Highlights
-
•
This study highlights dosimetric benefits of MRgART with and without rectal spacers.
-
•
Real-time adaptive planning results in similar dosimetric outcomes without spacers.
-
•
No significant toxicity differences were found between spacer and no-spacer groups.
-
•
MRgART offers a tailored and adaptive solution that may reduce the need for spacers.
Keywords: MR-Linac, Prostate cancer, Rectal spacers, Stereotactic ablative body radiotherapy
Abstract
Background and purpose
The use of stereotactic ablative radiotherapy (SABR) for prostate cancer has increased significantly. However, SABR can elevate the risk of moderate gastrointestinal (GI) side effects. Rectal spacers mitigate this risk by reducing the rectal dose. This study evaluates the impact of rectal spacers in MR-guided adaptive radiotherapy (MRgART) for prostate SABR.
Materials and methods
A retrospective analysis was conducted on twenty patients with localised prostate cancer treated on the Unity MR-Linac at a single centre. Half of the cohort (n = 10) had rectal spacers placed before treatment. The adapt-to-shape strategy was used for online MRgART, and non-adapted plans were later generated offline for comparison. Dosimetric assessments were made between spacer and no-spacer cohorts, and between online adapted and non-adapted plans. Clinician-reported outcomes for genitourinary (GU) and GI toxicity were assessed at 3-, 6-, and 12-months post-treatment using Common Terminology Criteria for Adverse Events v.5.0.
Results
No grade 2 or higher toxicity was observed in either cohort. Overall, the dosimetric analysis showed comparable results between the cohorts for target volumes, with D95% of 36.3 Gy in the spacer cohort and 36.0 Gy in the no-spacer cohort (p = 0.08). The spacer cohort demonstrated significant benefits in all rectal dose objectives (p < 0.0001) and in some bladder objectives (V40, p = 0.03; V36, p = 0.03). Failure rates for achieving planning objectives were similar between spacer and no-spacer groups for online adapted plans, with most rates ranging from 0 % to 4 % in both groups.
Conclusion
The findings from this cohort suggest that MRgART is safe and effective for prostate SABR, with comparable toxicity rates in both spacer and no-spacer cohorts. While rectal spacers offer dosimetric advantages, the adaptive nature of MRgART can mitigate some dosimetric disparities, potentially reducing the need for invasive spacer placement. However, further studies with larger patient populations are needed to confirm these results.
Introduction
The use of stereotactic ablative radiotherapy (SABR) for treating prostate cancer has substantially increased in recent years. Stereotactic radiotherapy (RT) is more convenient and cost-effective than conventional RT, as treatments can be completed in far less fractions compared to conventional RT. Literature, such as the PACE trial, has demonstrated the feasibility and safety of SABR, showing similar toxicity rates between SABR and conventional RT [1]. However, it has been reported that SABR techniques can increase the risk of moderate gastrointestinal (GI) side-effects [2]. The HYPO-RT-PC trial reported grade 2 and worse toxicity estimates for ultra-hypofractionation ranging from 1 to 16 % for GI toxicity, highlighting the potential for increased GI complications with SABR [3].
Rectal spacers have been associated with a reduced risk of GI toxicity after prostate RT [4], [5]. By creating increased distance between the prostate and rectum, spacers can reduce the rectal volume exposed to high doses of radiation, therefore minimising the risk of damage to the rectal tissue. The use of spacer has been shown to significantly improve dosimetric outcomes in the treatment of prostate cancers [6]. However, rectal spacers are an invasive procedure with potential risks, including complications such as rectal perforation or fistula formation [7], which may be underreported in literature. Furthermore, several factors can render patients ineligible for spacer placement, including anatomical constraints, previous surgeries, or other medical conditions that complicate the procedure. Additionally, poor spacer insertion can occur, leading to suboptimal placement that fails to achieve the intended separation between the prostate and rectum, thereby diminishing the protective benefits. Online MR-guided adaptive radiotherapy (MRgART) offers a promising alternative to rectal spacers, with the potential to achieve comparable dosimetric and toxicity outcomes. The adaptive nature of MRgART enables it to accommodate variations in the patient's anatomy that might otherwise compromise the effectiveness of a fixed treatment plan. By adapting to the daily anatomical changes, MRgART can potentially reduce the radiation dose to the rectum, even without the use of a spacer.
