Abstract
Mutations in CDKN1C, encoding p57KIP2, a canonical cell cycle inhibitor, underlie multiple pediatric endocrine syndromes. Despite this central role in disease, little is known about the structure and function of p57KIP2 in the human pancreatic beta cell. Since p57KIP2 is predominantly nuclear in human beta cells, we hypothesized that disease-causing mutations in its nuclear localization sequence (NLS) may correlate with abnormal phenotypes. We prepared RIP1 insulin promoter-driven adenoviruses encoding deletions of multiple disease-associated but unexplored regions of p57KIP2 and performed a comprehensive structure-function analysis of CDKN1C/p57KIP2. Real-time polymerase chain reaction and immunoblot analyses confirmed p57KIP2 overexpression, construct size, and beta cell specificity. By immunocytochemistry, wild-type (WT) p57KIP2 displayed nuclear localization. In contrast, deletion of a putative NLS at amino acids 278–281 failed to access the nucleus. Unexpectedly, we identified a second downstream NLS at amino acids 312–316. Further analysis showed that each individual NLS is required for nuclear localization, but neither alone is sufficient. In summary, p57KIP2 contains a classical bipartite NLS characterized by 2 clusters of positively charged amino acids separated by a proline-rich linker region. Variants in the sequences encoding these 2 NLS sequences account for functional p57KIP2 loss and beta cell expansion seen in human disease.
Keywords: beta cell, diabetes, Beckwith-Wiedemann syndrome
Structural and functional defects in the CDKN1C gene, located on chromosome 11p15.5, and its product p57KIP2, are central to rare but important pediatric endocrine syndromes. For example, Beckwith-Wiedemann syndrome (BWS) is a well-described multiorgan overgrowth disorder characterized by macrosomia, macroglossia, abdominal wall defects, and hemihypertrophy (1, 2). Affected patients may also present with facial anomalies, embryonal tumors, and/or neonatal hypoglycemia, the latter resulting from an expansion of pancreatic beta cell mass and hyperinsulinism (3). The most common molecular cause of BWS is an epigenetic imprinting error in the maternal 11p15.5 region, exemplified by either a gain of methylation in the imprinting control region-1 (ICR1) (described later) or loss of methylation in the imprinting control region-2 (ICR2), leading to loss of expression of p57KIP2 mRNA and p57KIP2 protein (1). Additional cases of BWS have been attributed to chromosomal rearrangements (including microdeletions, translocations, inversions, or duplications), paternal uniparental disomy for the 11p15.5 region, or inactivating point mutations in CDKN1C (1, 3-6). Interestingly, some individuals with BWS caused by CDKN1C mutations have hypoglycemia as a component of their presenting phenotype (3, 7).
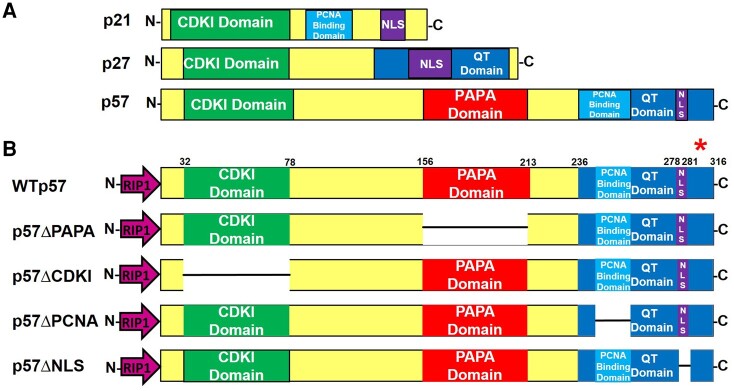
The human CDKN1C cDNA was cloned by Matsuoka and Elledge in 1995 using a mouse Cdkn1c DNA probe to screen a human embryonic cell cDNA library (2, 8). A cDNA clone encoding a 316 amino acid protein was identified,and named “p57KIP2” based on its apparent molecular weight on immunoblots and its homology with 2 previously identified CIP/KIP cell cycle inhibitors, p21CIP1 (CDKN1A) and p27KIP1 (CDKN1B) (Fig. 1A). Human p57KIP2 contains conserved amino- and carboxy-termini, comparable to mouse Cdkn1c. Surprisingly, however, the proline-rich and acidic regions in mouse Cdkn1c are replaced in humans with a domain of alternating proline-alanine repeats, termed the PAPA repeat domain (2). Thus, unlike p21CIP1 and p27KIP1, which are highly conserved between mouse and humans, p57KIP2 is uniquely human. Using fluorescent in situ hybridization, Matsuoko et al showed that the CDKN1C gene localizes to the telomeric end of chromosome 11 at 11p15.5, a well-described imprinted region. It contains 2 imprinting control regions: ICR1 and ICR2. ICR1 controls expression of the long noncoding RNA H19 and the insulin-like growth factor 2 (IGF2) gene and is methylated on the paternal allele. In a comparable manner, ICR2 controls expression of the KCNQ1and CDKN1C genes and is methylated on the maternal allele (9-11).
