Abstract
Most described Mesozoic ants belong to stem groups that existed only during the Cretaceous period. Previously, the earliest known crown ants were dated to the Turonian (Late Cretaceous, ca. 94–90 million years ago (Ma)) deposits found in the USA, Kazakhstan, and Botswana. However, the recent discovery of an alate male ant in Kachin amber from the earliest Cenomanian (ca. 99 Ma), representing a new genus and species, Antiquiformica alata, revises the narrative on ant diversification. Antiquiformica can be distinctly differentiated from all known male stem ants by its geniculate antennae with elongated scape, extending far beyond the occipital margin of the head and half the length of the funiculus, as well as its partly reduced forewing venation. Furthermore, the combination of a one-segmented waist with a well-developed node, elongated scape extending beyond the occipital margin, and reduced forewing venation, particularly the completely reduced m-cu and rs-m crossveins and absence of rm and mcu closed cells, firmly places the fossil within the extant subfamily Formicinae. Fourier transform infrared spectroscopy (FTIR) confirmed that the amber containing Antiquiformica alata originated from the Kachin mines in Myanmar. This discovery significantly revises our understanding of the early evolution of Formicinae. The presence of Antiquiformica in Cenomanian amber indicates that the subfamily Formicinae emerged at least by the start of the Late Cretaceous, with crown ants likely originating earlier during the earliest Cretaceous or possibly the Late Jurassic, although paleontological evidence is lacking to support the latter hypothesis.
Keywords: Formicidae, Formicinae, Morphology, Taxonomy, Antiquiformica alata, Paleontology, Kachin amber
INTRODUCTION
Ants (Formicidae) are an ecologically significant group, dominating terrestrial ecosystems worldwide. This lineage of eusocial insects is critical for understanding the evolution, maintenance, and diversity of eusociality. The fossil record of Formicidae is relatively extensive compared to other eusocial insects, although perspectives on their earliest evolution have been hindered by the diverse subfamilies of crown ants. The earliest fossil ants, dating from the Early Cretaceous, are all stem-group ants. These fossils have been described from the Crato Formation in Brazil (Late Aptian, ca. 116–113 million years ago (Ma)) (Brandão et al., 1989), Emanra Formation of the Khetana River in the Khabarovsk Territory, Russia (Middle-Early Albian, ca. 113–105 Ma) (Dlussky, 1999), Baikura amber from the Taimyr Peninsula, Russia (Albian-earliest Cenomanian, ca. 105–99 Ma) (Dlussky, 1987), and Charentese amber from France (Albian-earliest Cenomanian, ca. 100 Ma) (Nel et al., 2004; Perrichot et al., 2008; Perrichot, 2014).
Many fossil ants have also been recovered from deposits dating from the Cenomanian to Campanian (ca. 100–72 Ma) of the Late Cretaceous. A total of 76 formicid species from 40 genera have been described from 11 Cretaceous deposits (Barden, 2017; Boudinot et al., 2020, 2022; LaPolla et al., 2013), the vast majority of which are stem-group ants. Among these, the most significant and richest Cretaceous fossil ant fauna is found in Kachin amber, dated to the earliest Cenomanian (98.79±0.62 Ma) (Lin et al., 2019; Shi et al., 2012; Yang et al., 2019) or, less likely, the late Albian (Yu et al., 2019). Currently, 36 species from 15 genera have been recorded from this deposit (Barden, 2017; Boudinot et al., 2020; Zhuang et al., 2022), but none have yet belonged to the crown group of ants.
In the present study, we describe a definitive crown-group ant, Antiquiformica alata, based on an alate male specimen belonging to the extant subfamily Formicinae (Figure 1). This specimen was preserved in a piece of Kachin amber, as confirmed by Fourier transform infrared spectroscopy (FTIR). The discovery of this male ant, exhibiting morphological traits characteristic of the modern subfamily Formicinae, revises the timeline of ant diversification. This fossil, representing the earliest evidence of crown ants, indicates that crown ants coexisted with various stem lineages in the Kachin Forest ecosystem and that the crown group of Formicidae already existed by the end of the Early Cretaceous, possibly even predating it.
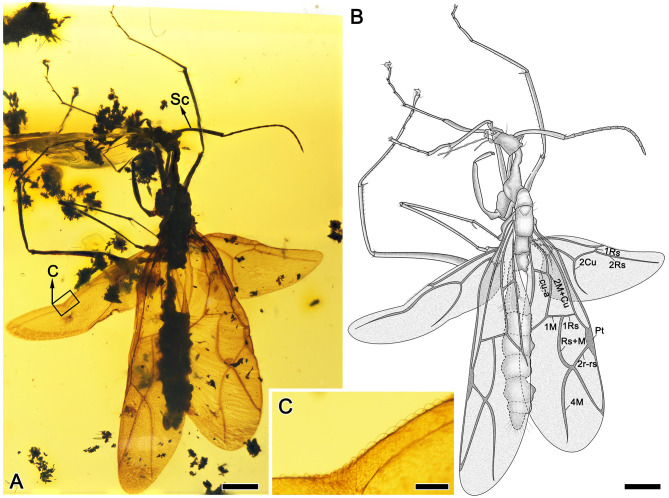
Figure 1.
