Abstract
Macronutrient intake impacts physiology, behavior, and gene expression in a wide range of organisms. We used the response surface methodology to compare how life history traits, lifespan, and reproduction differ as a function of protein and carbohydrate intakes under choice and no-choice feeding regimens in the fruit fly, Drosophila melanogaster. We found that when offered a choice of nutritionally complementary foods mated female flies regulated toward a protein to carbohydrate ratio (P:C) that was associated with shortened lifespan and maximal egg production when compared to response surfaces derived from flies fed 1 of a range of fixed diets differing in P:C (no-choice regimen). This difference in lifespan between choice and no-choice feeding was not seen in males or virgin flies, reflecting the fact that increased protein intake is triggered by mating to support egg production. However, whereas in mated females a higher P:C intake was associated with greater egg production under both choice and no-choice feeding, contrary to expectations, choice-fed mated flies laid fewer eggs than no-choice flies on equivalent macronutrient intakes, perhaps reflecting that they had to ingest twice the volume of food to attain an equivalent intake of nutrients than no-choice flies on a diet of equivalent P:C ratio.
Keywords: Dietary choice, Drosophila, Fecundity, Lifespan, Life-history trade-offs
Graphical Abstract
Graphical Abstract.
During recent decades there has been a focus on how animals make food-related decisions to satisfy their nutritional needs (1,2). Nutritional requirements are not static, they change over development and with a range of biotic and abiotic environmental circumstances (3). Additionally, different life history traits have different nutritional requirements, setting up functional trade-offs between competing demands. Among the best-studied examples of a functional trade-off between traits is between reproduction and lifespan, as explored experimentally in model systems including the fruit fly, Drosophila melanogaster (4,5).
A fundamental question is when given a choice of foods, which nutritional outcomes are prioritized by animals? The Geometric Framework for Nutrition (6) contributed significantly to our understanding of how organisms across many taxa possess nutrient-specific appetites, make food decisions, and control food intake in response to nutritional requirements. The Geometric Framework is based on the use of state space models comprising multiple nutrient dimensions. For example, Lee et al. (4) mapped how lifespan and egg production were influenced by the protein (P) and carbohydrate (C) content of the diet by confining female flies to 1 of 28 diets differing systematically in protein, carbohydrate, and total energy content. Flies lived longest on a diet containing a lower P:C than that which supported maximal lifetime egg production. When flies were offered a choice between nutritionally complementary diets they converged upon an intake of P and C that supported lifetime egg production, at the cost of longevity.
Here we have explored this question further, including studying whether equivalent nutrient intakes lead to the same lifespan and fecundity outcomes under both choice and no-choice feeding. To influence nutrient intake, we used choice and no-choice feeding assays. To study the influence of genetic background, sex, mating, and food perception on the outcome, we used males, virgin and mated females from several strains of D. melanogaster, including smell and taste mutants. Flies were provided with 1 of 9, no-choice diets containing a mixture of protein and carbohydrate, or else 1 of 9 choice food pairings that offered protein and carbohydrate separately. No-choice-fed flies could adjust the volume of food consumed but were constrained to ingest the fixed ratio of protein and carbohydrate provided in the diet. By contrast, flies offered a choice of foods were free to mix a dietary intake of protein and carbohydrate within the region in protein and carbohydrate intake space defined by the protein-to-carbohydrate (P:C) ratio in the 2 foods. They were, therefore, free both to select the balance of P:C ratio in their diet within that range and to adjust the volume eaten.
We fitted nutritional response surfaces mapped onto protein–carbohydrate intake arrays derived from the choice- and no-choice-fed flies, to see: (a) if flies provided with food choice consumed a combination of protein and carbohydrate intake that supported maximal lifespan, maximal egg production, or neither, and (b) whether lifespan and egg production for a particular intake of protein and carbohydrate differed under choice feeding versus no-choice conditions.
Materials and Methods
Insect Maintenance
Flies of wild-type Canton-S and Dahomey strains were used, as well as Gr5;Gr64 (7), and Orco (Or83b) (8) mutant strains. Stock flies were provided by the Bloomington Drosophila Stock Center (Indiana University, USA). Additional flies of the wild-type strain IF collected in Ivano-Frankivsk were used (9). Flies were cultured on a standard yeast–molasses medium (7.5% molasses, 5% yeast, 6% corn, 1% agar, and 0.18 nipagin as a mold inhibitor) at 25°C under a 12:12 light:dark cycle (10). Four-day-old flies were separated by sex and kept on standard medium for 24 hours to recover after CO2 anesthesia, then transferred at Day 5 into 1.5 L demographic cages at densities of 46–262 flies per cage (exact numbers are provided in Supplementary Table 1). Cages were supplemented with two 25-mL plastic vials filled with 5 mL of experimental medium.
