Abstract
Despite intensive characterization of immune responses after COVID-19 infection and vaccination, research examining protective correlates of vertical transmission in pregnancy are limited. Herein, we profiled humoral and cellular characteristics in pregnant women infected or vaccinated at different trimesters and in their corresponding newborns. We noted a significant correlation between spike S1–specific IgG antibody and its RBD-ACE2 blocking activity (receptor-binding domain–human angiotensin-converting enzyme 2) in maternal and cord plasma (P < .001, R > 0.90). Blocking activity of spike S1–specific IgG was significantly higher in pregnant women infected during the third trimester than the first and second trimesters. Elevated levels of 28 cytokines/chemokines, mainly proinflammatory, were noted in maternal plasma with infection at delivery, while cord plasma with maternal infection 2 weeks before delivery exhibited the emergence of anti-inflammatory cytokines. Our data support vertical transmission of protective SARS-CoV-2–specific antibodies. This vertical antibody transmission and the presence of anti-inflammatory cytokines in cord blood may offset adverse outcomes of inflammation in exposed newborns.
Keywords: antibody, cytokines, placental transmission, pregnancy, SARS-CoV-2
Results in this study suggest vertical transmission of SARS-CoV-2 spike S1–specific IgG antibodies from mothers to infants and an emergence of anti-inflammatory cytokines/chemokines in cord blood in the third trimester of pregnancy.
Although our understanding of human immunity to SARS-CoV-2 after infection and vaccination has increased markedly during the COVID-19 pandemic, identifying correlates of protection in the mother-infant dyad in pregnancy remains limited. In general, pregnant women are at increased risk of severe disease complications mainly due to immune adaptation during pregnancy [1–4]. This is underscored by a large study from the Centers for Disease Control and Prevention, which showed that among >450 000 women with symptomatic COVID-19 infection at reproductive ages who were admitted to an intensive care unit, death was more likely among pregnant than nonpregnant women [5]. However, data on vertical transmission in SARS-CoV-2–exposed infants have been more reassuring. When appropriate precautions were maintained in 116 mothers infected with SARS-CoV-2 who breastfed, 120 newborns all tested negative and remained asymptomatic [6]. Although a small number of individual case reports have documented vertical transmission, this occurs infrequently [7–9]. Mechanisms of protection underlying these clinical observations have not been elucidated but may involve a combination of humoral and cellular responses in the mother-infant dyad.
Previous data on vertical transmission of SARS-CoV-2–specific antibodies revealed reduced antibody transfer in neonates born to mothers with recent or ongoing infection and identified a transient response to maternal inflammation in the form of increased cytokines in cord plasma [10]. However, infection timing and the impact of maternal vaccination during gestation are variables that likely influence the extent of immune protection/modulation in newborns. These variables are largely dependent on maternal antibody titers, immunization status, and gestational age, as previously shown with measles vaccination [11] and recently with SARS-CoV-2, where specific antibodies waned by 6 months following infection [12] and vaccination [13]. Transplacental antibodies are also likely to wane but, during the newborn period, probably confer protection at a time of increased host susceptibility. Concomitantly, immunomodulatory cytokines may protect the neonate from the effects of associated inflammation following maternal SARS-CoV-2 infection in pregnancy [14], including invasive disease and multisystem inflammatory syndrome [15].
The magnitude and specificity of transplacental SARS-CoV-2–specific antibodies and perturbations in cytokines/chemokines have not been characterized by gestational age and maternal vaccination. We hypothesized that neonates of pregnant women infected/vaccinated early during pregnancy will have lower antibody titers due to waning immunity as reported in adults. Since the cytokine storm associated with severe, invasive COVID-19 can occur in pregnant women without affecting the fetus, we also hypothesized that elevated levels of anti-inflammatory cytokines in cord blood would lower the risk of adverse outcomes in exposed newborns. Herein, we characterized the antibody and cytokine/chemokine profiles of mother-infant dyads infected and/or vaccinated at various time points during gestation to test these hypotheses.
METHODS
Human Participants
The Mayo Clinic Institutional Review Board reviewed and approved this study (20-003014 and 20-003251).
Pregnant women were enrolled following infection or vaccination during gestation. Maternal and cord blood was collected upon admission and delivery, respectively, between June 2020 and August 2021 from the Maternal Fetal Medicine Division, Mayo Clinic. Paired maternal/fetal cord samples were categorized into 7 groups:
1T: first-trimester infection
2T: second-trimester infection
3T: third-trimester infection
2W: infection within 2 weeks prior to delivery
C: controls who were pregnant and uninfected
V: vaccinated (immunized with Pfizer mRNA vaccine)
I/V: infected and vaccinated during gestation
Trimesters were assigned as defined by the American College of Obstetricians and Gynecologists. Plasma samples from women who were nonpregnant and uninfected and patients who had recovered from COVID-19 were obtained from the Mayo Clinic Biobank as controls.
Blood Processing
Maternal and cord blood samples were processed in a biosafety level 2+ laboratory with heightened precautions. Blood was centrifuged for 8 minutes at 1000g. Plasma was collected and stored at −80 °C for future use.
Detection of SARS-CoV-2 Spike S1–Specific Antibodies
SARS-CoV-2 spike S1–specific IgG and IgM were measured in duplicate with commercially available enzyme-linked immunosorbent assay kits per the manufacturer's protocols (IEQ-CoVS1RBD-IgG-1 and IEQ-CoVS1RBD-IgM-1; RayBiotech). Plasma samples were 1:20 diluted for the detection of SARS-CoV-2–specific IgM and 4-fold diluted, starting from 1:20, for the detection of spike S1 IgG.
Surrogate Virus Neutralization Test
Neutralization antibodies against receptor-binding domain (RBD)–human angiotensin-converting enzyme 2 (hACE2) in maternal and fetal cord plasma were assessed with the SARS-CoV-2 Surrogate Virus Neutralization Test Kit (L00847-A; GenScript), following the manufacturer's protocol. The percentage inhibition of RBD-hACE2 binding was calculated with the provided equation: 100 × [1 − (optical density of serum sample / optical density of negative control)].
Detection of Plasma Cytokines/Chemokines
A panel of 74 plasma cytokines/chemokines was measured in duplicate with the Clinical Cytokine/Chemokine/Growth Factor 71-Plex Panel (HD71-CLIN) and 3-Plex TGF-β (TGFβ1-3) from Eve Technologies, following the manufacturer's protocols.