Alate male of †Antiquiformica alata gen. et sp. nov. (No. CNU-HYM-MA2015012)
A: Photograph of holotype male in dorsal view. B: Line drawing of the same specimen based on the photograph, with names of veins indicated. C: Photograph of hamuli. Scale bars: 1 mm in A, B; 0.2 mm in C. Photo by Q Wu.
MATERIALS AND METHODS
Materials availability
The holotype (Figure 1) was collected from Noije Bum hill, approximately 18 km southwest of Tanai Village in the Hukawng Valley, northern Myanmar (N26°21'33.41'', E96°43'11.88''). The specimen is stored in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University (CNUB; Dong Ren, Curator), Beijing, China. The nomenclature for wing venation follows Rasnitsyn (1980).
Optical microscopy and photography
The specimen was examined under a Nikon SMZ 25 stereomicroscope (Nikon, Japan) and photographed using a Nikon DS-Ri 2 digital camera system (NIS-Elements D 5.30.00) at the College of Life Sciences, Capital Normal University, Beijing China. To minimize light interference, the specimen was fixed to Blu-Tack and submerged in glycerol. All pictures were superimposed using the Z-Stack approach.
Confocal laser scanning microscopy
A Zeiss LSM 780 confocal laser scanning microscope (Zeiss, Germany) at the College of Life Sciences, Capital Normal University, Beijing, China was used to observe and photograph detailed characteristics of the specimen. The laser wavelength was set to 488 nm, with a green background. The specimen was attached to a coverslip in an oil-free state.
Micro-computed tomography (CT) scanning
The specimen was scanned at the micro-CT laboratory of the Yunnan Key Laboratory for Paleobiology using an X-ray microscope (3D-XRM, Zeiss Xradia 520 versa, Germany). The scanning parameters were as follows: beam strength: 60 kV/5 w, filter: no, resolution: 2.92 μm, exposure time: 5 s, and number of TIFF images: 993. Volume rendering and three-dimensional (3D) reconstruction were performed using the open-source software Drishti v.2.4 (Limaye, 2012) from the Australian National University. The 3D-reconstruction models of the ant’s head were saved as images in Figure 2A, and the original scan data were deposited in Dryad (Wu et al., 2024; https://doi.org/10.5061/dryad.0k6djhb7d).
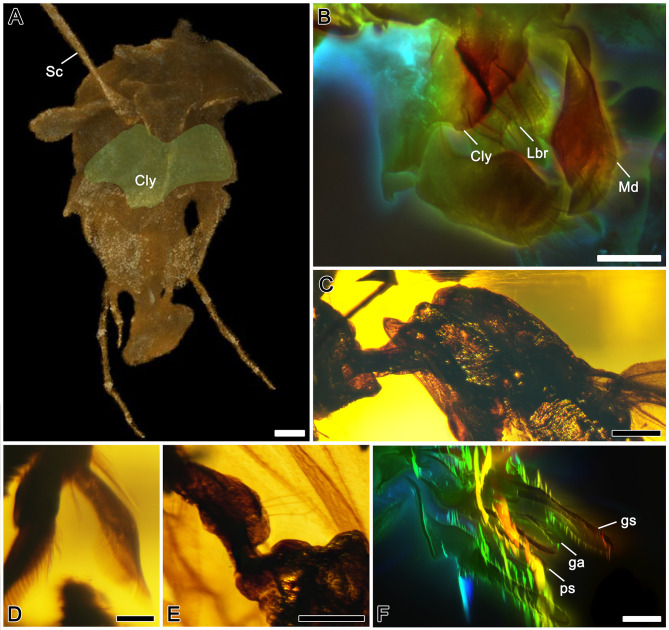
Figure 2.
Detailed structures of †Antiquiformica alata gen. et sp. nov.
A: 3D reconstructions of head; green area refers to clypeus. B: Confocal microscopy with depth color-coding of mouthparts. C: Photograph of promesonotum in lateral view. D: Photograph of protibial apex with calcar. E: Photograph of petiole in lateral view. F: Confocal microscopy with depth color-coding of genitalia. Sc, scape; Cly, clypeus; Lbr, labrum; Md, mandible; Pro, pronotum; ga, gonapophysis; gs, gonostylus; ps, penial sclerite. Scale bars: 0.2 mm in A, B; 0.5 mm in C, E; 0.1 mm in D, F. Photo by Q Wu and XQ Li.