Figure 1.
Protein structures of the CIP-KIP family of cell cycle inhibitors, p21CIP1, p27KIP1, and p57KIP2. (A) Comparison of p21CIP1, p27KIP1, and p57KIP2 illustrates their homologous features and emphasizes that p57KIP2 is approximately twice the size of the others and contains additional presumably different functional domains. (B) Protein maps of the p57KIP2 cDNA mutant constructs expressed in adenoviral constructs: Ad.p57WT, Ad.p57ΔPAPA, Ad.p57ΔCDKI, Ad.p57ΔPCNA, and Ad.p57ΔNLS. Each adenoviral construct was driven by the RIP1 promoter to provide beta cell specificity. The red asterisk above the p57KIP2 carboxy-terminus illustrates antibody epitope used for immunoblots and immunocytochemistry. The numbers above the constructs indicate amino acid locations. Abbreviations: CDKI, cyclin-dependent kinase inhibitory domain; NLS, nuclear localization sequence or signal; PCNA, proliferating cell nuclear antigen domain; QT, a region named by Matsuoka because of its repetitive glutamines and a threonine in the mouse sequence (2).
The functions of the unique and human-specific domains of p57KIP2 (Fig. 1A and 1B) are remarkably understudied. This in part reflects the facts that, unlike p21CIP1 and p27KIP1, for which mouse and human sequences are highly homologous, mouse and human p57KIP2 sequences are far less homologous. As an example, the large and human-unique PAPA domain does not exist in rodents and therefore cannot be inferred from mouse studies. Moreover, while human p21CIP1 and p27KIP1 are widely expressed in adult human tissues and are well-described cell cycle inhibitors and tumor suppressor proteins (12, 13), p57KIP2 is expressed principally in humans in embryonic tissues and placenta but is not expressed in most adult human cell types (6, 14-16). Finally, while CDKN1C/p57KIP2 is expressed in ∼40% of human pancreatic beta cells and its functional loss is clearly implicated in human endocrine syndromes, rodent beta cells do not express cdkn1c/p57KIP2 (17). Thus, if one wishes to explore the biology of CDKN1C/p57KIP2 in the beta cell, one must study human beta cells.
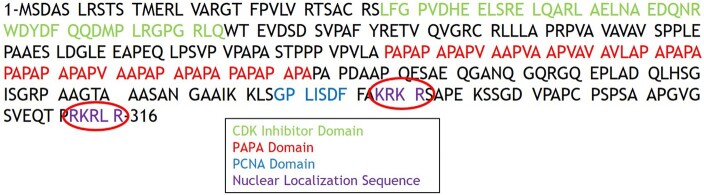
Another conundrum involves the putative NLS domain in CDKN1C/p57KIP2. Nuclear proteins translate into the nucleus via the nuclear pore complex, which requires interaction of a NLS in a cytoplasmic protein (18). Classical NLS sequences contain either a single cluster of positively charged amino acids (a monopartite NLS) or 2 clusters of positively charged amino acids, separated by a linker region of variable amino acid length (a bipartite NLS) (18). While both p21CIP1 and p27KIP1 contain putative monopartite NLS domains (Fig. 1), requirement and definition of the presumed functional NLS in p57KIP2 is unknown. The CDKN1C/p57KIP2 sequences suggested to Matsuoka et al in 1995 that the KRKR amino acid sequence at residues 278–281 was likely a bona fide NLS (Fig. 2), but this has never been confirmed experimentally. Thus, while p57KIP2 is abundant in approximately 40% of human beta cell nuclei (19-21), the mechanism by which it accesses the beta cell nucleus remains enigmatic.