Statistical Analysis
Maternal and fetal cord–paired analysis was carried out with the Wilcoxon rank sum test. Anti–spike S1 antibody titers and RBD-hACE2 blocking antibody activity comparisons among infection and vaccinated groups were conducted by the Mann-Whitney U test. Correlation analyses between maternal-fetal dyads were conducted via the Spearman method. P < .05 was considered significant. Figures and statistical analyses were computed with R (version 4.2.1).
RESULTS
Demographic and Clinical Characteristics of Study Cohort
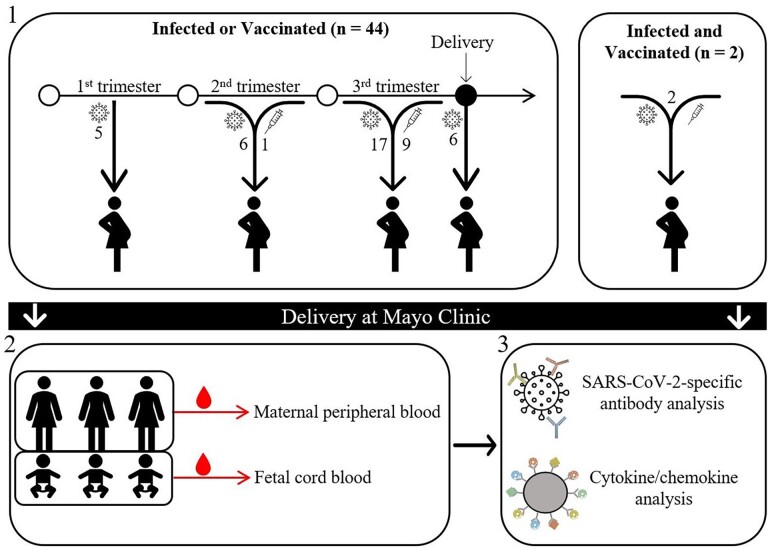
We conducted a cross-sectional cohort study of 57 pregnant women of mostly (79%) European descent (Figure 1). The demographic and clinical characteristics of the study cohort are summarized in Table 1. The median age at delivery was 31 years (range, 20–40), and the median gestation duration was 274 days (range, 239–290). Of the pregnant women who were infected, 5, 6, 17, and 6 tested positive in the 1T, 2T, 3T, and 2W groups, respectively. Of the vaccinated pregnant women, 1 and 9 were vaccinated during the second and third trimesters, respectively. The mean time during gestation for pregnant women receiving their most recent COVID-19 vaccination was 234 days, ranging from 184 to 261.
Figure 1.
Study design. (1) This study recruited pregnant women infected and/or vaccinated (n = 46) at various time points during gestation (first, second, and third trimesters and within 2 weeks prior to delivery), as well as those uninfected or vaccinated (controls, n = 11). (2) Maternal peripheral blood and fetal cord blood were collected at the time of birth. (3) Plasma was isolated and used for quantitative and functional SARS-CoV-2–specific antibody via enzyme-linked immunosorbent assay and cytokine/chemokine analysis via multiplex.
Table 1.
Demographic and Clinical Characteristics of Study Cohort
| Characteristic | 1T (n = 5) | 2T (n = 6) | 3T (n = 17) | 2W (n = 6) | I/V (n = 2) | V–All (n = 10) | V–3T (n = 9) | C (n = 11) | All (N = 57) |
|---|---|---|---|---|---|---|---|---|---|
| Maternal | |||||||||
| Age, y | 29 (21–36) | 35 (22–37) | 30 (20–37) | 31 (21–39) | 30 (27–32) | 36 (29–40) | 36 (29–40) | 29 (22–32) | 31 (20–40) |
| European descent | 2 (40) | 3 (50) | 14 (82) | 6 (100) | 2 (100) | 8 (80) | 7 (78) | 10 (91) | 45 (79) |
| Black/African American | 2 (40) | 2 (33) | 2 (12) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 6 (11) |
| Asian | 0 (0) | 0 (0) | 1 (6) | 0 (0) | 0 (0) | 2 (20) | 2 (22) | 0 (0) | 3 (5) |
| Race unknown/not reported | 1 (20) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 3 (5) |
| Hispanic or Latinx | 1 (20) | 0 (0) | 1 (6) | 3 (50) | 0 (0) | 1 (10) | 1 (11) | 1 (9) | 7 (12) |
| Not Hispanic or Latinx | 4 (80) | 6 (100) | 16 (94) | 3 (50) | 2 (100) | 9 (90) | 8 (89) | 10 (91) | 50 (88) |
| Asthma | 1 (20) | 2 (33) | 3 (18) | 1 (17) | 0 (0) | 2 (20) | 2 (22) | 2 (18) | 11 (19) |
| Obesity | 3 (60) | 3 (50) | 8 (47) | 4 (67) | 1 (50) | 2 (20) | 2 (22) | 8 (73) | 29 (51) |
| Hypertension | 0 (0) | 0 (0) | 0 (0) | 2 (33) | 1 (50) | 1 (10) | 1 (11) | 0 (0) | 4 (7) |
| Diabetes | 0 (0) | 0 (0) | 0 (0) | 1 (17) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 2 (4) |
| Cardiac conditions | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 1 (2) |
| Preeclampsia | 2 (40) | 1 (17) | 1 (6) | 3 (50) | 0 (0) | 1 (10) | 1 (11) | 4 (36) | 12 (21) |
| Gestational age, d | |||||||||
| PCR+ | 67 (53–95) | 146 (110–187) | 214 (196–263) | 263 (247–284) | 121 (96–146) | … | … | … | 205 (53–284) |
| Vaccination | … | … | … | … | 250 (247–253) | 238 (184–261) | 234 (184–261) | … | 243 (184–261) |
| COVID-19 symptoms | |||||||||
| Asymptomatic | 0 (0) | 1 (17) | 2 (12) | 4 (67) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (12) |
| Fever | 1 (20) | 2 (33) | 4 (24) | 1 (17) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 9 (16) |
| Cough | 1 (20) | 4 (67) | 8 (47) | 1 (17) | 1 (50) | 0 (0) | 0 (0) | 1 (9) | 16 (28) |