Experimental Media
During the experiment, the flies were provided with either of 2 food vials, where 1 of the vials contained solidified yeast suspension and the other solidified sucrose solution (“choice feeding”), or a single food vial (“no-choice” feeding), where sucrose and yeast were mixed together at certain ratios. We used 9 diets with different concentrations of yeast and sucrose for the media with mixed or separated macronutrients. Those diets contained dry yeast (at concentrations of 3, 6, or 12%) as a source of protein, vitamins and micronutrients, sucrose (at concentrations of 3, 6, or 12%) as a source of carbohydrate, 1.2% of agar, and 0.18% of nipagin as a mold inhibitor (Supplementary Table 1). It is worth noting, that the term “diet” in our study represents the concentration of nutrients provided in the experimental media, rather than actual intakes of these nutrients.
Lifespan Assay
Experimental flies were maintained in 1.5 L demographic cages under the conditions, described above. The “choice” cages were supplied with 2 food vials, each with either solidified (with agar) yeast or sucrose. In turn, “no-choice” vials were supplied with a single food vial with yeast and sucrose mixed at the defined proportions. The food vials were changed every second day and dead flies were removed and counted (11). The lifespan study for mated Canton-S female flies was run in 2 independent replicates.
Fecundity Test
Twenty, 5-day old flies were placed in 1.5 L demographic cages, and the number of eggs laid was counted 24 hours after the food change. Measurements were conducted at intervals of 3 days over a 30-day period (12). Values were expressed as an average number of eggs per female per 24 hours over the indicated period (either 10 or 30 days). In fecundity preference tests, the number of eggs laid over a 15-day period was used. The number of eggs laid followed similar patterns for different diets on the 10th, 15th, and 30th day of observation.
Food Intake Estimation Assay
Food consumption was determined on Day 10 and Day 30 according to the modified assay of Skorupa et al. (13), using erioglaucine. Twenty flies were allowed to feed for 75 minutes during daytime on the corresponding experimental medium supplemented with 0.5% of the dye. After feeding, flies were frozen in liquid nitrogen for further analysis. Flies were homogenized in 50 mM phosphate buffer (pH 7.5) and centrifuged twice (16 000g, 10 minutes, 25°C). Aliquots of final supernatants were used for the measurement. The absorbance of samples was determined on a spectrophotometer Spekol-211 (Carl Zeiss, Germany) at wavelength 629 nm. The amount of food consumed by 20 flies during 75 minutes was used to estimate consumption rate per fly.
Data Analysis
Survival data were fitted by an accelerated failure time model using the survreg function of the R package survival. A nested model was used to account for cohort effects in different demographic cages. The resulting statistical tables were generated by Anova and implemented in the R package car (Companion to Applied Regression). We have used the survdiff function from the survival package to calculate restricted mean survival times and median lifespans and to compare mortality curves on different diets. Multiple comparisons were performed by log-rank test implemented in the R package survival with following adjustment of p values by the Benjamini–Hochberg procedure. A validating set of multiple comparisons for the fitted data was conducted using the R package emmeans (p values were generated by the Tukey method). Hazard ratios were calculated by the log-rank approach using data on the numbers of observed and expected events for each cohort.
Feeding and fecundity data were analyzed using a 2-way analysis of variance followed by Sidak’s test for multiple comparisons.
Response surfaces were fitted using generalized additive regression models, implemented in the R package mgcv, and visualized via thin-plate splines with tools provided by R package fields (14).
Results
Choice Nutrition Leads to a Reduction in Lifespan in Female Drosophila
We first tested whether food regimen (choice vs no-choice) influenced Drosophila lifespan across a range of diets that varied in their protein, carbohydrate, and caloric content. Dietary regimen and dietary composition affected the lifespan of Canton-S mated females (Figure 1, Table 1). Significant differences between choice and no-choice curves were observed in all comparisons (Figure 1). Our results indicated that the lifespan of Canton-S females on choice nutrition was shorter compared with no-choice nutrition under all experimental diets (Figure 1).
Figure 1.
The type (no-choice or choice) of nutrition and concentration of macronutrients in the food altered the lifespan of mated female Canton-S Drosophila. Each curve represents percentage of individuals alive as a function of age for about 300 flies (in total) of 2 independent mortality cages. The curves were compared using pairwise log-rank test.
Table 1.
