Abstract
Cytochrome P-450-dependent digitoxin 12 beta-hydroxylase from cell cultures of foxglove (Digitalis lanata) was solubilized from microsomal membranes with CHAPS (3-[(3-cholamidopropyl)dimethylammonio]propane-1-sulphonic acid). Cytochrome P-450 was separated from NADPH: cytochrome c (P-450) reductase by ion-exchange chromatography on DEAE-Sephacel. NADPH:cytochrome c (P-450) reductase was further purified by affinity chromatography on 2',5'-ADP-Sepharose 4B. This procedure resulted in a 248-fold purification of the enzyme; on SDS/polyacrylamide-gel electrophoresis after silver staining, only one band, corresponding to a molecular mass of 80 kDa, was present. The digitoxin 12 beta-hydroxylase activity could be reconstituted by incubating partially purified cytochrome P-450 and NADPH:cytochrome c (P-450) reductase together with naturally occurring microsomal lipids and flavin nucleotides. This procedure yielded about 10% of the original amount of digitoxin 12 beta-hydroxylase.
Full text
PDF

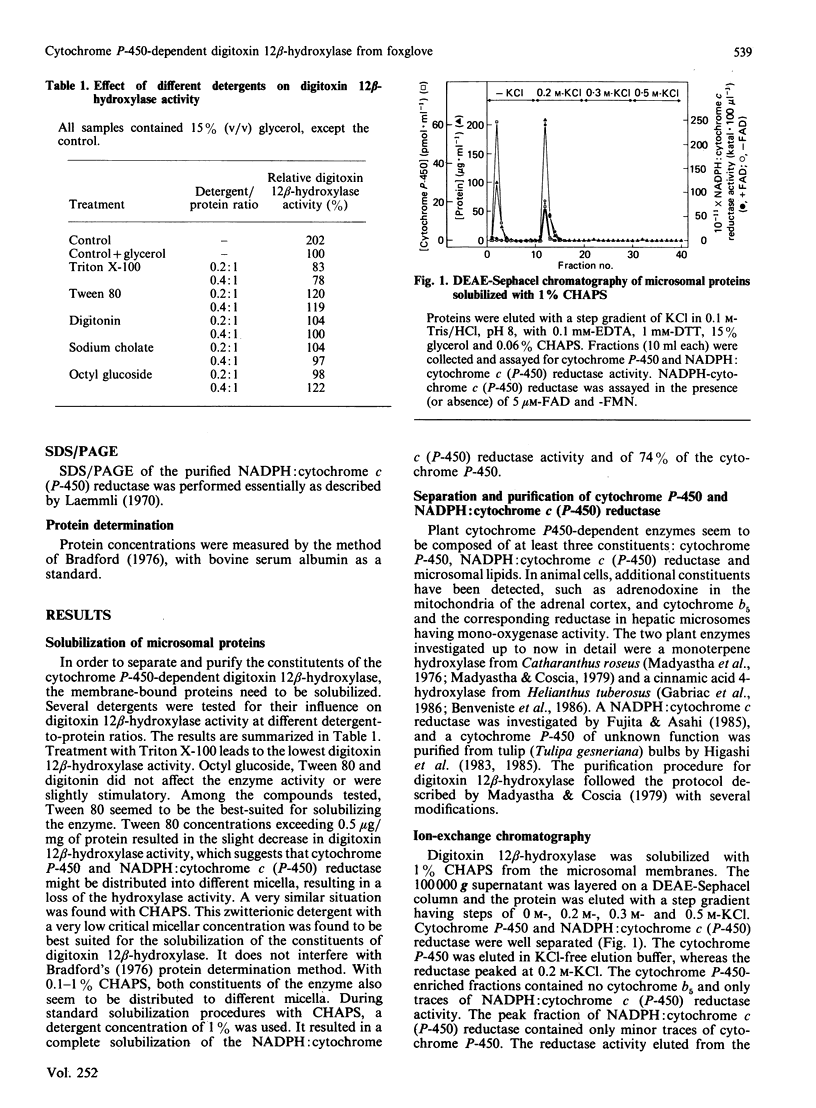
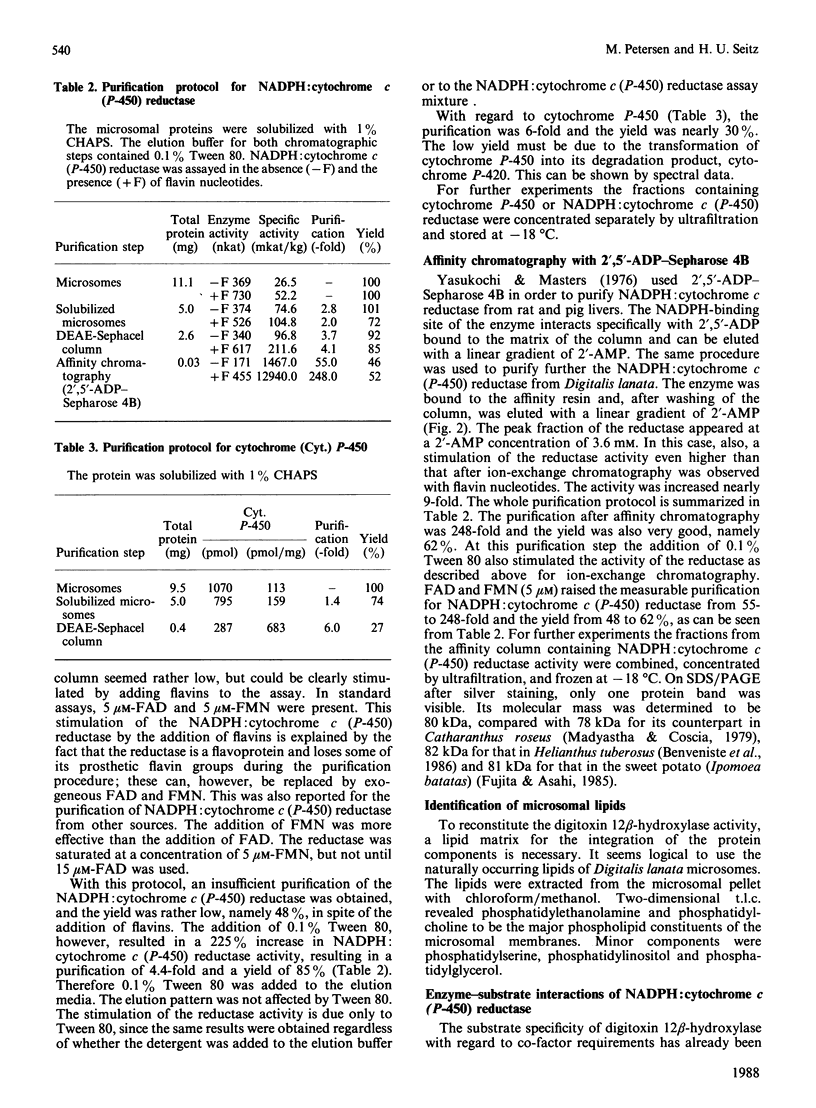
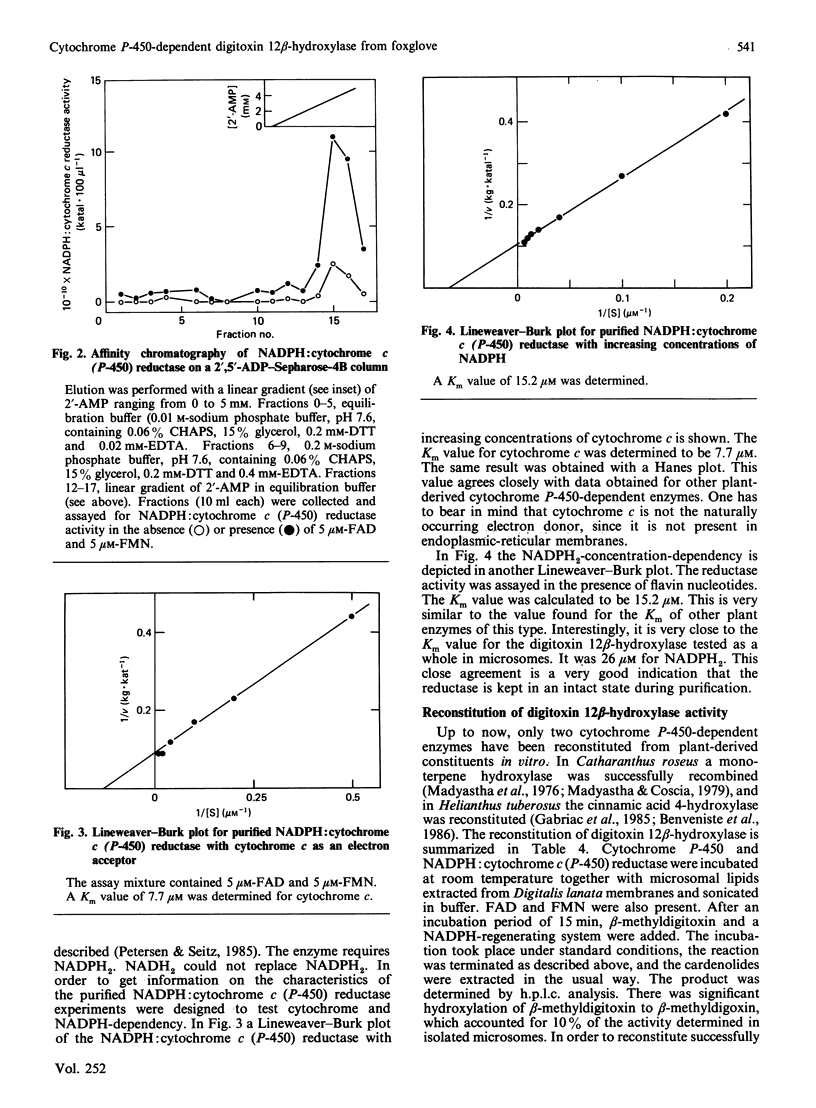


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benveniste I., Gabriac B., Durst F. Purification and characterization of the NADPH-cytochrome P-450 (cytochrome c) reductase from higher-plant microsomal fraction. Biochem J. 1986 Apr 15;235(2):365–373. doi: 10.1042/bj2350365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Estabrook R. W., Werringloer J. The measurement of difference spectra: application to the cytochromes of microsomes. Methods Enzymol. 1978;52:212–220. doi: 10.1016/s0076-6879(78)52024-7. [DOI] [PubMed] [Google Scholar]