| Sore throat | 2 (40) | 3 (50) | 6 (35) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 12 (21) |
| Dyspnea | 1 (20) | 0 (0) | 6 (35) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 1 (9) | 10 (18) |
| Myalgia | 1 (20) | 3 (50) | 4 (24) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 10 (18) |
| Fatigue | 0 (0) | 1 (17) | 4 (24) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 6 (11) |
| Diarrhea | 0 (0) | 2 (33) | 3 (18) | 2 (33) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 7 (12) |
| Anosmia | 1 (20) | 3 (50) | 9 (53) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 14 (25) |
| Ageusia | 1 (20) | 2 (33) | 8 (47) | 0 (0) | 1 (50) | 0 (0) | 0 (0) | 0 (0) | 12 (21) |
| Cardiac | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Neonatal | |||||||||
| Singleton | 5 (100) | 6 (100) | 17 (100) | 6 (100) | 2 (100) | 10 (100) | 9 (100) | 11 (100) | 57 (100) |
| Male | 2 (40) | 5 (83) | 10 (59) | 4 (67) | 1 (50) | 7 (70) | 6 (67) | 7 (64) | 36 (63) |
| Female | 3 (60) | 1 (17) | 7 (41) | 2 (33) | 1 (50) | 3 (30) | 3 (33) | 4 (36) | 21 (37) |
| Gestational age at delivery, d | 274 (273–280) | 273 (239–280) | 274 (265–278) | 267 (252–290) | 274 (274–274) | 274 (264–278) | 274 (264–278) | 276 (273–286) | 274 (239–290) |
| Birth weight, g | 3.1 (2.6–3.9) | 3.6 (3.1–3.9) | 3.4 (2.2–4.4) | 3.2 (2.3–3.9) | 3.7 (3.2–4.1) | 3.2 (2.9–4.2) | 3.2 (2.9–4.2) | 3.6 (2.8–4.2) | 3.5 (2.2–4.4) |
| Apgar score at 5 min | 9 (9–9) | 9 (7–9) | 9 (9–9) | 9 (8–9) | 8.5 (8–9) | 9 (9–9) | 9 (9–9) | 9 (8–9) | 9 (7–9) |
| Delivery | |||||||||
| Vaginal | 4 (80) | 5 (83) | 13 (76) | 4 (67) | 0 (0) | 8 (80) | 8 (89) | 6 (55) | 40 (70) |
| Cesarean section | 1 (20) | 1 (17) | 4 (24) | 2 (33) | 2 (100) | 2 (20) | 1 (11) | 5 (45) | 17 (30) |
| Asymptomatic, healthy | 5 (100) | 4 (67) | 13 (76) | 4 (67) | 2 (100) | 7 (70) | 6 (67) | 11 (100) | 46 (81) |
| Tachypnea | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (10) | 1 (11) | 0 (0) | 1 (2) |
| Respiratory distress syndrome | 0 (0) | 1 (17) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2) |
| Other/unknown outcome | 0 (0) | 1 (17) | 4 (24) | 2 (33) | 0 (0) | 2 (20) | 2 (22) | 0 (0) | 9 (16) |
Data are presented as median (range) or No. (%).
Abbreviations: 1T, first-trimester infection; 2T, second-trimester infection; 3T, third-trimester infection; 2W, infected within 2 weeks of delivery; C, noninfected/vaccinated pregnant controls; I/V, infected and vaccinated; PCR, polymerase chain reaction; V–All, all vaccinated; V–3T, third trimester vaccinated.
Pregnant Women Vaccinated in the Third Trimester Had Significantly Higher Anti–Spike S1 IgG Titers Than Pregnant Women Infected With SARS-CoV-2
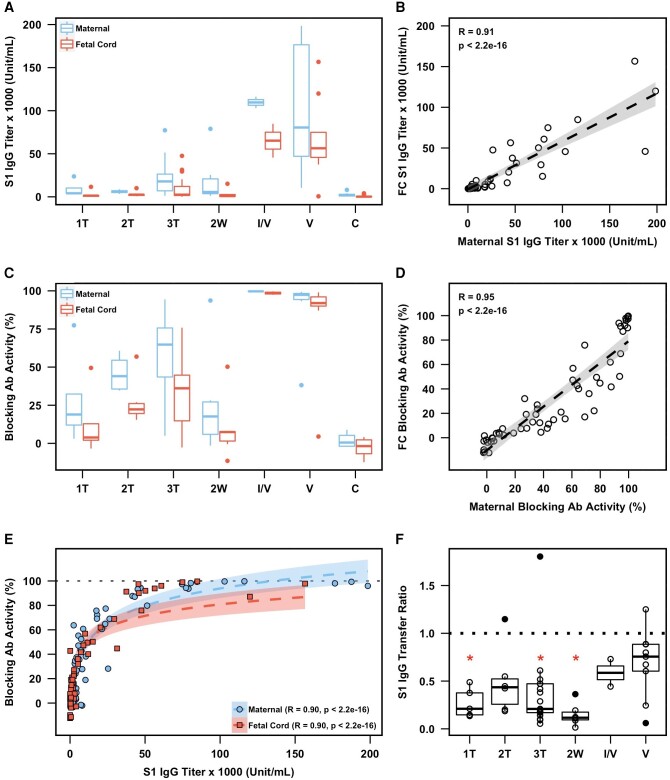
We measured anti–spike S1 IgG and IgM titers and the activity of RBD-hACE2 blocking antibody in maternal and cord plasma sampled at the time of delivery (Figure 2). Anti–spike S1 IgG titers were significantly higher in vaccinated maternal plasma samples (all within the third trimester) as compared with the infection groups (1T, P = .002; 2T, P = .0004; 3T, P = .004; 2W, P = .008; Figure 2A). Furthermore, the fetal cord plasma anti–spike S1 IgG titers from pregnant women who were vaccinated were significantly higher than those of pregnant women who were infected (1T, P = .01; 2T, P = .01; 3T, P = .0007; 2W, P = .005). We noted a comparable level (P > .05) of anti–spike S1 IgG in vaccinated pregnant women and corresponding fetal cord plasma both without prior infection (V group) and with it (I/V group). We also observed comparable levels (P > .05) of anti–spike S1 IgG in pregnant women and corresponding fetal cord plasma infected in each trimester. Importantly, there was a strong positive correlation in the titers of anti–spike S1 IgG between maternal and corresponding fetal cord plasma (R = 0.91, P < .001), suggesting vertical transmission (Figure 2B).
Figure 2.