This study aimed to evaluate the impact of spacer in MRgART for prostate SABR. Additionally, we compared the dosimetric differences between online adapted plan and non-adapted plans for both spacer and no-spacer cohorts. The non-adapted treatment plans were created to simulate conventional image guided RT (IGRT) in the absence of online adaptive treatment.
Methods
Patients
For this study, a cohort of twenty patients with localized prostate cancer, who had received clinical treatment on the Unity MR-Linac (Elekta AB, Stockholm, Sweden) was retrospectively selected. The study includes the prostate cancer subgroup of the ongoing GenesisCare Oncology Outcomes Protocol, with approval from the local ethics committee (reference number: 2022/ETH00247). Treatment inclusion criteria involved; age > 18 years, histologically proven prostate adenocarcinoma, low risk (all of PSA < 10 ng/mL, Gleason grade 6 and stage T1 or T2a) or intermediate risk (any or all of PSA 10–20 ng/mL, Gleason, grade 7 or Stage T2b-c) disease, and ECOG performance status 0–2. The exclusion criteria involved: N1 disease or high risk of nodal involvement whereby whole pelvis RT indicated, evidence of distant metastases, severe obstructive or irritative urinary symptoms, artificial hips, prostate volume > 100 cc, previous pelvic RT and MR imaging contraindications (electronic devices such as pacemakers, defibrillators, deep brain stimulators, cochlear implants or foreign metal bodies or aneurysm clips or severe claustrophobia). All eligible patients were offered spacer insertion as part of their treatment. For this study, a total of 10 patients who received spacers (SpaceOAR, Boston Scientific, Marlborough, MA, USA) and 10 patients who did not receive spacers were randomly selected for analysis.
Imaging and treatment planning
All patients underwent MR scans on the Unity MR-Linac and a planning computed tomography (CT) scan (Siemens Somatom Definition AS, Siemens Healthineers, Erlangen, Germany). Patients were instructed to follow bladder filling instructions (completely voiding bladder 30 min prior to the scan, and drinking 100 mL of water), and an empty rectum before scans, and before each fraction.
A T2-weighted MR scan (simulation MR) was acquired on the MR-Linac at time 0 and time 25 min (to assess bladder filling and rectal movement). Patients subsequently had a planning CT scan on the same day following standard protocols. The clinical target volume (CTV) included prostate and proximal 1 cm of seminal vesicles (SV). The planning target volume (PTV) was defined as CTV + 5 mm margins in all directions, except posteriorly where the margin was 3 mm. Organs at risk (OAR) delineated were rectum, bladder, penile bulb, urethra, proximal femur, and sigmoid colon. The CTVs were prescribed to receive 38–40 Gy, while the PTVs were prescribed to receive 36.25 in 5 fractions (1–3 fractions per week, once daily). All plans were generated using Monaco (version 5.40.01, Elekta AB, Stockholm, Sweden) with a 7-field, step-and-shoot intensity modulated RT (IMRT) plan with a maximum of 125 segments per plan and dose calculation grid of 2 mm. Treatment plans were generated in accordance with the clinical protocol as outlined in Table 1.
Table 1.
Plan compliance criteria.
| Structure | Objective | Protocol | Minor violation | Major violation |
|---|---|---|---|---|
| Clinical target volume | D95 | > 40 Gy | 38–40 Gy | < 38 Gy |
| Planning target volume | D95 | > 36.25 Gy | 34.5–36.25 Gy | < 34.5 Gy |
| D98 | > 34.5 Gy | 32.5–34.5 Gy | < 32.5 Gy | |
| Rectum | Dmax (0.1 cm3) | < 38 Gy | 38–40 Gy | > 40 Gy |
| V36Gy | < 1 cm3 | NA | > 1 cm3 | |
| V34Gy | < 3 cm3 | 3–4 cm3 | > 4 cm3 | |
| V32Gy | < 10 % | 10–20 % | > 20 % | |
| V20Gy | < 40 % | 40–50 % | > 50 % | |
| Bladder | Dmax (0.1 cm3) | < 42 Gy | NA | > 42 Gy |
| V40Gy | < 2 cm3 | 2–3 cm3 | > 3 cm3 | |
| V36Gy | < 5 cm3 | 5–10 cm3 | > 10 cm3 | |
| V32Gy | < 5 % | 5–10 % | > 10 % | |
| V20Gy | < 40 % | 40–50 % | > 50 % | |
| Penile bulb | Dmax (0.1 cm3) | < 36.25 Gy | NA | > 36.25 Gy |
| V20Gy | < 3 cm3 | 3–5 cm3 | > 5 cm3 | |
| Femurs | Dmax (0.1 cm3) | < 36.25 Gy | NA | > 36.25 Gy |
| V20Gy | < 3 cm3 | 3–5 cm3 | > 5 cm3 | |
| Urethra | Dmax (0.1 cm3) | < 42 Gy | NA | > 42 Gy |
Dmax: maximum dose; Dx: dose received by x% of the structure; Gy: Gray; Vx Gy: the volume of the structure receiving x Gy.