- Fujita M., Oba K., Uritani I. Properties of a Mixed Function Oxygenase Catalyzing Ipomeamarone 15-Hydroxylation in Microsomes from Cut-Injured and Ceratocystis fimbriata-Infected Sweet Potato Root Tissues. Plant Physiol. 1982 Aug;70(2):573–578. doi: 10.1104/pp.70.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction and characterization of a microsomal flavonoid 3'-hydroxylase from parsley cell cultures. Eur J Biochem. 1983 Aug 15;134(3):547–554. doi: 10.1111/j.1432-1033.1983.tb07601.x. [DOI] [PubMed] [Google Scholar]
- Hagmann M. L., Heller W., Grisebach H. Induction of phytoalexin synthesis in soybean. Stereospecific 3,9-dihydroxypterocarpan 6a-hydroxylase from elicitor-induced soybean cell cultures. Eur J Biochem. 1984 Jul 2;142(1):127–131. doi: 10.1111/j.1432-1033.1984.tb08259.x. [DOI] [PubMed] [Google Scholar]
- Heller W., Kühnl T. Elicitor induction of a microsomal 5-O-(4-coumaroyl)shikimate 3'-hydroxylase in parsley cell suspension cultures. Arch Biochem Biophys. 1985 Sep;241(2):453–460. doi: 10.1016/0003-9861(85)90570-3. [DOI] [PubMed] [Google Scholar]
- Higashi K., Ikeuchi K., Karasaki Y., Obara M. Isolation of immunochemically distinct form of cytochrome P-450 from microsomes of tulip bulbs. Biochem Biophys Res Commun. 1983 Aug 30;115(1):46–52. doi: 10.1016/0006-291x(83)90966-x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larson R. L., Bussard J. B. Microsomal flavonoid 3'-monooxygenase from maize seedlings. Plant Physiol. 1986 Feb;80(2):483–486. doi: 10.1104/pp.80.