Maternal and fetal cord blood antibody responses. A, Anti–spike S1 protein IgG titers in maternal plasma (blue) and FC plasma (red). B, Paired maternal and FC anti–spike S1 IgG titers, including best-fit linear regression. C, RBD-hACE2 blocking antibody activity in maternal plasma (blue) and FC plasma (red). D, Paired maternal and FC RBD-hACE2 blocking antibody activity, including best-fit linear regression. E, Paired anti–spike S1 IgG titers and RBD-hACE2 blocking antibody activity faceted by maternal (blue circles) and FC (red squares) plasma. Panel includes best-fit logarithmic regressions. F, Anti–spike S1 IgG transfer ratios (FC plasma to maternal plasma) of pregnant women infected in the first, second, and third trimesters (1T, 2T, 3T) and 2 weeks prior to delivery (2W), as well as infected and vaccinated (I/V) and vaccinated (V) individuals. Mann-Whitney U test (vs vaccinated group): *P < .05. A, C, F, Data are graphed by median (line) and IQR (box). B, D, E, Data include coefficient of correlation (R; dotted line), 95% CI (shading), and unadjusted P value computed by the Spearman rank correlation test. Ab, antibody; C, pregnant uninfected controls; FC, fetal cord; RBD-hACE2, receptor-binding domain–human angiotensin-converting enzyme 2.
Next, we examined the functional activities of anti–spike S1 IgG. Unlike its titer, blocking activity of anti–spike S1 IgG against RBD-hACE2 was significantly higher (P < .05) in pregnant women and paired fetal cord infected during the third trimester than in those infected during the first and second trimesters (Figure 2C), implying waning of humoral immunity to SARS-CoV-2 [13]. Consistent with the titer of anti–spike S1 IgG, RBD-hACE2 blocking antibody activity was significantly higher in vaccinated pregnant women than in the infected groups (1T, P = .002; 2T, P = .003; 3T, P = .0005; 2W, P = .002). RBD-hACE2 blocking antibody activity in fetal cord plasma from vaccinated pregnant women was also significantly higher than that from pregnant women who were infected (1T, P = .004; 2T, P = .01; 3T, P = .0005; 2W, P = .005). We observed a significant positive correlation (R = 0.95, P < .001) in the blocking activity of SARS-CoV-2–specific IgG between maternal and corresponding cord plasma samples (Figure 2D), confirming the transplacental transmission of RBD-hACE2 blocking antibody from mothers to newborns. Due to similar correlation trends in anti–spike S1 IgG titer and blocking antibody activity, we examined a potential relationship between antibody quantity and function. We found a strong correlation (R = 0.90, P < .001) between anti–spike S1 IgG titer and RBD-hACE2 blocking antibody activity in maternal and fetal cord plasma (Figure 2E), suggesting a mediating role of anti–spike S1 IgG antibodies in blocking activity.
We next examined a potential influence of type and timing of SARS-CoV-2 exposure on the vertical transmission rate of anti–spike S1 IgG. We found no significant differences among the transfer ratios of anti–spike S1 IgG (ie, fetal cord spike antibody to maternal spike antibody) in the 3 infected groups (1T, 2T, and 3T). We noted significantly higher transfer ratios in those vaccinated (all within the third trimester) as compared with those infected in the first (P = .04) and third trimesters (P = .02) and those who tested positive within 2 weeks prior to delivery (P = .01; Figure 2F). The transfer ratio of anti–spike S1 IgG in pregnant women who were infected and vaccinated (I/V group) was not significantly different when compared with vaccinated pregnant women (V group), although the sample size in this group was small (n = 2).
We observed the highest titer of spike S1–specific IgM in pregnant women infected in the third trimester, which was significantly greater than the vaccinated group (P = .014; supplementary information). However, anti–spike S1 IgM was detected at background levels in all fetal cord plasma, suggesting the absence of vertical transmission of this antibody class, as expected (Supplementary Figure 1).
Characteristics of the Cytokine/Chemokine Profile in Mothers Who Were Infected
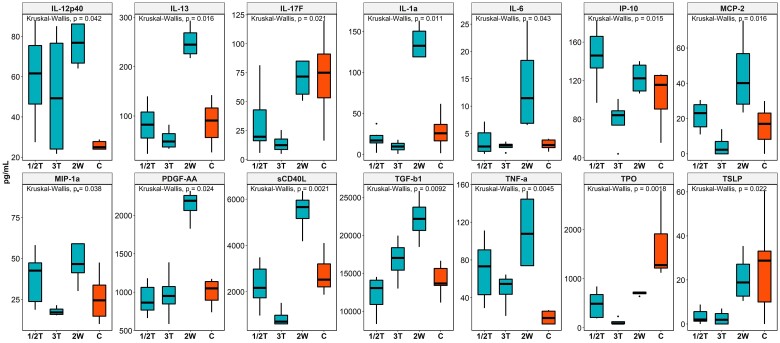
We measured plasma levels of 74 cytokines and chemokines in maternal blood samples. Of these analytes, 14 primarily inflammatory cytokines and chemokines—IL-12p40, IL-13, IL-17F, IL-1α, IL-6, IP-10, MCP-2, MIP-1α, PDGF-AA, sCD40L, TGF-β1, TNF-α, TPO, and TSLP—were upregulated in mothers infected within 2 weeks prior to delivery (2W group) as compared with uninfected pregnant controls (C group) and pregnant women infected earlier in gestation (1T and 2T groups; Figure 3). Strong positive correlations were noted among these upregulated analytes, which may reflect induction of signature cellular immune responses in mothers during pregnancy following infection with SAR-CoV-2 (Supplementary Figure 2).
Figure 3.
Plasma levels of cytokines and chemokines in mothers infected at different time points of pregnancy. Kruskal-Wallis test was used compare differences in cytokines/chemokine expression between groups. Data are graphed by median (line) and IQR (box). 1/2T, infected in the first and second trimesters; 2W, infected within 2 weeks before delivery; 3T, infected in the third trimester; C, control (pregnant women who were not infected during pregnancy).
We observed significant differences in the profiles of 8 cytokines/chemokines in mothers infected 2 weeks prior to delivery (2W group) vs immunized mothers during pregnancy (V group) (Supplementary Figure 3): IL-1α, IL-1RA, IL-6, IL-8, IL-10, IL-17F, IP-10, and MCP-2. These cytokines/chemokines are well known for their inflammatory or regulatory activities; therefore, our data suggest a greater degree of inflammation induced by infection than vaccination. These analytes—particularly, IL-17F, IP-10, MCP-2, IL-6, and IL-1RA—demonstrated strong positive correlation, reflecting their upregulations in response to infection vs vaccination (Supplementary Figure 4). These inflammatory and regulatory cytokines/chemokines were dysregulated in mothers infected within 2 weeks of delivery (2W group) as compared with mothers who were infected but recovered (Supplementary Figures 5 and 6) and nonpregnant women uninfected with SARS-CoV-2 (Supplementary Figures 7 and 8).