Online MR-guided adaptive radiotherapy
The adapt-to-shape (ATS) online adaptive strategy was employed for each fraction, where the daily MR image was recontoured to adapt to the anatomy of the day [8]. Prior to each fraction, a T2-wieghted MR scan was acquired and rigidly registered to the simulation MR. Following this, deformable registration was used to project the original set of contours onto the daily pre-treatment MR image, and if necessary, the contours were edited by the treating radiation therapists or oncologist. Plan re-optimisation was completed, and treatment was initiated while monitoring the patient using real-time cineMR imaging.
Non-adapted plans
The non-adapted treatment plans were generated in Monaco by employing a virtual isocentre shift technique to simulate conventional IGRT. This approach maintains the weights and shapes of the original treatment plan while shifting the treatment isocentre to align with the tumour position on the daily pre-treatment MR image. Consequently, the existing plan is effectively adjusted to match the daily tumour position.
Outcome measurements and dosimetric analysis
Clinician reported outcomes (CRO) were assessed at 3-, 6- and 12-months post-treatment. Patient toxicity with a focus on genitourinary (GU) and gastrointestinal (GI) symptoms were scored using Common Terminology Criteria for Adverse Events (CTCAE) v.5.0. All dose metrics from the online adapted plans were compared between the spacer and no-spacer cohorts. Additionally, dose metrics were compared between online adapted plans and non-adapted plans for both the spacer and no-spacer cohorts. Statistical analysis was performed using the Wilcoxon signed-rank test, with significance defined as p values ≤ 0.05.
Results
Baseline patient characteristics are shown in Table 2. The median age was 72 (range 66 – 85) years in the spacer cohort and 68 (range 61 – 87) years in the no-spacer cohort. Grade 1 GU toxicity was observed in 40 %, 40 %, and 50 % of the spacer cohort at 3-, 6-, and 12-month follow-up, respectively. In the no-spacer cohort, grade 1 GU toxicity was recorded in 20 %, 30 %, and 40 % at 3-, 6-, and 12-months, respectively. For GI toxicity, none was reported at any time point in the spacer cohort, while the no-spacer cohort reported grade 1 toxicity in 20 % at 3 months and 10 % at 12 months. No grade 2 or higher toxicity was observed in both cohorts at any time point in the study.
Table 2.
Baseline characteristics.
| Characteristic | Spacer (n = 10) | No-spacer (n = 10) |
|---|---|---|
| Age, median (range) | 72 (66–85) | 68 (61–87) |
| Risk group, n (%) | ||
| Favourable intermediate | 6 (60 %) | 3 (30 %) |
| Unfavourable intermediate | 3 (30 %) | 6 (60 %) |
| High risk | 1 (10 %) | 1 (10 %) |
| Prostate size, median (range), cm3 | 63 (26–75) | 60 (31–78) |
A total of 100 online adapted plans were analysed. Table 3 displays the online adaptive plan comparison between the spacer and no-spacer cohorts. The mean dose achieved over five fractions for all dose objectives were within the acceptable tolerance for both spacer and no-spacer cohorts. The results show dosimetric advantages favouring the spacer cohort for all rectum dose objectives. For bladder, differences were observed for V40 and V36, in favour of the spacer cohort. No significant difference was observed between the two cohorts for PTV, urethra and some bladder objectives (V32, V20 and D0.1 cm3).
Table 3.