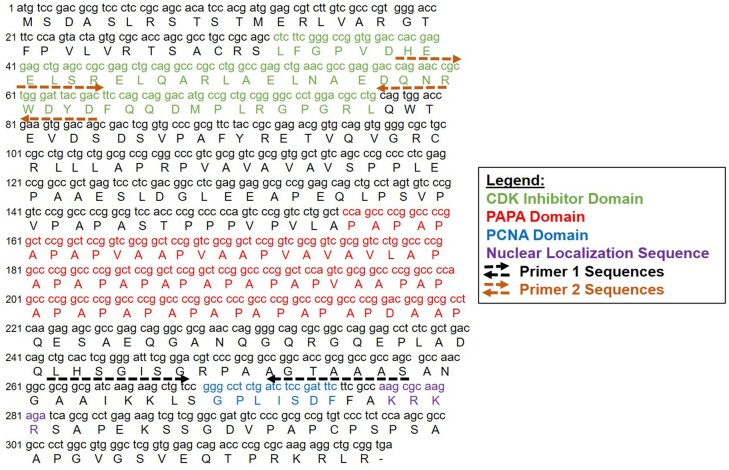
Figure 2.
Nucleotide sequence of p57KIP2 highlighting its distinct domains. The primer sequences in Fig. 3A and 3B are depicted. Note that the primer 1 sequences overlap with the proliferating cell nuclear antigen domain and the primer 2 sequences overlap with the CDKI domain, interfering with real-time polymerase chain reaction quantification. Abbreviations: CDKI, cyclin-dependent kinase inhibitory domain.
Accordingly, the initial goal of this study was to include or exclude the possibility that the KRKR amino acid sequence at residues 278–281 was a bona fide NLS. In 2021, while this study was in progress, Stampone et al, using HEK293 and HepG2 cells, reported the presence of a second potential NLS in p57KIP2 at amino acids 312–316 (Figs. 1 and 2). Here, we report that the Matsuoka 278–281 putative NLS is indeed required for nuclear entry of p57KIP2 into human beta cells. We also show that the 312–316 Stampone sequence is also required for p57KIP2 nuclear entry into human beta cells. Unexpectedly, we report that while the 2 sequences, 278–281 and 312–316, are each individually necessary for nuclear entry of p57KIP2, neither individually is sufficient to permit nuclear entry of p57KIP2 or heterologous proteins: both sequences must be present and intact for full nuclear transport capability. Notably, we find that this p57KIP2 bipartite NLS is also fully capable of transporting a heterologous cargo, GFP, into the nuclear compartment of human beta cells. Thus, we report for the first time that nuclear import of p57KIP2 into human beta cells requires a canonical, fully functional, and intact bipartite NLS.
Research Design and Methods
Human Islets
Human islets from 12 donors were obtained through the Naional Institutes of Health-supported Integrated Islet Distribution Program (https://iidp.coh.org), and Prodo Laboratories
(Aliso Viejo, CA). Islets were harvested from pancreata of deceased organ donors without identifying information and with informed consent and Institutional Review Board approval at the isolating centers. Donors ranged in age from 28 to 68 years old (mean ± SEM, 46.5 ± 3.1). One was female, and 11 were male. Body mass index ranged from 23.0 to 37.5 kg/m2 (mean ± SEM, 28.6 ± 1.2). Purity ranged from 80% to 95%, and viability ranged from 95% to 97% (mean ± SEM, 95 ± 0.2). Causes of death were anoxic event (6), head trauma (4), and stroke (2).
Cell Lines
Three cell lines were used for these studies: human embryonic kidney cells (HEK-293A) (ATCC Cat# CRL-1573, RRID:CVCL_0045), human hepatoma cells (HepG2) (ATCC Cat # HB-8065, RRID:CVCL_0027), and INS1 832/13 cells rat insulinoma cells, which were a generous gift from Dr. Christopher Newgard at Duke University (22). HEK-293A cells were cultured in DMEM supplemented with 10% FBS, 1% penicillin-streptomycin, and 1 × nonessential amino acids. HepG2 cells were cultured in Eagle’s minimum essential medium supplemented with 10% FBS and 1% penicillin-streptomycin. INS1 cells were cultured in RPMI 1640 medium containing 10% FBS, 1% penicillin-streptomycin, 2 mM L glutamine, 10 mM HEPES, 1 mM Na Pyruvate, and 0.05 mM beta-mercaptoethanol.
