Abstract
Background
The adipokine adiponectin (APN)’s role in Alzheimer’s disease (AD) is controversial. Some studies suggest APN is neuroprotective while others propose it has harmful effects. We have used Multiple Indicators Multiple Causes (MIMIC) models to evaluate the effects of serum protein biomarkers on cognitive performance in the Texas Alzheimer’s Research and Care Consortium (TARCC) (Royall DR, Bishnoi RJ, Palmer RF. Serum IGF-BP2 strongly moderates age’s effect on cognition: a MIMIC analysis. Neurobiol Aging. 2015;36:2232–2240; Bishnoi RJ, Palmer RF, Royall DR. Vitamin D binding protein as a serum biomarker of Alzheimer’s disease. J Alzheimers Dis. 2015;43:37–45; Bishnoi RJ, Palmer RF, Royall DR. Serum interleukin (IL)-15 as a biomarker of Alzheimer’s disease. PLoS One. 2015;10:e0117282).
Methods
MIMIC models were constructed and replicated in randomly selected 50% splits of TARCC’s data (Group 1 N = 1,691; Group 2 N = 1,690) and used to evaluate the relationship between serum APN levels and cognition. Our approach has been to divide general intelligence (Spearman’s g) (Spearman C. The Abilities of Man: Their Nature and Measurement. 1932) into two latent variables, δ (ie, a dementia-specific phenotype representing the disabling fraction of cognitive variance) and g prime (g′) (ie, the residual non-disabling fraction). Only effects on δ are likely to be dementing.
Results
Serum APN was significantly related to δ scores (r = .10, p = .015). APN had no significant effect on g′ (r = −.25, p = .66), nor did it have any independent direct effects on cognitive performance. These results were replicated across random subsets (ΔCHISQ = 2.8(7), p > .90).
Conclusions
APN’s effect on cognition is mediated through intelligence (ie, δ), likely to be disabling, and therefore to mediate one or more dementing processes. We have previously shown APN to partially mediate age’s-specific effect on δ (Royall DR, Al-Rubaye S, Bishnoi R, Palmer RF. Serum protein mediators of dementia and aging proper. Aging (Albany NY). 2016;8:3241–3254). However, because the current model is age adjusted, APN must mediate one or more additional age-independent dementing process(es), possibly AD.
Keywords: Biomarkers, Cognition, Functional performance, Metabolism
There has been growing interest in the role of obesity and adipocytokines in the pathogenesis of dementia and Alzheimer’s disease (AD). It has been proposed that adipose tissue is associated with cognitive impairment via cerebrovascular disease, alterations in brain structure, and the release of cytokines (1). Obesity and aging can lead to dysregulated adipokine release (2). Adiponectin (APN) is one of the most abundant adipocytokines. It is synthesized and secreted by white adipose tissue and acts by binding to APN receptors: AdipoR1, AdipoR2, and T-Cadherin which have been found throughout the body including in adipose tissue, skeletal muscle, liver, and endothelial cells (2–4 ). In the brain, APN’s receptors have been found in the nucleus basalis of Meynert, hypothalamus, pituitary, and hippocampus (4).
APN regulates glucose and fatty acid catabolism and sensitizes cells to insulin (1,2,4,5). Serum APN levels are inversely correlated with body mass index (BMI), insulin resistance, type 2 diabetes mellitus, and cardiovascular disease (1,2), all of which have been associated with cognition, dementia and/or dementia risk. APN exhibits anti-inflammatory effects by acting to decrease production of pro-inflammatory cytokines, including tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and interferon gamma (INFγ) and by increasing interleukin-10 (IL-10) and interleukin-1 (IL-1) expression (3).
Studies evaluating the role of APN in AD have had mixed results. Several have shown serum levels to be significantly lower in patients with Mild Cognitive Impairment (MCI) and AD (6,7). Other studies have suggested that higher APN levels adversely affect cognition or are associated with the pathogenesis of AD (5,8,9). Additional studies have found no differences in the levels of APN in patients with AD compared to controls (10,11). These conflicting data indicate a need for additional research evaluating APN’s role in dementia and AD.