Comparison of online adaptive plans between spacer and no-spacer cohorts.
| Planning objective | Mean (± Standard deviation) |
p value | |
|---|---|---|---|
| Spacer | No-spacer | ||
| Planning target volume | |||
| D95% > 36.25 Gy (−1.75 Gy*) | 36.29 (0.74) | 35.99 (0.93) | 0.08 |
| D98% > 34.5 Gy (−2 Gy*) | 35.17 (1.15) | 35.00 (1.24) | 0.48 |
| D2% < 42 Gy (+0.8 Gy*) | 41.96 (0.64) | 41.75 (0.90) | 0.19 |
| D0.1 cm3 < 42.8 Gy (+1.2 Gy*) | 42.52 (0.68) | 42.52 (1.05) | 0.99 |
| Rectum | |||
| V36 Gy < 1 cm3 | 0.20 (0.27) | 0.54 (0.34) | < 0.0001 |
| V34 Gy < 3 cm3 (+1 cm3*) | 0.51 (0.55) | 1.48 (0.75) | < 0.0001 |
| V32 Gy < 10 % (+10 %*) | 1.65 (1.34) | 5.33 (2.28) | < 0.0001 |
| V20 Gy < 40 % (+10 %*) | 16.53 (7.21) | 31.11 (8.12) | < 0.0001 |
| D0.1 cm3 < 38 Gy (+2 Gy*) | 34.37 (4.41) | 37.27 (1.01) | < 0.0001 |
| Bladder | |||
| V40 Gy < 2 cm3 (+1 cm3*) | 0.11 (0.27) | 0.35 (0.74) | 0.03 |
| V36 Gy < 5 cm3 (+5 cm3*) | 2.25 (1.58) | 3.07 (2.04) | 0.03 |
| V32 Gy < 5 % (+5%*) | 4.3 (2.48) | 4.65 (1.77) | 0.41 |
| V20 Gy < 40 % (+10 %*) | 17.92 (7.67) | 18.36 (5.47) | 0.73 |
| D0.1 cm3 < 42 Gy | 39.08 (1.64) | 39.52 (1.80) | 0.21 |
| Urethra | |||
| D0.1 cm3 < 42 Gy | 41.65 (0.58) | 41.36 (0.97) | 0.13 |
*Values show acceptable tolerance.
Table 4 presents an overview of the unmet planning objectives for both non-adapted and online adapted plans. For non-adapted plans, the failure rates for achieving planning objectives were consistent between the spacer and no-spacer groups for the PTV, bladder, and urethra. However, for rectum, the spacer cohort outperformed the no-spacer cohort in terms of fulfilling dose objectives within the non-adapted plans. For online adapted plans, the failure rates were comparable between the spacer and no-spacer groups.
Table 4.
Percentage of unmet dose objectives by non-adapted and online adapted plans.
| Structures | Dose Objectives | Non-adapted plan | Online adapted plan | ||
|---|---|---|---|---|---|
| Spacer | No-spacer | Spacer | No-spacer | ||
| Planning target volume | D95% | 94 % | 100 % | 4 % | 4 % |
| D98% | 100 % | 100 % | 4 % | 4 % | |
| D2% | 82 % | 73 % | 0 | 2 % | |
| D0.1 cm3 | 53 % | 58 % | 0 | 2 % | |
| Rectum | V36 Gy | 34 % | 68 % | 2 % | 6 % |
| V34 Gy | 26 % | 58 % | 0 | 0 | |
| V32 Gy | 2 % | 44 % | 0 | 0 | |
| V20 Gy | 4 % | 34 % | 0 | 0 | |
| D0.1 cm3 | 40 % | 54 % | 0 | 0 | |
| Bladder | V40 Gy | 14 % | 16 % | 0 | 2 % |
| V36 Gy | 14 % | 12 % | 0 | 0 | |
| V32 Gy | 18 % | 18 % | 0 | 0 | |
| V20 Gy | 8 % | 2 % | 0 | 0 | |
| D0.1 cm3 | 20 % | 16 % | 0 | 0 | |
| Urethra | D0.1 cm3 | 70 % | 74 % | 0 | 4 % |
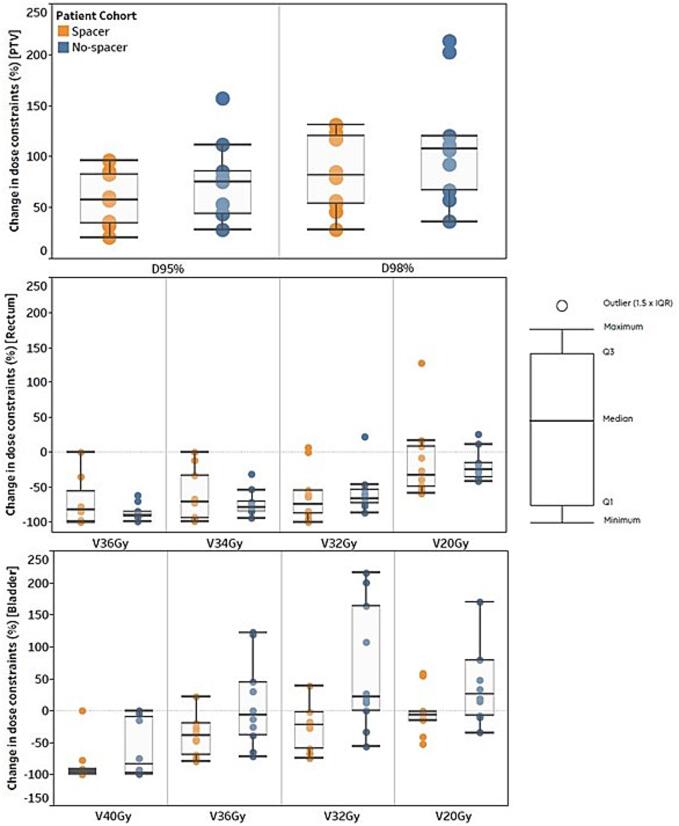
Fig. 1 represents the percentage change in dose constraints between online adapted and non-adapted plans. Improvement in dose to the PTV were higher in the no-spacer cohort when adapted. The reduction in dose to the rectum were comparable between the two cohorts when adapted. For bladder, higher reduction in dose was observed in the spacer cohort when adapted.
Fig. 1.
Percentage change in dose constraints between online adapted and non-adapted plans for patients treated with and without rectal spacers. Positive values for PTV reflect a percentage improvement in dose to the PTV when adapted, while negative values for the rectum and bladder signify a reduction in dose to OARs when plan adaptation is implemented.