Differential Profiles of Cytokines and Chemokines in Maternal and Cord Plasma
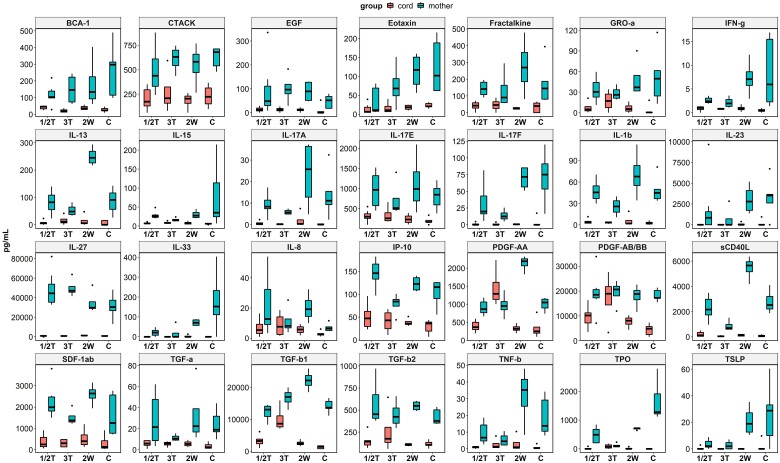
Of 74 cytokines/chemokines measured, 28 analytes were detected at elevated levels in maternal plasma but at background levels in corresponding cord blood samples (Figure 4). These included key proinflammatory cytokines/chemokines, such as IL-1β, IP-10, IFN-γ, eotaxin, PDGF-AA, PDGF-AB/BB, IL-8, IL-17A, IL-17E, and IL-17F, with strong mutual correlations (Supplementary Figure 9), suggesting a signature cellular response in pregnant women. Ten cytokines and chemokines were detected at higher concentrations in cord plasma than in corresponding maternal plasma: GM-CSF, IL-3, IL-12p40, MCP-1, RANTES, VEGF-A, I-309, SCF, TARC, and TRAIL (Supplementary Figure 10) with weak mutual correlations (Supplementary Figure 11). Three anti-inflammatory cytokines—IL-12p40, TGF-β1, and TGF-β2—were detected at significant levels in cord blood and may be immunoprotective. Other cytokines/chemokines were detected at background levels in maternal and cord plasma (data not shown).
Figure 4.
Plasma levels of cytokines and chemokines in maternal and cord blood at delivery. Wilcoxon rank sum test was used to examine differences in cytokine/chemokine expression between paired peripartum maternal and cord blood samples at delivery. Data are graphed by median (line) and IQR (box). 1/2T, infected in the first and second trimesters; 2W, infected within 2 weeks before delivery; 3T, infected in the third trimester; C, control (pregnant women who were not infected during pregnancy).
No Significant Difference in the Pattern of Cytokine/Chemokine Profiles Between Infected and Vaccinated Maternal/Cord Plasma
We compared levels of analyzed cytokines/chemokines in pregnant women and their newborns infected within 2 weeks prior to delivery and vaccinated pregnant women and their newborns. Among 15 cytokines/chemokines with dysregulated levels, similar patterns occurred between the groups: specifically, these cytokines/chemokines were detected at background levels in fetal cord plasma but at elevated levels in maternal plasma (Supplementary Figure 12). We also found weak correlations among these analytes (Supplementary Figure 13), implying that these cytokines/chemokines nonspecifically respond to either infection or vaccination.
Cytokine/Chemokine Comparisons Between Maternal Plasma and Plasma From Donors Who Recovered From SARS-CoV-2 Infection
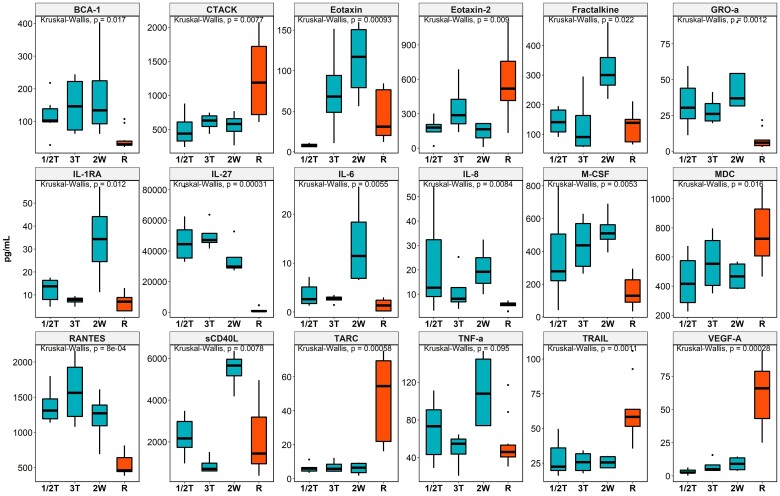
We compared levels of cytokines and chemokines between pregnant women infected at different time points during pregnancy and plasma samples in the Biobank from nonpregnant donors who had recovered from SARS-CoV-2 infection. We found 18 cytokines/chemokines significantly dysregulated in pregnant women infected within 2 weeks prior to delivery as compared with nonpregnant recovered women (Figure 5). As such, 12 cytokines and chemokines—sCD40L, eotaxin, fractalkine, IL-1RA, IL-6, IL-8, IL-27, GRO-α, M-CSF, RANTES, TNF-α, and BCA-1—were significantly higher in pregnant women who were infected than nonpregnant women who recovered. Six cytokines and chemokines—VEGF-A, MDC, CTACK, TRAIL, eotaxin 2, and TARC—were significantly lower in pregnant women who were infected than nonpregnant women who recovered. These cytokines/chemokines also showed strong mutual correlations, negative or positive, suggesting their mutual down- or upregulation in response to SARS-CoV-2 infection and recovery (Supplementary Figure 14).
Figure 5.
Comparison of cytokine/chemokine levels in pregnant women infected at different time points, including first and second trimesters (1/2T), third trimester (3T), within 2 weeks of delivery (2W), and nonpregnant recovered women (R). Kruskal-Wallis test was used to examine for differences in cytokine/chemokine expression between maternal and fetal cord blood. Data are graphed by median (line) and IQR (box).