We have advocated an empiric reconceptualization of dementia as “the cognitive correlates of functional status.” Our approach separates cognition into disabling and non-disabling factions through confirmatory factor analysis (CFA) in a Structural Equation Model (SEM) framework. One potential advantage of our method is that it generates latent variables that are relatively free of measurement error and may improve power to detect biomarker effects (ie, relative to categorical clinical diagnoses).
Our approach results in a latent variable δ (for dementia), which is empirically strongly correlated with dementia severity, as measured by Clinical Dementia Rating (CDR) scale scores (12) and achieves very high areas under the receiver operating curve (AUC /ROC) for dementia’s diagnosis (13–16). δ is derived from Spearman’s general intelligence factor g (17). δ’s empirically non-disabling residual in Spearman’s g is relabeled g prime (g′) by our method. g′ is empirically weakly related to dementia severity and poorly distinguishes demented persons 13,15,16,18). Because both δ and g′ are manifested independently in the same patients, our approach distinguishes dementia-specific cognitive performance from cognitive impairment. Biomarkers may influence cognitive performance via either compartment (19,20).
We have previously tested g′ and δ’s associations with serum protein biomarkers in the Texas Alzheimer’s Research and Care Consortium (TARCC) using Multiple Indicators Multiple Causes (MIMIC) models (19–22). The MIMIC model allows us to distinguish a serum protein’s direct effects on cognition from any indirect effects, mediated through either δ, g′, or both. APN is among the serum proteins in TARCC’s panel. This study aims to determine whether APN’s association with cognitive performance is mediated through δ, g′, or whether it might be independent of them. If APN has effects on δ, it could be a potential target for dementia-modifying interventions.
Method
Subjects
MIMIC models were constructed and replicated in randomly selected 50% splits of TARCC’s sample (Group 1 N = 1,691; Group 2 N = 1,690; Total N = 3,381, ca. 2016). The participants were examined annually in a standardized assessment, and a diagnosis of normal control (NC), MCI, or AD was made. Institutional Review Board (IRB) approval was obtained from each site. Written consent was obtained from all participants.
Clinical Variables
Dementia severity was evaluated using the Clinical Dementia Rating Sum of the Boxes (CDR-SB) scale (12) which assesses the patient’s cognitive ability in six domains including memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care through an interview with the caregiver (23). Depressive symptoms were evaluated using the Geriatric Depression Scale (GDS) (24).
Cognitive Battery
General cognitive ability was assessed using the Mini-Mental Status Exam (MMSE) (25). The Controlled Oral Word Association (COWA) test was used to evaluate verbal fluency (26), Digit Span test (DST) measured memory (27), The Boston Naming Test (BOSTON) evaluated verbal skills (28), and Trail-Making tests A and B measured nonverbal ability (29). Dementia severity was measured by the CDR-SB (12) which was used to validate the δ homolog.
Biomarkers
Non-fasting samples were collected, centrifuged, pipetted by aliquots, frozen, and sent in four batches for assay to Rules-Based Medicine (RBM) in Austin, TX. Over 100 proteins were quantified by fluorescent microspheres with protein-specific antibodies. A complete listing of the biomarker panel employed is available at http://www.rulesbasedmedicine.com. The TARCC methodology has been further described elsewhere (30).
Raw biomarker data were inspected to ascertain their normality, and data points beyond 3.0 standard deviations (SD) about the mean were labeled as “outliers” and deleted. Logarithmic transformation was used to normalize highly skewed distributions. The data were then standardized to a mean of zero and unit variance. All serum biomarkers were adjusted for age, gender, education, and ethnicity.
The serum biomarkers in TARCC have a significant batch effect which was primarily but incompletely explained by subject characteristics (age, gender, education, ethnicity, and APOEε4 status). The observed serum biomarker values were adjusted for batch effects using covariate-adjusted batch variables. Biomarker data from batch 1 was excluded after serial analyses could not be replicated. The missing biomarker data was handled using modern missing data methods including Full Information Maximum Likelihood (FIML) methods (31).