Discussion
The integration of rectal spacers in prostate cancer radiotherapy has been well-established as an effective strategy for reducing GI toxicity. This study aimed to evaluate the impact of rectal spacers in the context of MRgART for prostate SABR. Our findings indicate that the observed GU and GI toxicity rates remained at grade 1 for both spacer and no-spacer cohorts, demonstrating the safety of MRgART in delivering ablative doses in no-spacer cohort. Similarly, patient reported outcomes from prior research involving MRgART showed no significant differences between the spacer and no-spacer groups, reaffirming the comparable safety profiles of these treatment approaches [9]. In our study, the low rate of GI toxicity observed in the no-spacer cohort aligns with findings from the PACE-B trial (1), which also reported minimal GI toxicity in the absence of rectal spacers. These consistently low toxicity rates in both studies highlight the advancing potential of radiotherapy to safely deliver high-dose treatments with minimal side effects, even without the need for invasive interventions like rectal spacers. However, while these results are encouraging, it is important to recognise that they may not be generalisable to all patient populations or clinical settings. The potential to further reduce toxicity outcomes and improve patients' quality of life remains a critical area of investigation. The DESTINATION trial (NCT05709496) explores the feasibility of delivering high-precision, toxicity-minimising radiotherapy using the MR-Linac for patients with intermediate-risk localised prostate cancer. The treatment regimen includes 5 fractions, with 30 Gy delivered to the entire prostate without a margin, and a more targeted 45 Gy to the dominant lesion using a 4-mm margin. This study aims to further refine treatment delivery by increasing the precision of MRgART, which may lead to a reduction in both acute and late toxicities. The results of this trial will set an important benchmark for future development.
Our dosimetric analysis provided evidence to support the benefits of rectal spacers with improvements in dose to the rectum in the spacer cohort [9]. These outcomes align with existing literature emphasising the advantages of spacers in conventional prostate radiotherapy settings [10]. However, in the context of MRgART, where adaptive planning allows for real-time adjustments to the daily anatomy, the necessity of rectal spacers comes into question. Notably for online adapted plans in this study, the failure rates were comparable between spacer and no-spacer groups, suggesting that adaptive strategies may mitigate some dosimetric disparities associated with no-spacer placement in conventional settings. Online adaptive strategies, such as the ATS protocol employed in our study, modify the treatment plan based on daily imaging, offering a personalised and adaptive approach. Our findings indicate that MRgART without rectal spacers can achieve dosimetric outcomes comparable to those with spacers after online adaptation. Notably, MRgART without spacer demonstrates superior dosimetric outcomes compared to the use of spacers without MRgART. This suggests that the adaptive nature of MRgART is able to address some of the challenges posed by the proximity of the rectum to the prostate.