The Effects of Diet Components and the Feeding Regimen (Choice vs No-choice) on the Survival of Mated Canton-S Females
| χ2 | Degrees of freedom | p Value | |
|---|---|---|---|
| Sucrose | 8.15 | 2 | .01696 |
| Yeast | 224.12 | 2 | <2 × 10–16 |
| Regimen | 681.70 | 1 | <2 × 10–16 |
| Sucrose × yeast | 7.66 | 4 | .10503 |
| Sucrose × regimen | 0.09 | 2 | .95825 |
| Yeast × regimen | 8.48 | 2 | .01441 |
| Sucrose × yeast × regimen | 8.43 | 4 | .07715 |
| Sucrose × yeast × Regimen × cage | 318.41 | 18 | <2 × 10–16 |
Notes: Non-censored survival data obtained from mortality curves were fitted by an accelerated failure time model, assuming logistic distribution for the failure time. Table shows statistics of likelihood-ratio tests for the model terms.
Survival depended to a smaller extent on the concentration of sucrose in the medium (χ2 = 8.15, p < .017) and, to a larger extent, on the concentration of yeast (χ2 = 837.63, p < .0001) in Canton-S mated females (Table 1). Restricted mean survival time was shorter by 7.0%–12.5% in females that consumed media with 12% yeast than in those who consumed media with 3% and 6% yeast at the no-choice regimen (Figure 2). We detected a similar lifespan shortening by high protein-to-carbohydrate ratios (achieved by high concentrations of yeast in the diet) in the Canton-S mated females that could choose between eating from either sucrose- or yeast-containing vials (Figure 2). Flies that were kept in the cages with vials, containing 12% yeast, had 7%–13% shorter restricted mean survival time than flies that could choose to eat from vials, containing either 3% or 6% of yeast (Figure 2).
Figure 2.
Restricted mean survival times for Canton-S mated females on the diets with different contents of sucrose and yeast at options to eat food with sucrose and yeast mixed in 1 food vial (A) or to choose between sucrose- and yeast-containing vials (B). Coinciding letters over the bars denote the absence of significant difference between the means.
Shorter Lifespan in Choice-Fed Flies Was Associated With Selection of a Higher Protein Intake
We found a higher protein intake and P:C ratio consumed by the flies on choice nutrition on the 10th day versus 30th day regardless of dietary composition (Figure 3B and D). Notably, there was evidence of flies converging upon a common intake (“intake target”) for protein and carbohydrate, especially on Day 10 (see Figure 3A compared with Figure 3B). The clustering of intakes had dispersed somewhat by Day 30 (Figure 3D). That choice-fed flies selected a higher protein intake than predicted from random food selection was further supported by data on the number of flies that visited the feeding tubes during the day, which indicated a preference for yeast medium by flies reared on choice nutrition (Supplementary Figure 1).
Figure 3.
Protein (x-axis) and carbohydrate (y-axis) intake of mated 10- and 30-day-old Canton-S no-choice and choice fed female flies: 10-day-old no-choice-fed (A) and choice-fed (B) flies, and 30-day-old no-choice (C) and choice-fed (D) flies. Intake is expressed as micrograms of a macronutrient per fly, per 24 h.
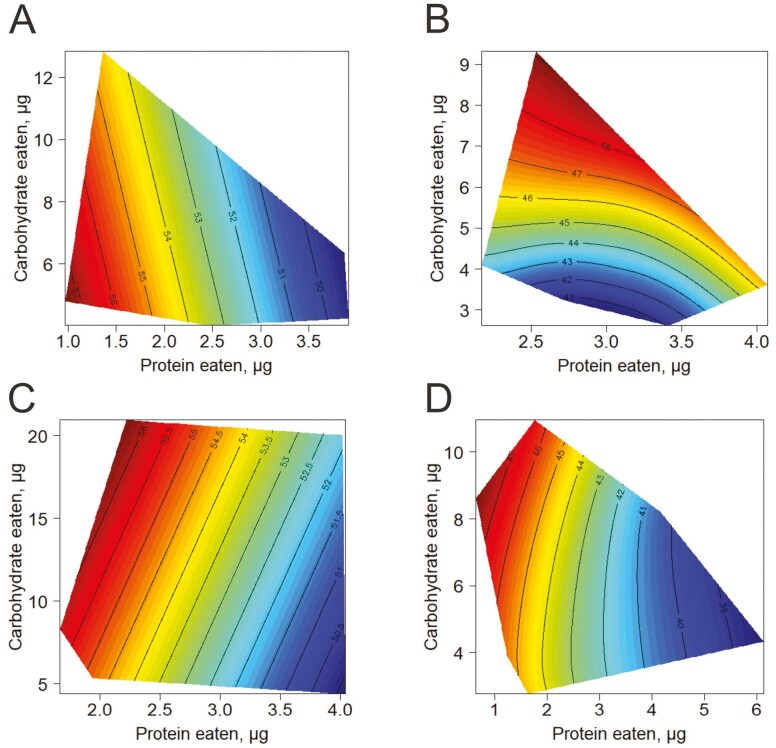
These patterns are visualized using nutritional geometry in Figure 4, showing a clear relationship between protein intake and longevity in both choice and no-choice flies. In summary, lifespan was shortened with higher protein intakes in both no-choice and choice-fed flies. This pattern was especially apparent when intake was measured on Day 30 (Figure 4C and D) and in no-choice flies when intake was measured on Day 10 (Figure 4A), whereas the median lifespan response surface for choice-fed flies fitted to Day-10 intakes indicated longer lifespans on higher carbohydrate intakes (Figure 4B).