DISCUSSION
Despite significant advances in understanding human immunity to SARS-CoV-2 infection/vaccination in a relatively short space of time, few studies have characterized the relationship between viral exposure in gestation and its immunologic impact in the mother-infant dyad during pregnancy. We therefore examined the impact of infection/vaccination on vertical transmission of SARS-CoV-2–specific antibodies and cytokines/chemokines in maternal and cord plasma. In agreement with previous observations [16, 17], we noted strong correlations in the titers of SARS-CoV-2–specific antibodies and RBD-hACE2 blocking antibody function. We also noted a trend toward higher antibody titer and better functionality in pregnant women infected in the third trimester. Results in our study confirmed the more robust vaccine-induced quantitative and functional antibody responses in maternal plasma [18–21]. Furthermore, we found antibody titer to be a strong positive predictor of blocking antibody activity. Last, we noted a significantly higher antibody transfer ratio for those vaccinated as compared with infected during the third trimester of gestation, suggesting the third trimester as optimal timing of SARS-CoV-2 vaccination.
Key proinflammatory cytokines/chemokines were detected in maternal plasma at elevated levels but at background levels in corresponding paired cord plasma: IL-1β, IL-17A, IL-17E, IL-17F IP-10, IFN-α2, PDGF-AA, and PDGF-AB/BB. PDGF-AA was significantly elevated in women who were infected with SARS-CoV-2 within 2 weeks of delivery when compared with uninfected pregnant women. Our observations were confounded by data showing that 18 cytokines/chemokines significantly increased in healthy controls who recovered from SARS-CoV-2 (Figure 5, Supplementary Figure 13). This observation may be explained by the fact that pregnancy per se can induce some degree of immune adaptation, as documented previously [9, 22, 23]. TGF isomers were not markedly different with concentrations detected in maternal plasma; however, their presence in cord blood points to a potential immunoregulatory role to offset the adverse effects of inflammation (Figure 4).
Numerous studies have documented a waning of SARS-CoV-2–specific antibody titer and its blocking activity following SARS-CoV-2 infection/vaccination in nonpregnant populations [13, 24–29]. In alignment with these data, we observed ∼2-fold lower antibody titers and blocking antibody activity in maternal and fetal cord plasma for those infected during the first trimester as compared with the third. This clear trend in waning immunity may affect protection in mother and infant, underscoring the potential importance of vaccination in the early third trimester so that the fetus can acquire adequate antibody titers transplacentally, conferring some degree of humoral protection in postnatal life. However, further work is required to assess vaccine-induced antibody responses across each trimester and determine the most beneficial gestation length for vertical transmission of functionally robust SARS-CoV-2 antibodies.
Through efficient presentation of essential epitopes to B-cell populations in the germinal center of draining lymph nodes, the generation of vaccine-induced antibodies likely promotes more robust quantitative and functional antibody responses than infection [30–32]. Although we did not examine the proportion of blocking antibody to anti–spike S1 antibody titers, we did observe higher antibody titers and better functionality for vaccinated pregnant mother-infant dyads as compared with those infected during the third trimester or those infected within 2 weeks of delivery. Antibody positivity has been shown to peak at approximately 4 weeks after the second dose [33], which may explain why vaccination or infection within 2 weeks of delivery does not allow for adequate transplacental transmission of vaccine-induced antibodies. Similarly, reduced antibody transfer to neonates of pregnant females was observed in recent or ongoing infection within 2 weeks of delivery, as compared with those who recovered from infection [10].
Interestingly, we found significantly higher transfer ratios for vaccinated mother-infant dyads when compared with those infected during the third trimester and those who tested positive within 2 weeks of delivery. Although transfer ratios were higher for mother-infant dyads who were vaccinated vs those who were infected with SARS-CoV-2 [34], results from other studies are conflicting [10, 16, 34]. For example, 1 study reported no difference in transfer ratio between infection and vaccination [16], whereas other studies noted the influence of gestational age of infection—specifically, a higher transfer ratio coincided with infection late in gestation [34]. Furthermore, Gee et al reported higher transfer ratios for maternal-infant dyads infected 22 to 221 days before delivery (ie, recovered) as compared with those infected within 2 weeks of delivery [10]. Despite similar observations between groups, the recovered group exhibited a much higher median transfer ratio than a comparable infected group (1.04 vs 0.25). Other factors might influence these differences between studies and the groups outlined in our study, including IgG subclass, placental integrity, Fcγ receptor expression on placental cells, and thymic dependency on an antigen [35]. Generally, the transfer efficiency hierarchy lists IgG1 as the most efficient subclass [36], which is preferentially produced upon vaccination as compared with infection [37]. Considering the unique glycosylation patterns of mRNA vaccine–produced antibodies, which are known to enhance their ability to bind Fcγ receptors [37], they might have enhanced ability to bind to the Fc receptor of the syncytiotrophoblast to reach the placental capillaries and eventually the fetus [38]. Future studies should consider IgG subclass when comparing transplacental antibody transfer ratios following SARS-CoV-2 infection/vaccination.
An immunoquiescent cytokine/chemokine milieu may protect the fetus from SARS-CoV-2 exposure during gestation. The presence of TGF-β and IL-10 with their well-known immunoregulatory activities at the maternal-fetal interface is important to not only support tolerance toward the haploidentical fetus but also potentially limit viral replication and inflammation during maternal infection [39–41]. Against this backdrop, a successful pregnancy outcome involves sequential, evolving immunity of placental cells, including macrophages (Hofbauer cells), through gestation, which is associated with dynamic cytokine changes that are only now being characterized. These include a proinflammatory immune profile early in gestation and an immunoregulatory anti-inflammatory predominance thereafter, ending with a proinflammatory phenotype again at the end of pregnancy. These fluctuations may be hormonally regulated. Perturbation in this dynamic from infection or a breakdown in maternal-fetal tolerance is associated with abortion, preterm labor, or transmission of viral pathogens [39, 40, 42]. Our data showed elevated levels of key proinflammatory cytokines/chemokines associated with maternal SARS-CoV-2 infection, which perhaps had little or no bearing on corresponding cord blood plasma.