While rectal spacers undoubtedly contribute to improved dosimetry, their application involves an additional invasive procedure and associated costs. The injection of hydrogel spacer is generally considered safe, however, complications following hydrogel implantations have been observed, including severe anaphylaxis, acute pulmonary embolism, prostatic or perineal abscess and sepsis, rectal wall erosion, and rectal ulceration and fistula [7], [11], [12]. It has also been reported that the routine use of hydrogel spacer is not always cost-effective [13]. A recent study considered the benefit and risk of SpaceOAR (hydrogel absorbable spacer) placing serious doubts on the real usefulness and impact of SpaceOAR in reducing rectal dose and toxicity [14]. In contrast, MRgART leverages advanced imaging and adaptive planning to tailor treatment to the unique daily anatomy of the patient, potentially eliminating the need for invasive interventions like spacer placement. The choice between incorporating rectal spacers or relying solely on MRgART should be carefully considered, weighing the benefits against the associated invasiveness and costs.
In considering margin selection, we adhered to our department’s standard protocol of 5 mm margins in all directions, except posteriorly where the margin was 3 mm. While the MIRAGE trial [15] employed smaller 2 mm margins isotropically on the MRIdian linac (ViewRay, Inc) with automated gating, this feature was not available on our Unity MR-linac during the study period. Although automated gating is now accessible with the Unity system, it was not an option at the time, necessitating the use of slightly larger margins to account for motion and setup uncertainties. Importantly, more evidence is needed to support the routine adoption of 2 mm margins in clinical practice to confirm the safety and efficacy of such margin reductions across diverse clinical contexts.
While our study provides valuable insights into the dosimetric benefits of MRgART with and without rectal spacers, there are several limitations that should be acknowledged. Firstly, the small sample size of only 20 patients, divided equally between the spacer and no-spacer cohorts, may limit the generalisability of our findings. A larger cohort would provide more robust data and potentially more definitive conclusions. Another limitation pertains to the methodology used for generating non-adapted treatment plans. In our study, we employed a virtual isocenter shift technique to simulate conventional IGRT. This approach maintains the weights and shapes of the original treatment plan while shifting the treatment isocenter to align with the tumour position on the daily pre-treatment MR image. This method, while practical, may not fully replicate all aspects of conventional non-adaptive radiotherapy. The specific details of this technique, including its validation and potential biases, should be carefully considered when interpreting the results.
This study highlights the evolving landscape of prostate cancer radiotherapy by considering the impact of rectal spacers and MRgART. While rectal spacers continue to play a vital role in optimising prostate radiotherapy, the emerging paradigm of MRgART offers a tailored and adaptive solution that may reduce the need for spacers in certain scenarios. However, given the small cohort size in this study, our findings should be interpreted with caution. Although MRgART shows promise in achieving comparable dosimetric outcomes without spacers, larger studies are needed to validate these results and assess the long-term implications. Future research should focus on exploring the comparative effectiveness of these approaches, considering factors such as patient comfort, resource utilisation, and treatment accessibility.
CRediT authorship contribution statement
Vikneswary Batumalai: Conceptualization, Methodology, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. David Crawford: Methodology, Writing – review & editing. Maddison Picton: Methodology, Writing – review & editing. Charles Tran: Methodology, Writing – review & editing. Urszula Jelen: Methodology, Writing – review & editing. Madeline Carr: Methodology, Writing – review & editing. Michael Jameson: Methodology, Writing – review & editing. Jeremy de Leon: Conceptualization, Methodology, Writing – review & editing.
Declaration of Competing Interest
GenesisCare and Elekta AB (Stockholm, Sweden) have a strategic research agreement.