Figure 4.
Response surfaces that show dependency of median lifespans for cohorts of Canton-S mated females on dietary carbohydrate and protein, when macronutrient intake was measured on the 10th or 30th day after eclosion: (A) and (B)—macronutrient intake was measured in no-choice and choice fed 10-day-old flies, respectively; (C) and (D)—macronutrient intake was measured in no-choice and choice fed 30-day-old flies, respectively. Lifespan data shown by terrain colors and contour lines were overlaid on the 2D scatter of the intake values measured on the day indicated.
That choice-fed flies had shorter lifespans than no-choice flies overall is, therefore, consistent with increased protein intakes in flies allowed to choose between yeast- and sugar-containing solid media.
The Amount of Food Consumed Depended on the Type of Nutrition
Dietary choice affected overall feeding rate, nutrient consumption and total calorie intake (Supplementary Figures 2–6). Dietary choice was associated with overall increased volume eaten relative to no-choice feeding, most notably over 10 days of the experiment when diets contained 3% or 6% of yeast (Supplementary Figure 2), whereas food consumption was not affected by the type of nutrition when the diets contained 12% yeast. Choice feeding was also associated with lower calorie intake when diets contained 6% or 12% of sucrose (Supplementary Figure 3). Together, these results reflect the need for choice-fed flies to ingest twice the total volume of food to achieve a similar nutrient intake to no-choice-fed flies.
Higher Protein Intakes Supported Higher Egg Numbers
The response surfaces for egg production differed significantly from the surfaces for lifespan (Figure 5A–D). The rate of egg production was greatest at a higher protein intake and lower carbohydrate intake under both choice and non-choice nutrition. However, the number of eggs laid was higher for flies on no-choice diets than under choice feeding conditions (F1,306 = 37.9, p < .0001; Supplementary Figure 7A). Choice of egg-deposition site was dependent on the diet (F8,306 = 11.87, p < .0001). When able to choose between a sucrose medium and a yeast medium, flies preferred to lay eggs on the sucrose medium when the alternative site contained 3% or 6% yeast (Supplementary Figure 7B). However, on the 3S vs 12Y choice, flies laid 2.4-fold more eggs on the yeast medium than on the sucrose medium (Supplementary Figure 7B; Sidak’s test: p = .0024). A similar tendency was observed in females with a choice between 12S and 6Y medium (Supplementary Figure 7B; Sidak’s test: p = .0009).
Figure 5.
Response surfaces that show dependency of fecundity (number of eggs laid by 1 female per 24 hours) for cohorts of Canton-S mated females on dietary carbohydrate and protein, when macronutrient intake was measured on the 10th or 30th day after eclosion: (A) and (B) —macronutrient intake was measured in no-choice and choice fed 10-day-old flies, respectively; (C) and (D)—macronutrient intake was measured in no-choice and choice fed 30-day-old flies, respectively. Fecundity data shown by terrain colors and contour lines were overlaid on the 2D scatter of the intake values measured on the day indicated. Open and closed dots show number of eggs laid at the ratios 2.5:8 and 3.5:5 between proteins and carbohydrates.
Gender-, Genotype- and Mating Status-Specific Effects of Food Choice on Lifespan
Mating causes many changes in physiology, behavior, and gene expression. Therefore, we determined how mating status modulated the effects of dietary choice on female lifespan. To do this, we employed a 2-choice assay offering foods of the same caloric concentration to virgin Canton-S females. We did not observe a general trend in the lifespan response to feeding regimen for virgin females regardless of the diet consumed, despite there being differences between flies on no-choice and choice regimens on certain diets (Figure 6B; Supplementary Figure 8). Together these results indicated that the effects of dietary choice on female lifespan depend on mating status. It is interesting that virgin Canton-S females also lost a strict dependence of survival on dietary yeast content, especially in the no-choice feeding regimen (Supplementary Figure 9). Effects of dietary yeast and sucrose contents were still detected by the regression analysis (Supplementary Table 2). However, interactions between feeding regimens and the effects of either dietary sucrose or yeast showed no significance (Supplementary Table 2).
Figure 6.