Ant specimen dating
A Bruker TENSOR 27 spectroscope (Bruker, Germany) was used to assess the hydrocarbon structure and geographic origin of the amber. Powder samples (2 mg) were mixed with KBr at a mass-fraction ratio of approximately 1:150 to prepare 12 pressed pellets for analysis. FTIR spectra were acquired in the range of 400 per cm to 4 000 per cm with a resolution of 2 per cm and by accumulating five scans. Amber attribution was determined by comparing the FTIR spectra of the samples with reference to pieces of Dominican amber and Kachin amber containing a stick insect (Supplementary Figure S1) (Chen et al., 2019; Li et al., 2023; Xing, 2016; Yang et al., 2023). The three specimens tested are stored in the Key Lab of Insect Evolution and Environmental Changes, College of Life Sciences, Capital Normal University, Beijing, China.
Measurements
Not all features of the examined specimen were clearly visible and measurable. Consequently, only clearly visible details were measured, including: HL: Maximum length of head in full-face view, measured in a straight line from anterior-most point of clypeus to mid-point of occipital margin; HW: Maximum head width in full-face view, excluding compound eyes; CW: Maximum width of clypeus in full-face view; SL: Maximum length of scape, measured in a straight line from its apex to articulation with condylar bulb neck; PDL: Length of pedicel; Funis 1–11: Length of funicular articles from 1st to 11th; MxpL 1–6: Length of maxillary palpomeres from 1st to 6th; LapL 1–4: Length of labial palpomeres from 1st to 4th; ML: Diagonal length of mesosoma seen in profile from anterior-upper margin of pronotum to posterior margin of propodeal lobes; MH: Height of mesosoma, measured from upper level of mesoscutum perpendicularly to level of lower margin of mesopleuron; MsL: Length of mesoscutum in dorsal view; MsW: Maximum width of mesoscutum in dorsal view; MscL: Length of mesoscutellum in dorsal view; MscW: Maximum width of mesoscutellum in dorsal view; PL: Maximum length of petiole, measured from posterodorsal margin of petiole to articulation with propodeum; PH: Maximum height of petiole in profile, measured from uppermost point of petiolar node perpendicularly to lowest point of petiole; FfL: Length of profemur; FmsL: Length of mesofemur; FmtL: Length of metafemur; TpL: Length of protibia; TmsL: Length of mesotibia; TmtL: Length of metatibia; FWL: Maximum length of forewing; and FWW: Maximum width of forewing. Approximate total length was calculated as the sum of HL+ML+PL+length of the gaster.
RESULTS
Systematic Paleontology
Family Formicidae Latreille, 1802
Subfamily Formicinae Latreille, 1802
Genus †Antiquiformica Wu, Radchenko & Engel gen. nov.
LSID:urn:lsid:zoobank.org:act:BED29755-93AA-4EFF-B231-E38148342EE0
Type species: †Antiquiformica alata Wu, Radchenko & Engel sp. nov. (hic designatus).
Etymology: The generic name is a combination of the Latin adjectives antīquus (ancient) and formīca (ant), emphasizing its ancient age. The gender of the name is feminine.
Diagnosis: Waist one-segmented (petiole), nodiform, with rather long anterior peduncle, narrowly attached to first gastral segment; helcium attached low on anterior surface of first gastral segment. Antennae distinctly geniculate, with 13 antennomeres, elongated scape, extending far beyond occipital margin, 1.5 times as long as head and about half as long as funiculus. Mandible subtriangular, massive, with well-developed, largely edentate masticatory margin, with single apical tooth. Maxillary palpus hexamerous (six palpomeres), labial palpus tetramerous (four palpomeres). Meso- and metatibiae each with one simple spur; at least metatrochantellus present; pretarsal claws well-developed, with preapical tooth. Forewing: m-cu and rs-m crossveins completely reduced, rm and mcu cells absent, 1+2r, 3r, and 2cua cells closed.
Remarks: Antiquiformica can be clearly differentiated from all known male stem ants based on two key features: distinctly geniculate antennae with elongated scape, extending far beyond occipital margin of head and half length of funiculus (Figure 1A); forewing venation reduced compared to stem ants, with completely reduced m-cu and rs-m crossveins and rm and mcu cells absent (Figure 1B). In contrast, the forewings of all known gyne and male stem ants contain a “full set” of closed cells (e.g., see Boudinot et al., 2020; Dlussky, 1987).
†Antiquiformica alata Wu, Radchenko & Engel sp. nov. (Figures 1, 2; Supplementary Figure S2)
LSID:urn:lsid:zoobank.org:act:AE03A064-D299-475B-92F2-5629A51BAEAC
Etymology: The specific epithet is the Latin adjective ālātus (winged), referring to the fact that the holotype is an alate male.