Statistical Analyses
We tested APN’s effects on cognitive performance in a MIMIC model incorporating the same δ homolog employed by Bishnoi and colleagues in previous studies (20). The bifactor δ homolog was constructed by CFA in an SEM framework and divides the cognitive battery’s shared variance (ie, Spearman’s g) into δ (ie, the portion of variance related to functional status, as estimated by CDR-SB) and g′ (ie, the variance unrelated to functional status and the dementing process). Similar δ homologs have been validated in multiple cohorts and validated as predictors of diagnostic outcomes in TARCC (13,18). This particular δ homolog has previously been associated with IL-15 in TARCC (20). The observed variables were fit to linear measurement models and adjusted for age, gender, education, ethnicity, and depression. Measurement errors were assumed uncorrelated, and latent variable means and variances were fixed allowing loadings to be freely estimated. Residual error for cognitive performance indicators and CDR-SB were unconstrained and co-variances were estimated. Serum APN levels were adjusted for batch effects as previously described (19,20).
Next, the model was used to apply δ and g′ as potential independent latent variable mediators of the direct associations between the serum biomarker APN and cognitive performance measures with the final model representing a set of nested MIMIC models (22,32). All indicator variables were adjusted for age, gender, education, and ethnicity, and biomarkers were further adjusted batch effects. Analysis of Moment Structures software (AMOS) was used to perform CFA and MIMIC analyses (33). The validity of the structural models was assessed by simultaneously considering chi-square, chi-square to degree of freedom ratio (CMIN/DF), comparative fit index (CFI), and root mean square error of approximation (RMSEA). Data is consistent with the model when chi-square is nonsignificant and CMIN/DF ration <5 (34). CFI, with values 0–1, compares the model with a null model, and values of 0.95 or greater indicate adequate or excellent fit (35). RMSEA evaluates closeness of fit to the data, and models below 0.05 are considered “good fit” and up to 0.08 are “acceptable” (36).
The models were constructed in randomly selected 50% splits of TARCC’s data (Group 1 N = 1,691; Group 2 N = 1,690). The Group 1 model was replicated in Group 2 by comparing the CHI SQ fit in unconstrained versus (cross-group) constrained versions of the model.
This method of analysis through MIMIC modeling of APN’s influences on cognitive and functional measures of dementia through latent variables δ and g′ allows for the assessment of APN as a biomarker for AD. The hypothesis was that serum APN levels could predict δ as well as performance on individual cognitive tests. Given APN’s inverse correlation with known risk factors for dementia including BMI, insulin resistance, type 2 diabetes mellitus, and cardiovascular disease (1,2) and its anti-inflammatory actions (3), it was hypothesized that APN levels would be inversely related to δ and poorer performance on cognitive tests.