Hazard ratios ± corresponding 95% confidence intervals for the association of mortality with feeding regimen (no-choice vs choice) in mated (A) and virgin (B) Canton-S females, Canton-S males (C), mated IF females (D), mated Dahomey females (E), mated females of Orco (F) and Gr5a;Gr64a (G) gustatory mutants on the diets with different ratios of dietary sucrose and yeast. The ratios that are greater than 1 mean increased risk of a fruit fly to die at the option to choose between sucrose- and yeast-containing vials.
We also determined the effects of fly sex. Feeding regimen (choice or no-choice) weakly influenced lifespans of Canton-S males (Figure 6C; Supplementary Figure 10). In particular, the high-protein no-choice diets 3S:12Y and 12S:12Y shortened male lifespan (Figure 6C). The no-choice regimen favored longer lifespans in males reared on 6S-6Y diet, but there were no other significant differences between no-choice and choice survival curves of Canton-S males. Like the situation with virgin females, the interactions between the effects of dietary components and feeding regimen were not statistically significant (Supplementary Table 3 and Supplementary Figure 11). The strength of interaction between P:C and feeding regimen was greater in males than in the virgin females, whereas the effect of sucrose on the survival of virgin females was greater (χ2 = 23.49, p < 0.0001) than in males (χ2 = 11.71, p < 0.01). In turn, yeast content affected males’ lifespan to a greater extent than that of virgin females.
To test differences among genotypes of fly, we used wild-type and outbred populations of D. melanogaster. In all comparisons from all diets studied, survival of laboratory wild-type mated females of line IF (9) that were allowed to choose between dietary macronutrients was consistently and significantly shortened as compared with counterparts on the no-choice regimen (Figure 6D; Supplementary Figure 12). Survival of this group of flies was dependent on the content of dietary components, feeding regimen and interactions between them (Supplementary Table 4 and Supplementary Figure 13). Similar effects were observed for outbred wild-type Dahomey mated females that all exhibited lifespan shortening under the choice regimen as compared with no-choice (Figure 6E; Supplementary Figures 14 and 15). Mated females of olfactory mutant strain Orco (8) had lower a risk of dying on 3S:3Y, 6S:6Y, and 12S:12Y diets under the choice regimen as compared with counterparts reared on the same diets under the no-choice regimen. However, Orco flies on 12S:3Y choice diet exhibited larger hazard ratios as compared with equivalents on the same no-choice diet (Figure 6F; Supplementary Figures 16 and 17).
Finally, we tested the effects of dietary choice on the strain with mutations in genes Gr5a and Gr64a (7). These flies do not perceive most of sugars. The option of dietary choice led to a decreased hazard ratio (ie, reduced risk of dying) in mated females of the gustatory mutant fed on 3S:3Y and 12S:12Y diets (Figure 6G; Supplementary Figures 18 and 19). However, dietary choice promoted extended lifespan in the gustatory mutants fed on 12S:3Y diet. Hence, smell- and taste-impaired mutant females responded differently to diet composition; this indicates that dietary-dependent changes in lifespan involve fly olfaction and gustation.
Discussion
Animals integrate responses to foods and food cues with their internal physiological state when making feeding decisions (1,2). We have recently reported effects of dietary choice on Drosophila metabolism that are predicted to be dependent on both the quantity and quality of the chosen diet (15). Here, we have explored how the dietary regimen affects lifespan, feeding behavior, and reproduction across diets with different contents of carbohydrate and yeast.
Lyu et al. have shown that having the choice between yeast- and sucrose-containing media fly changed behavior and metabolism and shortened lifespan, independent of the amount of food consumed (16). Our current study provided similar findings. One possibility is that choice- and no-choice-fed flies responded differently to nutrients, however, we can see that this is not the case according to the surface plots in Figure 4. The surfaces look essentially the same—in both cases (choice and no-choice) lifespan shortening was associated primarily with increased yeast (protein) intake. This means that the difference between choice and no-choice nutrition is related to the fact that choice flies could select their intake of protein and carbohydrates within the bounds of the choice diets, whereas no-choice flies were confined to their fixed ratio of yeast-to-sugar and could only adjust total food intake.