Our study had a few limitations. First, the small sample sizes of some infection/vaccination groups reduced statistical power; therefore, the comparison between groups may be overestimated. Second, the study cohort has poor representation from Black and Hispanic populations, for which the prevalence of SARS-CoV-2 infection and severe disease had been well documented in the United States [43, 44]. However, these limitations reflect the difficult and challenging nature of recruiting pregnant women into a study characterizing infection and vaccination during a dynamic pandemic. Third, group designation of pregnant women who were infected was based on a positive polymerase chain reaction test result. Each pregnant woman was tested during a hospital visit or if there were suggestive symptoms of SARS-CoV-2 infection. Therefore, the numbers of individuals experiencing asymptomatic infection may have been overestimated. Fourth, these births took place between June 2020 and August 2021; hence, the impact of new SARS-CoV-2 variants was not accounted for during our study timeline. Finally, IgG subclasses should be considered for future studies assessing SARS-CoV-2 transplacental antibody transmission, as different subclasses have been implicated in protection from severe COVID-19 [45, 46].
In summary, we assessed for the transplacental transmission of anti–spike S1 antibodies and cytokines/chemokines in pregnant women after SARS-CoV-2 infection or vaccination. Our data support vertical transmission of potentially protective humoral responses against SARS-CoV-2, especially following maternal vaccination. Our data also demonstrated more efficient IgG transfer to the fetus in the third trimester, underscoring the importance of maternal vaccination against SARS-CoV-2 during this time. Furthermore, the detection of anti-inflammatory cytokines in cord blood, despite maternal SARS-CoV-2 infection, may offset the adverse outcomes of inflammation in pregnancy for the neonate.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jonathon M Monroe, Vaccine Research Group, Department of Internal Medicine.
Huy Quang Quach, Vaccine Research Group, Department of Internal Medicine.
Sohan Punia, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Elizabeth Ann L Enninga, Graduate School of Biomedical Sciences.
Yaroslav Fedyshyn, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
James H Girsch, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine; Graduate School of Biomedical Sciences.
Bohdana Fedyshyn, Division of Obstetrics, Department of Obstetrics and Gynecology.
Maureen Lemens, Division of Obstetrics, Department of Obstetrics and Gynecology.
Dawn Littlefield, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Supriya Behl, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Elise Sintim-Aboagye, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Maria C Mejia Plazas, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Satoko Yamaoka, Department of Molecular Medicine.
Hideki Ebihara, Department of Molecular Medicine.
Akhilesh Pandey, Division of Clinical Biochemistry and Immunology, Department of Laboratory Medicine and Pathology, Center for Individualized Medicine, Mayo Clinic, Rochester, Minnesota; Center for Molecular Medicine, National Institute of Mental Health and Neurosciences, Bangalore; Department of Community Medicine, Manipal Academy of Higher Education, Manipal, India.
Cristina Correia, Department of Molecular Pharmacology and Experimental Therapeutics.
Choong Yong Ung, Department of Molecular Pharmacology and Experimental Therapeutics.
Hu Li, Department of Molecular Pharmacology and Experimental Therapeutics.
Robert Vassallo, Thoracic Diseases Research Unit, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Department of Immunology, College of Medicine and Science, Mayo Clinic, Rochester, Minnesota.
Jie Sun, Thoracic Diseases Research Unit, Division of Pulmonary and Critical Care Medicine, Department of Medicine, Department of Immunology, College of Medicine and Science, Mayo Clinic, Rochester, Minnesota; Carter Immunology Center, School of Medicine, University of Virginia, Charlottesville.
Erica L Johnson, Department of Microbiology, Biochemistry, and Immunology, Morehouse School of Medicine, Atlanta, Georgia.
Janet E Olson, Biobank.
Elitza S Theel, Division of Clinical Microbiology, Department of Laboratory Medicine and Pathology.
Andrew D Badley, Division of Infectious Diseases, Department of Medicine, Mayo Clinic, Rochester, Minnesota.
Richard B Kennedy, Vaccine Research Group, Department of Internal Medicine.
Regan N Theiler, Division of Obstetrics, Department of Obstetrics and Gynecology.
Rana Chakraborty, Children Research Center, Division of Pediatric Infectious Diseases, Department of Pediatric and Adolescent Medicine.
Notes
Author contributions . H. Q. Q., J. M. M., and S. P. were involved in collecting data, reviewed and analyzed data, and wrote the manuscript. E. A. L. E. designed the experiments, was involved in collecting and processing samples, and reviewed the manuscript. Y. F., J. H. G., B. F., and S. B. collected and processed samples. M. L., D. L., and R. N. T. coordinated patient recruitment. E. S.-A. and M. C. M. P. collected and analyzed clinical data. S. Y., H. E., A. P., C. C., C. Y. U., H. L., R. V., J. S., E. L. J., J. E. O., E. S. T., A. D. B., and R. N. T. designed the experiments and reviewed and edited the manuscript. R. B. K. supervised the antibody analysis and edited the manuscript. R. C. designed the experiments, reviewed and edited the manuscript, and supervised the overall project.
Disclaimer . This research and its activities have been reviewed by the Mayo Clinic Conflict of Interest Review Board and were conducted in compliance with Mayo Clinic Conflict of Interest policies.
Financial support . This work was supported by the National Institutes of Health (1D71TW012265-01, 1U01AI131566-01, 1R01HD097843-01, and 1R21HD103498-01 to R. C.), the National Institute of Allergy and Infectious Diseases (AI110173 and AI120698 to A. D. B.), amfAR (109593 to A. D. B.), and the Mayo Clinic (HH Sheikh Khalifa Bib Zayed Al-Nahyan Professorship of Infectious Diseases to A. D. B.).