Results
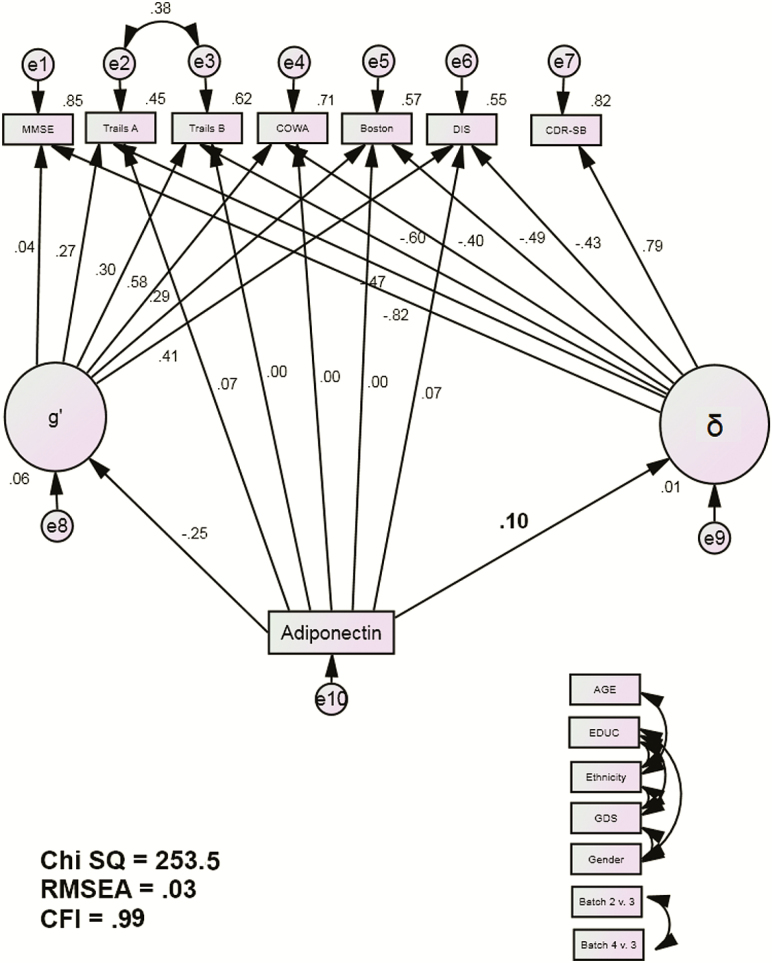
The demographic characteristics of TARCC’s sample are presented in Table 1. The latent variables δ and g′, which are orthogonal to each other and together represent the latent shared variance across observed cognitive performance variables (Spearman’s g), were again replicated by CFA in this larger TARCC sample (13). δ was significantly negatively associated with performance on each of its cognitive indicators (all p < .001) and was strongly related to CDR-SB (r = .79, p < .001) (Figure 1).
Table 1.
Descriptive Statistics
| Variable | N | Mean (SD) |
|---|---|---|
| Age (observed) | 3,381 | 70.88 (9.48) |
| Boston (observed) | 3,228 | 7.88 (4.26) |
| CDR (sum of boxes) | 3,306 | 2.42 (3.35) |
| COWA | 3,381 | 8.41 (3.49) |
| DIS | 3,381 | 8.89 (3.01) |
| EDUC (observed) | 3,381 | 13.24 (4.25) |
| Ethnicity (1 = MA, n = 1,189) | 3,381 | 0.36 (0.47) |
| GDS30 (observed) | 3,005 | 5.60 (5.25) |
| Gender (♂ = 1, n = 1,281) | 3,312 | 0.39 (0.49) |
| IADL (summed) | 3,381 | 10.48 (4.52) |
| MMSE (observed) | 3,311 | 25.52 (4.76) |
| Trails A (observed) | 3,106 | 8.25 (3.74) |
| Trails B (observed) | 2,807 | 7.95 (3.82) |
| Complete cases | 2,861 |
Note: CDR = Clinical Dementia Rating scale; COWA = Controlled Oral Word Association Test; DIS = Digit Span Test; GDS = Geriatric Depression Scale (24); IADL = Instrumental Activities of Daily Living; MMSE = Mini-mental State Exam (25); SD = standard deviation; Trails A = Trail-Making Test part A; Trails B = Trail-Making Test part B.
Figure 1.
APN MIMIC Model. Abbreviations: BOSTON, Boston Naming Test; CDR-SB, Clinical Dementia Rating Sum of Boxes; COWA, Controlled Oral Word Association Test; DIS, Digit Span Test; MMSE, Mini-Mental Status Examination; Trails A, Trail-Making Test A; Trails B, Trail-Making Test B. Observed indicators are adjusted for age, education, ethnicity, Geriatric Depression Scale (GDS) scores, gender, and batch effects (paths not shown for clarity).
APN was then added into the model as a predictor of g′, δ, and the cognitive performance measures (Figure 1). The fit indices were evaluated. The chi-square test (χ2 = 253.4 (52), p < .001) rejects the model. However, that statistical measure is sensitive to large sample sizes. Alternative fit indices which are not sensitive to sample size (CMIN /DF = 4.88; CFI = 0.99; RMSEA = 0.03) showed that the model fit the data well.
APN was significantly related to δ scores (r = .10, p = .015). APN was not significantly related to g′ (r = −.25, p = .66), nor did it have and independent direct effects on cognitive performance. These results were replicated across random subsets (ΔCHISQ = 2.8(7), p > .90). Since only δ’s variance is related to functional status, APN’s effect on cognition is likely to be disabling.