Flies have the capacity to regulate their intake of both protein and carbohydrates separately via separate nutrient-specific appetites (2). As seen in Figure 3, when given a choice, flies preferred a higher protein intake than those flies confined to lower P:C mixed diets—which explains their shorter lifespan. Food choice allowed flies to regulate their P:C intake toward a much tighter region (intake target) than was possible for no-choice flies. This is because choice-fed flies could adjust both the volume eaten and the ratio of foods eaten, whereas no-choice flies could adjust volume eaten but not nutrient ratio, which was fixed. This was especially evident on Day 10, but less so at later ages—Day 30 (Supplementary Figure 2). Were choice-fed flies simply to have eaten the 2 foods provided indiscriminately and to the same total volume as on the equivalent no-choice diet, they would have ended up consuming the same P:C ratio as the equivalent no-choice diet but would have ingested only half as much protein and carbohydrate. This is because arriving at the same amount of protein and carbohydrate under choice and no-choice feeding requires choice-fed flies to eat twice the volume of food in total, that is, ingesting 3 μg of protein (P) and 3 μg of carbohydrates (C) requires eating 100 μL of 3P and 100 μL of 3C (ie, 200 μL in total) under choice conditions, as compared with 100 μL of a 3P:3C mixed food. Flies under dietary choice given high-sugar diets did not double intake and hence ingested fewer total calories from the diet as compared to the equivalent no-choice diet. Our study is in good agreement with previous studies demonstrating that calorie restriction under ad libitum feeding was not responsible for extending the lifespan of Drosophila (4,9,17). Rather, the balance between protein and carbohydrates plays a pivotal role in determining longevity (4,9), with amounts of consumed protein having a greater effect on Drosophila survival than carbohydrates (4,17).
Dietary choice causes a neuronal response that reshapes metabolic networks in peripheral tissues via serotonin receptor 5A (5-HT2A), which is widely expressed in the central nervous system (16). The metabolomics data of Lyu et al. revealed that a key component of TCA cycle α-ketoglutarate (αKG) is involved in the effect of dietary choice on lifespan (16). Indeed, metabolisms of amino acids, derived from protein digestion, and metabolism of ketoacids, derived from carbohydrate digestion, converge at the points of transamination. Transamination serves to transfer intermediates for the tricarboxylic acid cycle and reduced nicotinamide adenine dinucleotide into mitochondrial matrix. At the same time, αKG serves as a key substrate for transaminases.
Why did flies under choice nutrition select a diet composition higher in protein that shortened lifespan? The obvious answer would be to increase egg production (4,18,19). This is because egg production in Drosophila has been closely associated with increased consumption of nutrients (specifically, increased consumption of protein-rich sources) (20,21). Flies selected a higher protein intake under choice feeding conditions. However, our data showed that choice nutrition led to reduced egg production relative to no-choice-fed flies for a given macronutrient intake (Figure 5, Supplementary Figure 7). The patterns of response for longevity (Figure 4) and reproduction (Figure 5) were as expected given that choice-fed flies selected a higher P:C diet than if they had fed indiscriminately between the 2 foods provided. However, they showed lower reproductive output than expected. This could have been for several reasons, including showing incomplete compensation in terms of volume of diets consumed (see above), especially with age, and hence achieving lower absolute intakes of nutrients than no-choice flies on equivalent P:C ratios. Flies respond to nutrient stressors by reduction in fecundity rate through elevation of the steroid hormone ecdysone (22); perhaps food choice under our conditions was stressful. An additional reason may be that females also invest more consumed nutrients into somatic tissue for survival rather than into eggs (23,24). Coupling the consumption of certain foods to maintain optimal P:C balance might be costly to the flies. Moreover, long-term decision-making conditions are metabolically stressful for the flies (16). Thus, under the same P:C values it was previously observed an almost 2-fold reduction of TAG content in flies given a food choice (15). On the other hand, several reports showed that Drosophila are not necessarily maximizing either their own survival or survival of their progeny (25,26).
Choice nutrition had no impact on the lifespan of males and virgin females. It was previously shown that dietary choice influenced lifespan in both male and female laboratory flies and that 5-HT2A is required for this effect in both sexes (16). The nutritional requirements of female Drosophila change after mating. Mated females consume more protein and carbohydrate, and require higher P:C ratios than virgin females (27,28). Presumably, therefore, a lack of difference in lifespan between choice- and no-choice-fed male and virgin females reflect lower protein requirements in the absence of egg production, thereby selecting a lower protein intake than mated females, which of itself is associated with a shortened lifespan (29). The substantial difference between male and female Drosophila in gut physiology promotes sex differences in susceptibility to environmental factors including nutrition (30,31). The sex differences are significantly exacerbated by mating. Reproductive status affects lifespan, metabolism, and the antioxidant system in Drosophila (10). Virgin females have the longest lifespan, whereas polygamous males and females had the shortest lifespan (10). Feeding behavior and lifespan patterns are similar in virgin females and males (4). Virgin female flies display a different survival trajectory as compared to males or mated females. Consequently, sex-specific nutrient preference is modulated by mating and determines longevity in Drosophila.
We examined the change in lifespan pattern between choice and no-choice-fed flies across several genotypes. Our data show that food choice decreased lifespan in females of wild-type strains Drosophila including strains IF and Dahomey. We were unable to detect any significant correlations between the lifespan of females carrying a loss-of-function mutation in Orco or GR and diet, suggesting that Orco, Gr5a, and Gr64a genes, play a role in the effect of nutrients on lifespan. Moreover, the effects of different macronutrients varied among different strains (32). Flies of different genotypes differed in longevity response to diet, rendering it impossible to know a priori how a certain dietary composition would contribute to longevity. According to Libert and co-authors, these effects are mostly dependent on serotonin signaling (8), consequently, the perceived rather than actual composition of food ingested modulates aging. Our results also suggest that metabolic pathways of the fly are coupled to the olfactory and gustatory sensing genes as flies actively make dietary food choices based on their genotype.