References
- 1. Lokken EM, Walker CL, Delaney S, et al. Clinical characteristics of 46 pregnant women with a severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol 2020; 223:911.e1–.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Panagiotakopoulos L, Myers TR, Gee J, et al. SARS-CoV-2 infection among hospitalized pregnant women: reasons for admission and pregnancy characteristics—eight US health care centers, March–May 30, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1355–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Villar J, Ariff S, Gunier RB, et al. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021; 175:817–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Woodworth KR, Olsen EO, Neelam V, et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy—SET-NET, 16 jurisdictions, March 29–October 14, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1635–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zambrano LD, Ellington S, Strid P, et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status—United States, January 22–October 3, 2020. MMWR Morb Mortal Wkly Rep 2020; 69:1641–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Salvatore CM, Han JY, Acker KP, et al. Neonatal management and outcomes during the COVID-19 pandemic: an observation cohort study. Lancet Child Adolesc Health 2020; 4:721–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dong L, Tian J, He S, et al. Possible vertical transmission of SARS-CoV-2 from an infected mother to her newborn. JAMA 2020; 323:1846–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fenizia C, Biasin M, Cetin I, et al. Analysis of SARS-CoV-2 vertical transmission during pregnancy. Nat Commun 2020; 11:5128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun 2020; 11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gee S, Chandiramani M, Seow J, et al. The legacy of maternal SARS-CoV-2 infection on the immunology of the neonate. Nat Immunol 2021; 22:1490–502. [DOI] [PubMed] [Google Scholar]
- 11. Coban SC, Temel F, Duman P, et al. Prevalence of protective measles virus antibody levels in umbilical cord blood samples and sera of mothers and transplacental transport ratio in Turkey. Jpn J Infect Dis 2019; 72:185–92. [DOI] [PubMed] [Google Scholar]
- 12. Bloise S, Marcellino A, Testa A, et al. Serum IgG levels in children 6 months after SARS-CoV-2 infection and comparison with adults. Eur J Pediatr 2021; 180:3335–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Haralambieva IH, Monroe JM, Ovsyannikova IG, Grill DE, Poland GA, Kennedy RB. Distinct homologous and variant-specific memory B-cell and antibody response over time after SARS-CoV-2 mRNA vaccination. J Infect Dis 2022; 226:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. More K, Aiyer S, Goti A, et al. Multisystem inflammatory syndrome in neonates (MIS-N) associated with SARS-CoV2 infection: a case series. Eur J Pediatr 2022; 181:1883–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pawar R, Gavade V, Patil N, et al. Neonatal multisystem inflammatory syndrome (MIS-N) associated with prenatal maternal SARS-CoV-2: a case series. Children (Basel) 2021; 8:572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beharier O, Plitman Mayo R, Raz T, et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J Clin Invest 2021; 131:e154834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kugelman N, Nahshon C, Shaked-Mishan P, et al. Third trimester messenger RNA COVID-19 booster vaccination upsurge maternal and neonatal SARS-CoV-2 immunoglobulin G antibody levels at birth. Eur J Obstet Gynecol Reprod Biol 2022; 274:148–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Assis R, Jain A, Nakajima R, et al. Distinct SARS-CoV-2 antibody reactivity patterns elicited by natural infection and mRNA vaccination. NPJ Vaccines 2021; 6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Israel A, Shenhar Y, Green I, et al. Large-scale study of antibody titer decay following BNT162b2 mRNA vaccine or SARS-CoV-2 infection. Vaccines 2021; 10:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2—preliminary report. N Engl J Med 2020; 383:1920–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Walsh EE, Frenck Jr RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N Engl J Med 2020; 383:2439–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kareva I. Immune suppression in pregnancy and cancer: parallels and insights. Transl Oncol 2020; 13:100759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mor G, Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol 2010; 63:425–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grigoryan L, Pulendran B. The immunology of SARS-CoV-2 infections and vaccines. Semin Immunol 2020; 50:101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020; 383:1724–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dan JM, Mateus J, Kato Y, et al. Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 2021; 371:eabf4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang H, Yuan Y, Xiao M, et al. Dynamics of the SARS-CoV-2 antibody response up to 10 months after infection. Cell Mol Immunol 2021; 18:1832–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lumley SF, Wei J, O'Donnell D, et al. The duration, dynamics, and determinants of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) antibody responses in individual healthcare workers. Clin Infect Dis 2021; 73:e699–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wei J, Matthews PC, Stoesser N, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun 2021; 12:6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turner JS, O’Halloran JA, Kalaidina E, et al. SARS-CoV-2 mRNA vaccines induce persistent human germinal centre responses. Nature 2021; 596:109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yu Y, Esposito D, Kang Z, et al. mRNA vaccine-induced antibodies more effective than natural immunity in neutralizing SARS-CoV-2 and its high affinity variants. Sci Rep 2022; 12:2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Halasa NB, Olson SM, Staat MA, et al. Maternal vaccination and risk of hospitalization for COVID-19 among infants. N Engl J Med 2022; 387:109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ward H, Whitaker M, Flower B, et al. Population antibody responses following COVID-19 vaccination in 212,102 individuals. Nat Commun 2022; 13:907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Otero S, Miller ES, Sunderraj A, et al. Maternal antibody response and transplacental transfer following severe SARS-CoV-2 infection or vaccination in pregnancy. Clin Infect Dis 2023; 76:220–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012; 2012:985646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Clements T, Rice TF, Vamvakas G, et al. Update on transplacental transfer of IgG subclasses: impact of maternal and fetal factors. Front Immunol 2020; 11:1920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Farkash I, Feferman T, Cohen-Saban N, et al. Anti-SARS-CoV-2 antibodies elicited by COVID-19 mRNA vaccine exhibit a unique glycosylation pattern. Cell Rep 2021; 37:110114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Leach JL, Sedmak DD, Osborne JM, Rahill B, Lairmore MD, Anderson CL. Isolation from human placenta of the IgG transporter, FcRn, and localization to the syncytiotrophoblast: implications for maternal-fetal antibody transport. J Immunol 1996; 157:3317–22. [PubMed] [Google Scholar]
- 39. Girsch JH, Mejia Plazas MC, Olivier A, et al. Host-viral interactions at the maternal-fetal interface: what we know and what we need to know. Front Virol 2022; 2:833106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Johnson EL, Chakraborty R. Placental Hofbauer cells limit HIV-1 replication and potentially offset mother to child transmission (MTCT) by induction of immunoregulatory cytokines. Retrovirology 2012; 9:101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yockey LJ, Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity 2018; 49:397–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Swieboda D, Johnson EL, Beaver J, et al. Baby's first macrophage: temporal regulation of Hofbauer cell phenotype influences ligand-mediated innate immune responses across gestation. J Immunol 2020; 204:2380–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Elkafrawi D, Sisti G, Mercado F, et al. Hispanic race is a risk factor for COVID-19 during pregnancy: data from an urban New York City hospital. J Obstet Gynaecol 2022; 42:1054–7. [DOI] [PubMed] [Google Scholar]
- 44. Pressman A, Lockhart SH, Wilcox J, et al. COVID-19 in pregnancy by race and ethnicity: implications for development of a vaccination strategy. Womens Health (Lond) 2021; 17:17455065211063300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Korobova ZR, Zueva EV, Arsentieva NA, et al. Changes in anti-SARS-CoV-2 IgG subclasses over time and in association with disease severity. Viruses 2022; 14:941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Atyeo CG, Shook LL, Brigida S, et al. Maternal immune response and placental antibody transfer after COVID-19 vaccination across trimester and platforms. Nat Commun 2022; 13:3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.