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu A. Y., Levin W. The resolution and reconstitution of the liver microsomal hydroxylation system. Biochim Biophys Acta. 1974 Sep 16;344(2):205–240. doi: 10.1016/0304-4157(74)90004-5. [DOI] [PubMed] [Google Scholar]
- Madyastha K. M., Coscia C. J. Detergent-solubilized NADPH-cytochrome c(P-450) reductase from the higher plant, Catharanthus roseus. Purification and characterization. J Biol Chem. 1979 Apr 10;254(7):2419–2427. [PubMed] [Google Scholar]
- Madyastha K. M., Meehan T. D., Coscia C. J. Characterization of a cytochrome P-450 dependent monoterpene hydroxylase from the higher plant Vinca rosea. Biochemistry. 1976 Mar 9;15(5):1097–1102. doi: 10.1021/bi00650a023. [DOI] [PubMed] [Google Scholar]
- Murphy P. J., West C. A. The role of mixed function oxidases in kaurene metabolism in Echinocystis macrocarpa Greene endosperm. Arch Biochem Biophys. 1969 Sep;133(2):395–407. doi: 10.1016/0003-9861(69)90468-8. [DOI] [PubMed] [Google Scholar]
- OMURA T., SATO R. THE CARBON MONOXIDE-BINDING PIGMENT OF LIVER MICROSOMES. I. EVIDENCE FOR ITS HEMOPROTEIN NATURE. J Biol Chem. 1964 Jul;239:2370–2378. [PubMed] [Google Scholar]
- Vaskovsky V. E., Kostetsky E. Y. Modified spray for the detection of phospholipids on thin-layer chromatograms. J Lipid Res. 1968 May;9(3):396–396. [PubMed] [Google Scholar]
- Yasukochi Y., Masters B. S. Some properties of a detergent-solubilized NADPH-cytochrome c(cytochrome P-450) reductase purified by biospecific affinity chromatography. J Biol Chem. 1976 Sep 10;251(17):5337–5344. [PubMed] [Google Scholar]


