. L., Katz E. P., Henmi M., Noyes C., Yamauchi M. Locus of a histidine-based, stable trifunctional, helix to helix collagen cross-link: stereospecific collagen structure of type I skin fibrils. Biochemistry. 1987 Jun 16;26(12):3500–3509. doi: 10.1021/bi00386a038. [DOI] [PubMed] [Google Scholar]
- Moriguchi T., Fujimoto D. Age-related changes in the content of the collagen crosslink, pyridinoline. J Biochem. 1978 Oct;84(4):933–935. doi: 10.1093/oxfordjournals.jbchem.a132206. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- Robins S. P. Analysis of the crosslinking components in collagen and elastin. Methods Biochem Anal. 1982;28:329–379. doi: 10.1002/9780470110485.ch8. [DOI] [PubMed] [Google Scholar]
- Robins S. P., Shimokomaki M., Bailey A. J. The chemistry of the collagen cross-links. Age-related changes in the reducible components of intact bovine collagen fibres. Biochem J. 1973 Apr;131(4):771–780. doi: 10.1042/bj1310771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STEGEMANN H. Mikrobestimmung von Hydroxyprolin mit Chloramin-T und p-Dimethylaminobenzaldehyd. Hoppe Seylers Z Physiol Chem. 1958;311(1-3):41–45. [PubMed] [Google Scholar]
- Walters C., Eyre D. R. Collagen crosslinks in human dentin: increasing content of hydroxypyridinium residues with age. Calcif Tissue Int. 1983 Jul;35(4-5):401–405. doi: 10.1007/BF02405067. [DOI] [PubMed] [Google Scholar]