Diagnosis: As for the genus.
Material: Holotype male, No. CNU-HYM-MA2015012.
Locality and horizon: Northern Myanmar, Kachin (Hukawng Valley), lowermost Cenomanian, dated 98.79±0.62 Ma (Cruickshank & Ko, 2003; Shi et al., 2012).
Description: Head small (Figure 1A) (due to specimen distortion, its proper shape is difficult to describe accurately), but head appears ca. 1.2 times as long as wide, somewhat narrowed anteriorly, with subparallel sides and straight occipital margin, bearing sparse, long, thin setae (Figure 1B). Size and correct position of compound eyes indeterminable due to distortion. Antennal scape feebly curved, somewhat widened to apex, with short suberect setae on inner margin; funiculus filiform, without apical club, pedicel almost straight, longer than each subsequent funicular article, article III longest, except for pedicel; all articles more than three times as long as wide (Figure 1A). Clypeus convex, with long medial carina and two short lateral carinae, anterior margin almost straight, shallowly emarginate medially, with row of long, coarse setae. Labrum large, bilobed, with rows of setae of various lengths along edges. Mandible with two rows of coarse setae apically, inner margin with numerous short, thin setae (Figure 2B). Maxillary palpus long, reaching occipital foramen, palpomere III longest, approximately 1.4 times as long as palpomere IV. Apical labial palpomere longest, approximately 3.5 times as long as preapical palpomere. Both maxillary and labial palpi with short, erect setae.
Mesosoma long and slender, 2.75 times as long as high, mesoscutum weakly convex, with parapsidal furrows, 2.38 times as long as wide, with sparse, thin, erect setae (Figure 2C). Dorsum of mesoscutellum convex. Dorsal surface of propodeum merging with posterior surface at obtuse, rounded angle, both surfaces of equal lengths, propodeal lobes well-developed and narrowly rounded. Legs long and slender, tibiae and tarsi with suberect setae on inner margins (Figure 2D), apices of tarsomeres with two pairs of fine, long setae; arolia of pretarsi large.
Forewing venation: pterostigma well-developed, narrow, approximately four times as long as wide; cell 1+2r approximately 2.8 times as long as wide, Rs+M arched; cell 3r relatively short, approximately 0.25 times chord length of wing; abscissae 1M and 1Rs almost forming straight line; abscissae 5Rs and 4M diverging from same knot; cu-a located somewhat proximally toward wing base, ratio of lengths of abscissae 1M+Cu and 2M+Cu approximately 0.85. Hind wing with 18 well-developed hamuli (Figure 1C), with venation of “typology II” (Cantone, 2017); 2M+Cu distinctly longer than cu-a, 1Rs and 2Cu curved.
Petiole long and low, 2.17 times as long as high, with small ventral process, node regularly rounded dorsally, anterior surface gradually sloping (Figure 2E); node with three long setae dorsally and four long setae ventrally. Gaster elongated, with seven segments, total length of 1st and 2nd segments approximately 0.36 times total gastral length; both tergites and sternites with sparse, thick, long setae. Genitalia not retractable. Apex of penial sclerite rounded in lateral view, without apicoventral tooth. Pygostyles present, with pubescence and single long seta. Gonapophysis (=digitus) robust and clavate. Gonostylus 12 times as long as high, with short setae; base of gonostylus with single, small tubercle. Cuspis robust, in dorsal view digitiform (Figure 2F).
Measurements (in mm): Total length 9.20, HL 1.01, HW 0.86, CW 0.43, SL 1.52, PDL 0.34, Funis 1–11 0.29, 0.32, 0.31, 0.28, 0.25, 0.25, 0.22, 0.21, 0.20, 0.20, 0.27, MxpL 1–6 0.13, 0.21, 0.32, 0.23, 0.29, 0.23, LapL 1–4 0.17, 0.09, 0.07, 0.24, ML 3.91, MH 1.25, MsL 1.52, MsW 0.64, MscL 0.61, MscW 0.40, PL 1.02, PH 0.47, FfL 2.87, FmsL 2.84, FmtL 2.98, TpL 3.01, TmsL 2.65, TmtL 2.86, FWL 7.5, FWW 1.90; approximate length of gaster 3.26.