Discussion
Previous studies have had conflicting results regarding the relationship between APN and cognitive function. We found APN to be directly related to dementia exclusively through the latent variable δ. The association was positive (adverse). This finding is consistent with some previous studies in which higher APN serum levels were significantly related to cognitive impairment (5,8,9). This was, however, contrary to the original hypothesis that APN levels would be inversely correlated to δ which was based on the inverse relationship between APN levels and known risk factors for dementia (BMI, insulin resistance, type 2 diabetes mellitus, and cardiovascular disease) (1,2) as well as APN’s anti-inflammatory properties (3). This would suggest that APN may have effects on cognition independent of these properties and dementia risk factors.
There are several theories connecting higher levels of APN with increased risk of AD. Waragai et al., have suggested a “gain of function” and “loss of function” hypothesis in which APN is sequestered by tau causing neurotoxic aggregation as well as APN misfolding impairing its intended neuroprotective and neurotrophic activities (37). Others have theorized that elevated APN levels represented and contributed to the weight loss which occurs in patients with dementia (5,8). In the Mayo Clinic Study of Aging, a gender-specific finding among elders without dementia showed that women with higher APN levels had smaller hippocampal volume (9). APN has receptors in the brain including in the nucleus basalis of Meynert and hippocampus (4) which are known areas of involvement in AD. We have previously shown APN to partially mediate age’s specific effect on δ (38). However, because the current model is age-adjusted, APN must mediate one or more additional age-independent dementing process(es), possibly AD.
Another notable feature of our analysis is that the MIMIC model suggests that APN’s effects on observed cognitive performance are mediated exclusively through δ and neither by g′ nor via direct effects. This constrains serum APN’s impact to an effect on intelligence. The association(s), if any, between APN in other tissues or biofluids (eg, brain or cerebrospinal fluid [CSF]) are not addressed by this analysis and remain unconstrained in this regard.
Regardless, g and δ are indifferent to their indicators and are unlikely to be impacted by local CNS dysfunctions. Instead, δ appears to be agnostic to dementia’s etiology, while domain-specific cognitive performance appears to be disease-specific (39). APN’s unique association with g supports that it may contribute to all-cause dementia risk, rather than an AD-specific one. This finding is consistent with the prospective results from the Framingham Heart Study in which APN level was an independent risk factor for development of AD as well as all-cause dementia in women (5).
While the association between APN and δ is weak, it may yet be meaningful for several reasons. By definition, the latent variable δ represents the functionally-salient and therefore dementia-relevant cognitive variance. Any biomarker associated with δ, even by a weak relationship, is potentially clinically meaningful because it is inherently related to disability and therefore dementia. Biomarkers likely have multiple functions, and similarly, dementia severity as measured by δ is likely to reflect multiple independent δ-related processes mediated through unique sets of biomarkers. Therefore, knowledge of δ-related biomarkers may provide clues as to a dementia’s etiology and/or its mechanism(s) which could provide further information on potential interventions. It may seem counterintuitive that the correlation between APN and δ (the weaker correlation) is significant while the correlation between APN and g′ (the stronger correlation) is not. This is because the variance of the two variables is different, and the weaker correlation’s sample had less error. This magnitude is similar to other biomarkers we have studied (20).
There were certain limitations of our study including our adjustment for gender and ethnicity. This may have affected our findings as δ’s serum protein biomarkers in TARCC appear to be ethnicity-specific (40). Similarly, the Framingham Heart and Mayo Clinic studies found serum APN to predict AD and all-cause dementia in women only (5,9), and our analysis was adjusted for gender. An additional limitation to our analysis is that CSF samples are not available in TARCC. While some studies have shown that APN, at certain molecular weights, is able to cross the blood–brain barrier, there has been debate as to whether APN has a separate intrathecal synthesis which could cause an inconsistency between serum and CSF levels (8). Thus, our findings do not preclude an effect of CNS APN expression on dementia. This study also does not differentiate between different isoforms of APN.
The MIMIC model offers an advantageous approach to biomarker associations because it clarifies the potentially complex paths by which a biomarker might be associated with cognitive test performance. Our analysis is additionally advantaged by TARCC’s large sample size and the potential for longitudinal follow-up. Future studies can assess APN’s possible role in determining the rate of change in cognitive performance. δ’s rate of change is strongly associated with concurrent change in dementia severity as measured by CDR (15,18), independently of δ’s intercept, while the intercept and slope of g′ make very little independent contribution (15).