References
- 1.Brand D.H., Tree A.C., Ostler P., van der Voet H., Loblaw A., Chu W., et al. Intensity-modulated fractionated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): acute toxicity findings from an international, randomised, open-label, phase 3, non-inferiority trial. Lancet Oncol. 2019;20(11):1531–1543. doi: 10.1016/S1470-2045(19)30569-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tree A.C., Ostler P., van der Voet H., Chu W., Loblaw A., Ford D., et al. Intensity-modulated radiotherapy versus stereotactic body radiotherapy for prostate cancer (PACE-B): 2-year toxicity results from an open-label, randomised, phase 3, non-inferiority trial. Lancet Oncol. 2022;23(10):1308–1320. doi: 10.1016/S1470-2045(22)00517-4. [DOI] [PubMed] [Google Scholar]
- 3.Widmark A., Gunnlaugsson A., Beckman L., Thellenberg-Karlsson C., Hoyer M., Lagerlund M., et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 4.Mariados N.F., Orio P.F., Schiffman Z., Van T.J., Engelman A., Nurani R., et al. Hyaluronic acid spacer for hypofractionated prostate radiation therapy: A randomized clinical trial. JAMA Oncol. 2023;9(4):511–518. doi: 10.1001/jamaoncol.2022.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamstra D.A., Mariados N., Sylvester J., Shah D., Karsh L., Hudes R., et al. Continued benefit to rectal separation for prostate radiation therapy: final results of a phase III trial. Int J Rad Oncol Biol Phys. 2017;97(5):976–985. doi: 10.1016/j.ijrobp.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 6.Whalley D., Hruby G., Alfieri F., Kneebone A., Eade T. SpaceOAR hydrogel in dose-escalated prostate cancer radiotherapy: rectal dosimetry and late toxicity. Clin Oncol. 2016;28(10):e148–e154. doi: 10.1016/j.clon.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 7.McLaughlin M.F., Folkert M.R., Timmerman R.D., Hannan R., Garant A., Hudak S.J., et al. Hydrogel spacer rectal wall infiltration associated with severe rectal injury and related complications after dose intensified prostate cancer stereotactic ablative radiation therapy. Adv Radiat Oncol. 2021;6(4) doi: 10.1016/j.adro.2021.100713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M., et al. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alongi F., Rigo M., Figlia V., Cuccia F., Giaj-Levra N., Nicosia L., et al. Rectal spacer hydrogel in 1.5 T MR-guided and daily adapted SBRT for prostate cancer: dosimetric analysis and preliminary patient-reported outcomes. Br J Radiol. 2021;94(1117) doi: 10.1259/bjr.20200848. 20200848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey M., Ong W.L., Chao M., Udovicich C., McBride S., Bolton D., et al. Comprehensive review of the use of hydrogel spacers prior to radiation therapy for prostate cancer. BJU Int. 2023;131(3):280–287. doi: 10.1111/bju.15821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ardekani M.A., Ghaffari H. Optimization of prostate brachytherapy techniques with polyethylene glycol–based hydrogel spacers: a systematic review. Brachytherapy. 2020;19(1):13–23. doi: 10.1016/j.brachy.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 12.Sanei M., Ghaffari H., Ardekani M.A., Mahdavi S.R., Mofid B., Abdollahi H., et al. Effectiveness of rectal displacement devices during prostate external-beam radiation therapy: a review. J Cancer Res Ther. 2021;17(2):303–310. doi: 10.4103/jcrt.JCRT_841_19. [DOI] [PubMed] [Google Scholar]
- 13.Giuliani J., Fiorica F. Cost-effectivess of SpaceOAR system during prostate cancer radiation therapy: really helpful or excess of expectations? Brachytherapy. 2021;20(6):1341–1342. doi: 10.1016/j.brachy.2021.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Hall W.A., Tree A.C., Dearnaley D., Parker C.C., Prasad V., Roach M., et al. Considering benefit and risk before routinely recommending SpaceOAR. Lancet Oncol. 2021;22(1):11–13. doi: 10.1016/S1470-2045(20)30639-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishan A.U., Ma T.M., Lamb J.M., Casado M., Wilhalme H., Low D.A., et al. Magnetic resonance imaging–guided vs computed tomography–guided stereotactic body radiotherapy for prostate cancer: the MIRAGE randomized clinical trial. JAMA Oncol. 2023;9(3):365–373. doi: 10.1001/jamaoncol.2022.6558. [DOI] [PMC free article] [PubMed] [Google Scholar]