DISCUSSION
The majority of Cretaceous Formicidae are classified within stem groups. However, 13 described species from 11 genera, dating from the Turonian (ca. 94–90 Ma) to Campanian (ca. 84–72 Ma) deposits in the USA, Kazakhstan, Botswana, and Canada, belong to the ant crown group (Dlussky, 1975, 1999; Dlussky et al., 2004; Engel & Grimaldi, 2005; Grimaldi & Agosti, 2000; McKellar et al., 2013). In addition, six undescribed species from three genera tentatively assigned to Dolichoderinae and Ponerinae have been recorded from Tilin amber in Myanmar (latest Campanian, ca. 72.1 Ma) (Zheng et al., 2018). Although their classification within these subfamilies is uncertain, they are certainly crown ants. Thus, the prior oldest crown ants have hitherto been from the Turonian. However, the discovery of Antiquiformica alata now extends the clade to the boundary of the Early and Late Cretaceous epochs.
FTIR provides fast infrared spectroscopy detection of structural information about the near-surface or internal organic components of a sample based on its reflection signals. FTIR has become widely used in amber research, as the spectral information can determine the location and age of amber specimens (Chen et al., 2019; Li et al., 2023; Musa et al., 2021). Given the significance of Antiquiformica alata, FTIR was applied to confirm the age and origin of the piece of amber containing the specimen. Previous studies have established that the main absorption peaks of Kachin amber are at approximately 3 442 per cm, 2 925 per cm, 2 866 per cm, 1 724 per cm, 1 696 per cm, 1 630 per cm, 1 458 per cm, and 1 380 per cm (Chen et al., 2019; Heinrichs et al., 2013; Li et al., 2023). The main absorption peaks of the piece of amber containing Antiquiformica alata were 2 928.6 per cm, 1 449.1 per cm, 3 434.8 per cm, 1 385.3 per cm, 1 569.0 per cm, 1 037.2 per cm, and 1 727.2 per cm (Supplementary Figure S1), closely matching those of previously analyzed Kachin amber specimens. Furthermore, a piece of confirmed Kachin amber containing a stick insect was used as a control group (Yang et al., 2023), showing absorption peaks of 2 927.3 per cm, 1 455.2 per cm, 2 867.7 per cm, 3 434.8 per cm, 1 382.7 per cm, 1 568.8 per cm, and 1 130.4 per cm (Supplementary Figure S1), consistent with the peaks of the amber containing Antiquiformica alata. In contrast, the main absorption peak differences between Dominican and Kachin amber are that the former has stronger peaks between 1 220 per cm and 1 250 per cm and between 886 per cm and 887 per cm. Our control specimen of Dominican amber displayed absorption peaks at 887.3 per cm and 1 242.5 per cm (Supplementary Figure S1), consistent with the known absorption peaks of Dominican amber (Chen et al., 2019). The FTIR spectral results demonstrate that the amber containing the Antiquiformica alata holotype is from the Kachin amber deposit.
The combination of a one-segmented waist (petiole) with a well-developed node and an elongated antennal scape extending far beyond the occipital margin of the head restricts the assignment of Antiquiformica to the extant ant subfamilies Formicinae or Dolichoderinae. Among Dolichoderinae males, only Tapinoma species have a scape extending beyond the occipital margin. However, in all males of this genus, the petiolar scale is somewhat reduced and strongly inclined forward. In addition, the masticatory margin of their mandibles contains a row of teeth (Shattuck, 1992; Yoshimura & Fisher, 2011). In contrast, Antiquiformica has a weakly nodiform petiole and an edentate masticatory margin on the mandible (Figure 2B).
The morphology of Antiquiformica is consistent with the characteristics of the subfamily Formicinae. Males of many formicine genera, such as those in the tribes Camponotini, Lasiini, Melophorini, and Plagiolepidini (sensu Ward et al., 2016), exhibit a long scape extending beyond the occipital margin and a forewing venation pattern similar to that of Antiquiformica (see Bolton, 2003; Boudinot, 2015). Notably, Antiquiformica shares several traits with Camponotini, including 13-segmented antennae with a long scape, well-developed but edentate masticatory mandibular margins, comparable general genital structure, and forewing venation pattern characterized by the complete reduction of m-cu and rs-m crossveins and the formation of a nearly straight line by 1Rs and 1M abscissae (Bolton, 2003; Boudinot, 2015; Ward & Boudinot, 2021), although the wing venation is not unique to this tribe. In addition, the articulation of the antennae distinctly behind the posterior clypeal margin is a synapomorphy of Camponotini. However, due to the preservation state of the specimen, it is unclear whether this character state is present in Antiquiformica. Therefore, while assignment to the subfamily Formicinae is confirmed, a more precise tribal attribution cannot be made for Antiquiformica.