Present results extend our understanding of how animals match their metabolic requirements to prevailing dietary circumstances to satisfy their physiological needs and prioritize different life history outcomes. Results highlight how expanding our understanding of metabolic and signaling interconnections will provide insight into the molecular and cellular processes that underlie aging in humans. Thus, additional research on the influences of the food decision-making process in humans could be beneficial for understanding the connection between uptake of specific macronutrients and longevity in a more naturalistic, choice-based food environment and will provide targets for healthy eating interventions.
Supplementary Material
Contributor Information
Olha Strilbytska, Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine.
Ihor Yurkevych, Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine.
Uliana Semaniuk, Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine.
Dmytro Gospodaryov, Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine.
Stephen J Simpson, Charles Perkins Centre, University of Sydney, Sydney, Australia.
Oleh Lushchak, Department of Biochemistry and Biotechnology, Vasyl Stefanyk Precarpathian National University, Ivano-Frankivsk, Ukraine; Research and Development University, Ivano-Frankivsk, Ukraine.
David G Le Couteur, (Biological Sciences Section).
Funding
U.S. and D.G. were supported by the National Research Foundation of Ukraine (grant number 2020.02/0118).
Conflict of Interest
None.
Author Contributions
O.L. and S.J.S. conceived and designed the research; O.S., I.Y. and U.S. conducted the experiments; O.S., O.L., D.G. and U.S. analyzed data; O.S. and O.L. prepared the original draft; S.J.S. and D.G. reviewed, and edited the manuscript. All authors read and approved the manuscript.
References
- 1. Simpson SJ, Raubenheimer D. The nature of nutrition: A unifying framework from animal adaptation to human obesity. Princeton University Press; 2012. [Google Scholar]
- 2. Münch D, Ezra-Nevo G, Francisco AP, Tastekin I, Ribeiro C. Nutrient homeostasis – translating internal states to behavior. Curr Opin Neurobiol. 2020;60:67–75. 10.1016/j.conb.2019.10.004 [DOI] [PubMed] [Google Scholar]
- 3. Raubenheimer D, Senior AM, Mirth C, et al. An integrative approach to dietary balance across the life course. iScience. 2022;25(5):104315. 10.1016/j.isci.2022.104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lee KP, Simpson SJ, Clissold FJ, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci USA. 2008;105(7):2498–2503. 10.1073/pnas.0710787105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tatar M, Post S, Yu K. Nutrient control of Drosophila longevity. Trends Endocrinol Metab. 2014;25(10):509–517. 10.1016/j.tem.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Raubenheimer D, Simpson SJ. Integrative models of nutrient balancing: Application to insects and vertebrates. Nutr Res Rev. 1997;10(1):151–179. 10.1079/NRR19970009 [DOI] [PubMed] [Google Scholar]
- 7. Dahanukar A, Lei YT, Kwon JY, Carlson JR. Two Gr genes underlie sugar reception in Drosophila. Neuron. 2007;56(3):503–516. 10.1016/j.neuron.2007.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Libert S, Zwiener J, Chu X, Vanvoorhies W, Roman G, Pletcher SD. Regulation of Drosophila life span by olfaction and food-derived odors. Science. 2007;315(5815):1133–1137. 10.1126/science.1136610 [DOI] [PubMed] [Google Scholar]
- 9. Lushchak O, Gospodaryov D, Rovenko B, et al. Balance between macronutrients affects life span and functional senescence in fruit fly Drosophila melanogaster. J Gerontol A Biol Sci Med Sci. 2012;67(2):118–125. 10.1093/gerona/glr184 [DOI] [PubMed] [Google Scholar]
- 10. Koliada A, Gavrilyuk K, Burdylyuk N, et al. Mating status affects Drosophila lifespan, metabolism and antioxidant system. Comp Biochem Physiol A. 2020;246:110716. 10.1016/j.cbpa.2020.110716 [DOI] [PubMed] [Google Scholar]