In conclusion, this study applied CFA and the MIMIC model in SEM to clarify the potential paths by which APN, a serum protein biomarker, can be associated with cognitive performance. We found APN to be directly related to δ through a statistically weak relationship. This adds to a growing literature of analyses linking serum proteins with cognitive performance. More research is needed to clarify the particular mechanism(s) by which APN and other serum proteins produce their effects on cognition.
Funding
This work was supported by the Julia and Van Buren Parr endowment for the study of Alzheimer’s Disease and by a generous gift of Mr. Charles Butt in the memory of Mrs. Littlie Littrell. This project was also supported in part by funding provided to the Texas Alzheimer’s Research and Care Consortium by the Darrell K Royal Texas Alzheimer’s Initiative, directed by the Texas Council on Alzheimer’s Disease and Related Disorders. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
Investigators from the Texas Alzheimer’s Research and Care Consortium: Baylor College of Medicine: Valory Pavlik PhD, Paul Massman PhD, Eveleen Darby MA/MS, Monica Rodriguear MA, Aisha Khaleeq Ansari MD; Texas Tech University Health Sciences Center: John C. DeToledo MD, Hemachandra Reddy PhD, Henrick Wilms MD, PhD, Kim Johnson PhD, Victoria Perez; University of North Texas Health Science Center: Thomas Fairchild PhD, Janice Knebl DO, Sid E. O’Bryant PhD, James R. Hall PhD, Leigh Johnson PhD, Robert C. Barber PhD, Douglas Mains DrPH, Lisa Alvarez; University of Texas Southwestern Medical Center: Munro Cullum PhD, Roger Rosenberg MD, Benjamin Williams MD, PhD, Mary Quiceno MD, Joan Reisch PhD, Linda S. Hynan PhD, Ryan Huebinger PhD, Janet Smith, Trung Nguyen MD, PhD; University of Texas Health Science Center – San Antonio: Donald Royall MD, Raymond Palmer PhD, Marsha Polk; Texas A&M University Health Science Center: Alan Stevens PhD, Marcia Ory PhD/MPH; University of Texas at Austin/Dell Medical School: David Paydarfar MD, John Bertelson MD, Martin Woon PhD, Gayle Ayres DO; Alyssa Aguirre LCSW; University of North Carolina: Kirk C. Wilhelmsen MD, PhD, Jeffrey L. Tilson PhD.
Contributor Information
Kimberly S K Benavente, Department of Psychiatry, UT Health San Antonio, San Antonio, Texas.
Raymond F Palmer, Family and Community Medicine, UT Health San Antonio, San Antonio, Texas.
Donald R Royall, Department of Psychiatry, UT Health San Antonio, San Antonio, Texas; Family and Community Medicine, UT Health San Antonio, San Antonio, Texas; Audie L. Murphy Division GRECC, The South Texas Veterans’ Health System, San Antonio, Texas.
Conflict of Interest
None reported.