Antibody
Antibodies used were as follows: p57KIP2 (2557, Cell Signaling, RRID:AB_2291591), glyceraldehyde 3 phosphate dehydrogenase (FL-335, 25778, Santa Cruz, RRID:AB_10167668), C-peptide (GN-ID4, Developmental Studies Hybridoma Bank, RRID:AB_2255626), glucagon (ab10988, abcam, RRID:AB_297642).
Human Islet Dispersion
On arrival, islets were centrifuged at 1000 rpm for 5 minutes, washed twice in PBS, resuspended in 1 mL Accutase (Corning 25-058-Cl), and incubated for 10 minutes at 37 °C. During this digestion, the islets were dispersed by gentle pipetting every 5 minutes. Complete RPMI medium containing 5.5 mM glucose and 1% penicillin/streptomycin with 10% FBS was added to stop the digestion. The cells were then centrifuged for 5 minutes at 1000 rpm, the supernatants removed, and the pellet resuspended in complete RPMI medium described earlier.
Plasmid Construction
peGFP C1 plasmid DNA (Clontech # V012024, San Jose, CA) was used as the backbone for the plasmid constructs. WTp57, p57ΔNLS1, p57ΔNLS2, and p57ΔNLS1 + 2 cDNAs were generated by polymerase chain reaction (PCR) mutagenesis using the primers and DNA templates described in Table 1. PCR products were digested with EcoRI and BamHI and then ligated with peGFP C1 previously digested with the same restriction enzymes.
Table 1.
Primer sequences and DNA templates used in polymerase chain reaction mutagenesis to generate plasmid constructs
| Construct | Forward Primer | Reverse Primer | cDNA Template |
|---|---|---|---|
| p57WT | TACTACGAATTCAATGTCCGACGCGTCCCTCCGCA (EcoRI) | TACTACGGATCCTCACCGCAGCCTCTTGCGC (BamHI) | WTp57 |
| p57ΔNLS1 | TACTACGAATTCAATGTCCGACGCGTCCCTCCGCA (EcoRI) | TACTACGGATCCTCACCGCAGCCTCTTGCGC (BamHI) | p57ΔNLS1 mutant |
| p57ΔNLS2 | TACTACGAATTCAATGTCCGACGCGTCCCTCCGCA (EcoRI) | TACTACGGATCCTCACGGGGTCTGCTCCACCGA (BamHI) | WTp57 |
| p57ΔNLS1 + 2 | TACTACGAATTCAATGTCCGACGCGTCCCTCCGCA (EcoRI) | TACTACGGATCCTCACGGGGTCTGCTCCACCGA (BamHI) | p57ΔNLS1 mutant |
Transient Transfection
Transfection was performed with Lipofectamine 3000 (Invitrogen, Waltham, MA) following the manufacturer's instructions. HEK-293A cells were seeded into a clear thin-walled 96-well plate. Plasmids peGFPWTp57, peGFPp57ΔNLS1, peGFPp57ΔNLS2, and peGFPp57ΔNLS1 + 2 were transfected into HEK-293A cells on the same day. After 48 hours, live cell confocal microscopy (Leica SP5 DM) was used to track p57KIP2 localization.
Adenovirus Construction
Adenoviruses were prepared using the pAd/PL-DEST Gateway recombination system (Life Technologies, Carlsbad, CA), cloning WT and mutant p57KIP2 cDNA constructs driven by the RIP1 promoter into a modified pENTR vector (Fig. 1B) as detailed previously (21). Briefly, the RIP1-mini cytomegalovirus (CMV) promoter includes 177 bases of the hCMV IE-1 promoter ClaI-SpeI fragment ligated to 438 bases of the RIP1 promoter. The beta cell fraction has been confirmed to be >92% pure by immunolabeling of sorted cells with insulin, by quantitative real-time PCR (RT-PCR) and by RNaseq (23). pENTR.p57ΔPAPA, pENTR.p57ΔCDKI, pENTR.p57ΔPCNA, and pENTR.p57ΔNLS were prepared using site-directed mutagenesis. Corresponding primers are shown in Table 2.
Table 2.