- 11. Strilbytska OM, Semaniuk UV, Storey KB, Yurkevych IS, Lushchak O. Insulin signaling in intestinal stem and progenitor cells as an important determinant of physiological and metabolic traits in Drosophila. Cells. 2020;9(4):803. 10.3390/cells9040803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Strilbytska OM, Zayachkivska A, Koliada A, et al. Anise Hyssop Agastache foeniculum increases lifespan, stress resistance, and metabolism by affecting free radical processes in Drosophila. Front Physiol. 2020;11:596729. 10.3389/fphys.2020.596729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Skorupa DA, Dervisefendic A, Zwiener J, Pletcher SD. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell. 2008;7(4):478–490. 10.1111/j.1474-9726.2008.00400.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Solon-Biet SM, McMahon AC, Ballard JW, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014;19(3):418–430. 10.1016/j.cmet.2014.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Strilbytska O, Semaniuk U, Bubalo V, Storey KB, Lushchak O. Dietary choice reshapes metabolism in Drosophila by affecting consumption of macronutrients. Biomolecules. 2022;12(9):1201. 10.3390/biom12091201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lyu Y, Weaver KJ, Shaukat HA, et al. Drosophila serotonin 2A receptor signaling coordinates central metabolic processes to modulate aging in response to nutrient choice. Elife. 2021;10:e59399. 10.7554/eLife.59399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol. 2005;3(7):e223. 10.1371/journal.pbio.0030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Piper MD, Blanc E, Leitão-Gonçalves R, et al. A holidic medium for Drosophila melanogaster. Nat Methods. 2014;11(1):100–105. 10.1038/nmeth.2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ribeiro C, Dickson BJ. Sex peptide receptor and neuronal TOR/S6K signaling modulate nutrient balancing in Drosophila. Curr Biol. 2010;20(11):1000–1005. 10.1016/j.cub.2010.03.061 [DOI] [PubMed] [Google Scholar]
- 20. Bradley TJ, Simmons FH. An analysis of resource allocation in response to dietary yeast in Drosophila melanogaster. J Insect Physiol. 1997;43(8):779–788. 10.1016/s0022-1910(97)00037-1 [DOI] [PubMed] [Google Scholar]
- 21. Terashima J, Bownes M. Translating available food into the number of eggs laid by Drosophila melanogaster. Genetics. 2004;167(4):1711–1719. 10.1534/genetics.103.024323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chakraborty TS, Gendron CM, Lyu Y, et al. Sensory perception of dead conspecifics induces aversive cues and modulates lifespan through serotonin in Drosophila. Nat Commun. 2019;10(1):2365. 10.1038/s41467-019-10285-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Meiselman MR, Kingan TG, Adams ME. Stress-induced reproductive arrest in Drosophila occurs through ETH deficiency-mediated suppression of oogenesis and ovulation. BMC Biol. 2018;16(1):18. 10.1186/s12915-018-0484-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. O’Brien DM, Min KJ, Larsen T, Tatar M. Use of stable isotopes to examine how dietary restriction extends Drosophila lifespan. Curr Biol. 2008;18(4):R155–R156. 10.1016/j.cub.2008.01.021 [DOI] [PubMed] [Google Scholar]
- 25. Rodrigues MA, Martins NE, Balancé LF, et al. Drosophila melanogaster larvae make nutritional choices that minimize developmental time. J Insect Physiol. 2015;81:69–80. 10.1016/j.jinsphys.2015.07.002 [DOI] [PubMed] [Google Scholar]
- 26. Chess KF, Ringo JM. Oviposition site selection by Drosophila melanogaster and Drosophila simulans. Evolution. 1985;39(4):869–877. 10.1111/j.1558-5646.1985.tb00428.x [DOI] [PubMed] [Google Scholar]
- 27. Camus MF, Huang CC, Reuter M, Fowler K. Dietary choices are influenced by genotype, mating status, and sex in Drosophila melanogaster. Ecol Evol. 2018;8(11):5385–5393. 10.1002/ece3.4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ganguly A, Pang L, Duong VK, et al. A molecular and cellular context-dependent role for Ir76b in detection of amino acid taste. Cell Rep. 2017;18:737–750. 10.1016/j.celrep.2016.12.071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Rogina B, Wolverton T, Bross TG, Chen K, Müller H-G, Carey JR. Distinct biological epochs in the reproductive life of female Drosophila melanogaster. Mech Ageing Dev. 2007;128(9):477–485. 10.1016/j.mad.2007.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hudry B, Khadayate S, Miguel-Aliaga I. The sexual identity of adult intestinal stem cells controls organ size and plasticity. Nature. 2016;530(7590):344–348. 10.1038/nature16953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Regan JC, Khericha M, Dobson AJ, Bolukbasi E, Rattanavirotkul N, Partridge L. Sex difference in pathology of the ageing gut mediates the greater response of female lifespan to dietary restriction. Elife. 2016;5:e10956. 10.7554/eLife.10956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jin K, Wilson KA, Beck JN, et al. Genetic and metabolomic architecture of variation in diet restriction-mediated lifespan extension in Drosophila. PLoS Genet. 2020;16(7):e1008835. 10.1371/journal.pgen.1008835 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.