References
- 1. Gustafson DR. Adiposity hormones and dementia. J Neurol Sci. 2010;299:30–34. doi: 10.1016/j.jns.2010.08.036 [DOI] [PubMed] [Google Scholar]
- 2. Kiliaan AJ, Arnoldussen IA, Gustafson DR. Adipokines: a link between obesity and dementia? Lancet Neurol. 2014;13:913–923. doi: 10.1016/S1474-4422(14)70085-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brochu-Gaudreau K, Rehfeldt C, Blouin R, Bordignon V, Murphy BD, Palin MF. Adiponectin action from head to toe. Endocrine. 2010;37:11–32. doi: 10.1007/s12020-009-9278-8 [DOI] [PubMed] [Google Scholar]
- 4.Letra L, Rodrigues T, Matafome P, Santana I, Seica R. Adiponectin and sporadic Alzheimer’s disease: clinical and molecular links. Front Neuroendocrinol. 2019;52:1–11. doi:10.1016/j.yfrne.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 5. van Himbergen TM, Beiser AS, Ai M, et al. Biomarkers for insulin resistance and inflammation and the risk for all-cause dementia and Alzheimer disease: results from the Framingham Heart Study. Arch Neurol. 2012;69:594–600. doi: 10.1001/archneurol.2011.670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teixeira AL, Diniz BS, Campos AC, et al. Decreased levels of circulating adiponectin in mild cognitive impairment and Alzheimer’s disease. Neuromolecular Med. 2013;15:115–121. doi: 10.1007/s12017-012-8201-2 [DOI] [PubMed] [Google Scholar]
- 7. Gorska-Ciebiada M, Saryusz-Wolska M, Borkowska A, Ciebiada M, Loba J. Adiponectin, leptin and IL-1 β in elderly diabetic patients with mild cognitive impairment. Metab Brain Dis. 2016;31:257–266. doi: 10.1007/s11011-015-9739-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Une K, Takei YA, Tomita N, et al. Adiponectin in plasma and cerebrospinal fluid in MCI and Alzheimer’s disease. Eur J Neurol. 2011;18:1006–1009. doi: 10.1111/j.1468-1331.2010.03194.x [DOI] [PubMed] [Google Scholar]
- 9. Wennberg AM, Gustafson D, Hagen CE, et al. Serum adiponectin levels, neuroimaging, and cognition in the Mayo Clinic Study of Aging. J Alzheimers Dis. 2016;53:573–581. doi: 10.3233/JAD-151201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bigalke B, Schreitmüller B, Sopova K, et al. Adipocytokines and CD34 progenitor cells in Alzheimer’s disease. PLoS One. 2011;6:e20286. doi: 10.1371/journal.pone.0020286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Warren MW, Linda SH, Weiner MF; Texas Alzheimer’s Research Care Consortium . Lipids and Adipokines as risk factors for Alzheimer’s disease. J Alzheimers Dis. 2012;29:151–157. doi:10.3233/JAD-2012-111385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140:566–572. [DOI] [PubMed] [Google Scholar]
- 13. Royall DR, Palmer RF, O’Bryant SE; Texas Alzheimer’s Research and Care Consortium . Validation of a latent variable representing the dementing process. J Alzheimers Dis. 2012;30:639–649. doi: 10.3233/JAD-2012-120055 [DOI] [PubMed] [Google Scholar]
- 14. Royall DR, Palmer RF, Vidoni ED, Honea RA, Burns JM. The default mode network and related right hemisphere structures may be the key substrates of dementia. J Alzheimers Dis. 2012;32:467–478. doi:10.3233/JAD-2012-120424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Palmer RF, Royall DR. Future dementia severity is almost entirely explained by the latent variable δ’s intercept and slope. J Alzheimers Dis. 2016;49:521–529. doi: 10.3233/JAD-150254 [DOI] [PubMed] [Google Scholar]
- 16. Koppara A, Wolfsgruber S, Kleineidam L, et al. The latent dementia phenotype δ is associated with cerebrospinal fluid biomarkers of Alzheimer’s disease and predicts conversion to dementia in subjects with mild cognitive impairment. J Alzheimers Dis. 2016;49:547–560. doi: 10.3233/JAD-150257 [DOI] [PubMed] [Google Scholar]
- 17. Spearman C. The Abilities of Man: Their Nature and Measurement. London, UK: Macmillan & Co; 1932. [Google Scholar]
- 18. Gavett BE, Vudy V, Jeffrey M, John SE, Gurnani A, Adams J. The δ latent dementia phenotype in the NACC UDS: cross-validation and extension. Neuropsychology. 2015;29:344–352. doi:10.1037/neu0000128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bishnoi RJ, Palmer RF, Royall DR. Vitamin D binding protein as a serum biomarker of Alzheimer’s disease. J Alzheimers Dis. 2015;43:37–45. doi: 10.3233/JAD-140042 [DOI] [PubMed] [Google Scholar]