The origin of ants has been actively studied for more than a century, initially based on morphological analyses and later incorporating molecular approaches. Estimates of the origin of crown ants vary widely, with median ages spanning from the Early Jurassic to the Cenomanian (185–94 Ma) (Borowiec et al., 2019; Brady et al., 2006; Crozier et al., 1997; Moreau et al., 2006; Moreau & Bell, 2013; Peters et al., 2017). Such variability in estimates arises from the use of different analytical methods and the inclusion of different sets of taxa in the analyses. Additionally, time estimates for the origin for the subfamily Formicinae vary significantly, from the Paleocene (60 Ma; Borowiec et al., 2019) to the Aptian (ca. 118 Ma; Blaimer et al., 2015). Clearly, these estimates lack precision, a challenge compounded by the lack of key calibration points for the extant subfamilies.
A single fossil cannot definitively resolve the question of the origin of ants. Nevertheless, the discovery of Antiquiformica in Cenomanian amber indicates that the subfamily Formicinae emerged at least by the earliest part of the Late Cretaceous, with crown ants certainly arising earlier, possibly in the earliest Cretaceous or even the Late Jurassic, although there is currently no paleontological evidence to support the latter hypothesis. Regardless of these challenges, it is evident that stem ants and crown ants coexisted during the Late Cretaceous, with their overlap period lasting approximately 20 Ma and spanning the episode of significant angiosperm diversification, which may have influenced this faunal turnover. Crown ants must have been present in the Early Cretaceous, and future excavations will be crucial in enriching our understanding of the earliest origins and diversification of these ecologically ubiquitous eusocial insects.
In conclusion, ants are currently one of the most ecologically dominant terrestrial lineages, but their Cretaceous counterparts were comparatively rare. We report the discovery of the oldest known crown ant, preserved in Kachin amber (Myanmar). Antiquiformica alata extends the earliest record of crown ants, specifically within the subfamily Formicinae, to the earliest Cenomanian (ca. 99 Ma). This new fossil reveals that the morphology of early crown ants already exhibited marked differences from that of stem-ants, especially in antennal structure and wing venation. These findings suggest that crown ants may have evolved as early as the earliest Cretaceous or even the latest Jurassic.
SUPPLEMENTARY DATA
Supplementary data to this article can be found online.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
T.P.G. designed the research. Q.W., A.G.R., M.S.E., and T.P.G. performed the analyses. Q.W. and X.Q.L. prepared the photographs and line drawings. X.R.L. helped with spectroscopy. Q.W., A.G.R., M.S.E., and T.P.G. wrote the manuscript with editing by all authors. Q.W., A.G.R., M.S.E., H.R.Y., C.K.S., D.R. and T.P.G. reviewed and edited the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
We are sincerely grateful to Dr. Brendon E. Boudinot (Senckenberg Research Institute and Natural History Museum of Germany) for his help in specimen identification and guidance with the description, and to Dr. Alexander P. Rasnitsyn (Borissiak Palaeontological Institute of Russia) for his advice regarding certain morphological details. We thank Dr. Xian-Yong Sheng and Ms. Yan-Jie Zhang (Capital Normal University of China) for their helpful advice with the use of a Zeiss LSM 780 inverted confocal laser microscope (CLM), and Mr. Kaitao Chen for his help in spectral analysis. We appreciate Ms. Xiao-Ran Zuo for an ecological reconstruction picture. We express our gratitude to three anonymous reviewers for their valuable comments and suggestions.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32270467, 32020103006), National Research Foundation of Ukraine (2020/02/0369 to A.G.R.), and CONCYTEC through the PROCIENCIA “Interinstitutional Alliances for Doctorate Programs” (PE501084299-2023-PROCIENCIA-BM to M.S.E.)
References
- Barden P Fossil ants (Hymenoptera: Formicidae): ancient diversity and the rise of modern lineages. Myrmecological News. 2017;24:1–30. [Google Scholar]
- Blaimer BB, Brady SG, Schultz TR, et al Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: a case study of formicine ants. BMC Evolutionary Biology. 2015;15:271. doi: 10.1186/s12862-015-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton B Synopsis and classification of Formicidae. Memoirs of the American Entomological Institute. 2003;71:1–370. [Google Scholar]
- Borowiec ML, Rabeling C, Brady SG, et al Compositional heterogeneity and outgroup choice influence the internal phylogeny of the ants. Molecular Phylogenetics and Evolution. 2019;134:111–121. doi: 10.1016/j.ympev.2019.01.024. [DOI] [PubMed] [Google Scholar]
- Boudinot BE Contributions to the knowledge of Formicidae (Hymenoptera, Aculeata): a new diagnosis of the family, the first global male-based key to subfamilies, and a treatment of early branching lineages. European Journal of Taxonomy. 2015;120:1–62. [Google Scholar]
- Boudinot BE, Perrichot V, Chaul JCM. 2020. Camelosphecia gen. nov. , lost ant-wasp intermediates from the mid-Cretaceous (Hymenoptera, Formicoidea). ZooKeys, 1005 : 21–55.