- 20. Bishnoi RJ, Palmer RF, Royall DR. Serum interleukin (IL)-15 as a biomarker of Alzheimer’s disease. PLoS One. 2015;10:e0117282. doi: 10.1371/journal.pone.0117282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Royall DR, Bishnoi RJ, Palmer RF. Serum IGF-BP2 strongly moderates age’s effect on cognition: a MIMIC analysis. Neurobiol Aging. 2015;36:2232–2240. doi: 10.1016/j.neurobiolaging.2015.04.003 [DOI] [PubMed] [Google Scholar]
- 22. Proitsi P, Hamilton G, Tsolaki M, et al. A multiple indicators multiple causes (MIMIC) model of behavioural and psychological symptoms in dementia (BPSD). Neurobiol Aging. 2011;32:434–442. doi: 10.1016/j.neurobiolaging.2009.03.005 [DOI] [PubMed] [Google Scholar]
- 23. O’Bryant SE, Waring SC, Cullum CM, et al. ; Texas Alzheimer’s Research Consortium. Staging dementia using clinical dementia rating scale sum of boxes scores: a Texas Alzheimer’s research consortium study. Arch Neurol. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. Clin Gerontologist. 1986;5:165–173. [Google Scholar]
- 25. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 26. Benton A, Hamsher K. Multilingual Aphasia Examination. Iowa City, IA: AJA Associates; 1989. [Google Scholar]
- 27. Wechsler D. Wechsler Memory Scale. 3rd ed. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 28. Kaplan EF, Goodglass H, Weintraub S. The Boston Naming Test. 2nd ed. Philadelphia, PA: Lea & Febiger; 1983. [Google Scholar]
- 29. Reitan RM. Validity of the Trail Making test as an indicator of organic brain damage. Percept Mot Skills. 1958;8:271–276. [Google Scholar]
- 30. Waring S, O’Bryant SE, Reisch JS, Diaz-Arrastia R, Knebl J, Doody R; for the Texas Alzheimer’s Research Consortium . The Texas Alzheimer’s Research Consortium longitudinal research cohort: study design and baseline characteristics. Texas Pub Health J. 2008;60:9–13. [Google Scholar]
- 31. Graham JW. Missing data analysis: making it work in the real world. Annu Rev Psychol. 2009;60:549–576. doi: 10.1146/annurev.psych.58.110405.085530 [DOI] [PubMed] [Google Scholar]
- 32. Muthén BO. Some uses of structural equation modeling in validity studies: extending IRT to external variables. In: Wainer H, Braun HI, eds. Test Validity. Hillsdale, NJ: Lawrence Erlbaum; 1988:213–238. [Google Scholar]
- 33. Arbuckle JL. Analysis of Moment Structures-AMOS (Version 7.0) [Computer Program]. Chicago, IL: SPSS; 2006. [Google Scholar]
- 34. Bollen KA, Long JS. Testing Structural Equation Models. Thousand Oaks, CA: Sage Publications; 1993. [Google Scholar]
- 35. Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–246. [DOI] [PubMed] [Google Scholar]
- 36. Browne M, Cudeck R. Alternative ways of assessing model fit. In: Bollen KA, Long JS, eds. Testing Structural Equation Models. Thousand Oaks, CA: Sage Publications; 1993:136–162. [Google Scholar]
- 37. Waragai M, Ho G, Takamatsu Y, et al. Importance of adiponectin activity in the pathogenesis of Alzheimer’s disease. Ann Clin Transl Neurol. 2017;4:591–600. doi: 10.1002/acn3.436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Royall DR, Al-Rubaye S, Bishnoi R, Palmer RF. Serum protein mediators of dementia and aging proper. Aging (Albany NY). 2016;8:3241–3254. doi: 10.18632/aging.101091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. John SE, Gurnani AS, Bussell C, Saurman JL, Griffin JW, Gavett BE. The effectiveness and unique contribution of neuropsychological tests and the δ latent phenotype in the differential diagnosis of dementia in the uniform data set. Neuropsychology. 2016;30:946–960. doi: 10.1037/neu0000315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Royall DR, Palmer RF; Texas Alzheimer’s Research and Care Consortium . Does ethnicity moderate dementia’s biomarkers? Neurobiol Aging. 2014;35:336–344. doi: 10.1016/j.neurobiolaging.2013.08.006 [DOI] [PubMed] [Google Scholar]