- Boudinot BE, Richter A, Katzke J, et al Evidence for the evolution of eusociality in stem ants and a systematic revision of †Gerontoformica (Hymenoptera: Formicidae) Zoological Journal of the Linnean Society. 2022;195(4):1355–1389. doi: 10.1093/zoolinnean/zlab097. [DOI] [Google Scholar]
- Brady SG, Schultz TR, Fisher BL, et al Evaluating alternative hypotheses for the early evolution and diversification of ants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(48):18172–18177. doi: 10.1073/pnas.0605858103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandão CRF, Martins-Neto RG, Vulcano MA The earliest known fossil ant (first Southern Hemisphere Mesozoic record) (Hymenoptera: Formicidae: Myrmeciinae) Psyche. 1989;96(3-4):086043. [Google Scholar]
- Cantone S. 2017. Winged Ants. The Male. Dichotomous Key to Genera of Winged Male Ants in the World. Behavioral Ecology of Mating Flight. São Paulo: Autopubblicato.
- Chen D, Zeng QS, Yuan Y, et al Baltic amber or Burmese amber: FTIR studies on amber artifacts of Eastern Han Dynasty unearthed from Nanyang. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2019;222:117270. doi: 10.1016/j.saa.2019.117270. [DOI] [PubMed] [Google Scholar]
- Crozier RH, Jermiin LS, Chiotis M Molecular evidence for a Jurassic origin of ants. Naturwissenschaften. 1997;84(1):22–23. doi: 10.1007/s001140050341. [DOI] [Google Scholar]
- Cruickshank RD, Ko K Geology of an amber locality in the Hukawng Valley, northern Myanmar. Journal of Asian Earth Sciences. 2003;21(5):441–455. doi: 10.1016/S1367-9120(02)00044-5. [DOI] [Google Scholar]
- Dlussky GM. 1975. Superfamily formicoidea latreille, 1802. Family Formicidae Latreille, 1802. In: Rasnitsyn AP. Hymenoptera Apocrita of Mesozoic. Trudy Paleontologicheskogo Instituta, Akademiya Nauk SSSR, 114–122. (in Russian
- Dlussky GM. 1987. New formicoidea (hymenoptera) of the upper cretaceous. Paleontologicheskii Zhurnal, 21 (1): 131–135. (in Russian
- Dlussky GM New ants (Hymenoptera, Formicidae) from Canadian amber. Paleontological Journal. 1999;33(4):409–412. [Google Scholar]
- Dlussky GM, Brothers DJ, Rasnitsyn AP The first Late Cretaceous ants (Hymenoptera: Formicidae) from southern Africa, with comments on the origin of the Myrmicinae. Insect Systematics & Evolution. 2004;35(1):1–13. [Google Scholar]
- Engel MS, Grimaldi DA Primitive new ants in Cretaceous amber from Myanmar, New Jersey, and Canada (Hymenoptera: Formicidae) American Museum Novitates. 2005;2005(3485):1–24. [Google Scholar]
- Grimaldi D, Agosti D A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(25):13678–13683. doi: 10.1073/pnas.240452097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs J, Vitt DH, Schäfer-Verwimp A, et al The moss Macromitrium richardii (Orthotrichaceae) with sporophyte and calyptra enclosed in Hymenaea resin from the Dominican Republic. Polish Botanical Journal. 2013;58(1):221–230. doi: 10.2478/pbj-2013-0022. [DOI] [Google Scholar]
- Lapolla JS, Dlussky GM, Perrichot V Ants and the fossil record. Annual Review of Entomology. 2013;58:609–630. doi: 10.1146/annurev-ento-120710-100600. [DOI] [PubMed] [Google Scholar]
- Li XP, Wang YM, Shi GH, et al Evaluation of natural ageing responses on burmese amber durability by FTIR spectroscopy with PLSR and ANN models. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy. 2023;285:121936. doi: 10.1016/j.saa.2022.121936. [DOI] [PubMed] [Google Scholar]
- Limaye A. 2012. Drishti: a volume exploration and presentation tool. In: Proceedings of SPIE 8506, Developments in X-ray Tomography VIII. San Diego: SPIE, 85060X.
- Lin XD, Labandeira CC, Shih C, et al Life habits and evolutionary biology of new two-winged long-proboscid scorpionflies from mid-Cretaceous Myanmar amber. Nature Communications. 2019;10(1):1235. doi: 10.1038/s41467-019-09236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar RC, Glasier JRN, Engel MS New ants (Hymenoptera: Formicidae: Dolichoderinae) from Canadian Late Cretaceous amber. Bulletin of Geosciences. 2013;88(3):583–594. [Google Scholar]
- Moreau CS, Bell CD Testing the museum versus cradle tropical biological diversity hypothesis: phylogeny, diversification, and ancestral biogeographic range evolution of the ants. Evolution. 2013;67(8):2240–2257. doi: 10.1111/evo.12105. [DOI] [PubMed] [Google Scholar]
- Moreau CS, Bell CD, Vila R, et al Phylogeny of the ants: diversification in the age of angiosperms. Science. 2006;312(5770):101–104. doi: 10.1126/science.1124891. [DOI] [PubMed] [Google Scholar]
- Musa M, Kaye TG, Bieri W, et al Burmese amber compared using micro-attenuated total reflection infrared spectroscopy and ultraviolet imaging. Applied Spectroscopy. 2021;75(7):839–845. doi: 10.1177/0003702820986880. [DOI] [PubMed] [Google Scholar]
- Nel A, Perrault G, Perrichot V, et al The oldest ant in the Lower Cretaceous amber of Charente-maritime (SW France) (Insecta: Hymenoptera: Formicidae) Geologica Acta. 2004;2(1):23–29. [Google Scholar]
- Perrichot V A new species of the Cretaceous ant Zigrasimecia based on the worker caste reveals placement of the genus in the Sphecomyrminae (Hymenoptera: Formicidae) Myrmecological News. 2014;19:165–169. [Google Scholar]
- Perrichot V, Nel A, Néraudeau D, et al New fossil ants in French Cretaceous amber (Hymenoptera: Formicidae) Naturwissenschaften. 2008;95(2):91–97. doi: 10.1007/s00114-007-0302-7. [DOI] [PubMed] [Google Scholar]
- Peters RS, Krogmann L, Mayer C, et al Evolutionary history of the Hymenoptera. Current Biology. 2017;27(7):1013–1018. doi: 10.1016/j.cub.2017.01.027. [DOI] [PubMed] [Google Scholar]
- Rasnitsyn AP. 1980. Origin and evolution of hymenoptera. Trudy Paleontologicheskogo Instituta, 174 : 1–192. (in Russian
- Shattuck SO Generic revision of the ant subfamily Dolichoderinae. Sociobiology. 1992;21:1–181. [Google Scholar]
- Shi GH, Grimaldi DA, Harlow GE, et al Age constraint on Burmese amber based on U-Pb dating of zircons. Cretaceous Research. 2012;37:155–163. doi: 10.1016/j.cretres.2012.03.014. [DOI] [Google Scholar]
- Ward PS, Blaimer BB, Fisher BL. 2016. A revised phylogenetic classification of the ant subfamily Formicinae (Hymenoptera: Formicidae), with resurrection of the genera Colobopsis and Dinomyrmex. Zootaxa, 4072 (3): 343–357.
- Ward PS, Boudinot BE Grappling with homoplasy: taxonomic refinements and reassignments in the ant genera Camponotus and Colobopsis (Hymenoptera: Formicidae) Arthropod Systematics & Phylogeny. 2021;79:37–56. [Google Scholar]
- Wu Q, Radchenko AG, Engel MS, et al. 2024. Scan mCT data of Cretaceous ant Antiquiformica alata[Dataset]. Dryad, https://doi.org/10.5061/dryad.0k6djhb7d.
- Xing YY Application of attenuated total reflectance infrared spectroscopy (ATR-FTIR) for amber identification and research. Spectroscopy and Spectral Analysis. 2016;36(7):2066–2070. [PubMed] [Google Scholar]
- Yang HR, Engel MS, Shih C, et al Independent wing reductions and losses among stick and leaf insects (Phasmatodea), supported by new Cretaceous fossils in amber. BMC Biology. 2023;21(1):210. doi: 10.1186/s12915-023-01720-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang HR, Yin XC, Lin XD, et al Cretaceous winged stick insects clarify the early evolution of Phasmatodea. Proceedings of the Royal Society B: Biological Sciences. 2019;286(1909):20191085. doi: 10.1098/rspb.2019.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Fisher BL A revision of male ants of the Malagasy region (Hymenoptera: Formicidae): Key to genera of the subfamily Dolichoderinae. Zootaxa. 2011;2794(1):1–34. [Google Scholar]
- Yu TT, Thomson U, Mu L, et al An ammonite trapped in Burmese amber. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(23):11345–11350. doi: 10.1073/pnas.1821292116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng DR, Chang SC, Perrichot V, et al A late cretaceous amber biota from central Myanmar. Nature Communications. 2018;9(1):3170. doi: 10.1038/s41467-018-05650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang YH, Ran H, Li XQ, et al A new species of the iron maiden ant based on an alate female from mid-Cretaceous Burmese amber (Hymenoptera: Formicidae: †Zigrasimecia) Cretaceous Research. 2022;130:105056. doi: 10.1016/j.cretres.2021.105056. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data to this article can be found online.