Primer sequences used during site-directed mutagenesis for adenovirus construction
| Construct | Forward Primer | Reverse Primer |
|---|---|---|
| pENTR.p57ΔPAPA | CCTCAAGAGAGC GCCGAGCAGGGC |
GACCGGGACACT AGGCAGCTGCTC |
| pENTR.p57ΔCDKI | CAGTGGACCGAA GTGGACAGC |
GCTGCGGCAGGC GCTGGTGCGCACTAG |
| pENTR.p57ΔPCNA | TTCGCCAAGCGC AAGAGATCA |
GGACAGCTTCTT GATCGCCGC |
| pENTR.p57ΔNLS | TCAGCGCCTGAG AAGTCGTCGGGC |
GGCGAAGAAATC GGAGATCAGAGG |
To create the pENTR.GFPp57WT and mutant constructs (pENTR.GFPp57ΔNLS1, pENTR.GFPp57ΔNLS2, pENTR.GFPp57ΔNLS1 + 2) the peGFP plasmid constructs described earlier were digested with NheI and DraIII, and DNA was then purified. T4 DNA polymerase was used to blunt both ends and ligated with a modified pENTR.RIP promoter gateway vector. All of the gateway vectors were recombined with the pAd/PL-DEST Gateway recombination system. Details of the CMV-promoter driven Ad.shp57 virus have been reported previously (21). Adenoviruses were packaged and produced in HEK-293A cells and then purified on a PD-10 column (17085101, GE Healthcare). Titers (PFU) were determined by plaque assay.
Viral Transduction
Dispersed human islets on coverslips were transduced with either experimental (Ad.p57WT, Ad.p57ΔPAPA, Ad.p57ΔCDKI, Ad.p57ΔPCNA, Ad.p57ΔNLS, Ad.p57sH, Ad.p57ΔNLS1, Ad.p57ΔNLS2, Ad.p57ΔNLS1 + 2) or control adenoviruses (Ad.Cre or Ad.LacZ) at a multiplicity of infection of 200 in serum-free medium for 2 hours. Transduction was stopped by adding complete medium containing 10% FBS, and cells were cultured for 48 to 96 hours.
Immunocytochemistry
Dispersed islet cells on coverslips were fixed in fresh 4% paraformaldehyde for 10 minutes at room temperature, washed with PBS, and incubated in blocking buffer (1% BSA, 0.5% Triton, and 5% normal goat serum in PBS) for 1 hour at room temperature. Cells on coverslips were incubated with primary antibody for 2 hours at room temperature in blocking buffer. Secondary antibodies were added for 1 hour at room temperature in blocking buffer. Primary antibodies were p57KIP2 (2557, Cell Signaling, RRID:AB_2291591), C-peptide (GN-ID4, Developmental Studies Hybridoma Bank, RRID:AB_2255626), and glucagon (ab10988, abcam, RRID:AB_297642). Labeled cells were visualized using laser confocal microscopy (Leica SP5 DM) Results shown are representative of 3 to 6 separate human islet preparations.
Immunoblotting
Islet, HepG2, and INS1 cell extracts (20–50 μg) were resolved using 10% SDS-PAGE and transferred to Immobilon-P membranes (Millipore). Primary antibodies were p57KIP2 (2557, Cell Signaling, RRID:AB_2291591) and glyceraldehyde 3 phosphate dehydrogenase (FL-335, 25778, Santa Cruz, RRID:AB_10167668).
Gene Expression
RNA was purified from dispersed islets using the Qiagen RNeasy Micro Kit (Qiagen, Germantown, MD) according to the manufacturer's instructions. cDNA was prepared using the SuperScript IV VILO (Life Technologies). Gene expression in dispersed islets was analyzed by RT-PCR using the Bio Rad SYBRGreen system (BioRad, Hercules, CA) on an ABI 7500 System machine (Life Technologies). Relative quantification of gene expression was analyzed by the comparative cycle threshold method with cyclophilin A as the reference. The 2 primer pair sequences for RT-PCR used for CDKN1C are shown in Table 3 and Fig. 2.
Table 3.
Primer sequences used for real-time polymerase chain reaction of CDKN1c
| Primer Name | Sequence |
|---|---|
| Primer 1 forward | GCGGCGATCAAGAAGCTGT |
| Primer 1 reverse | GCTTGGCGAAGAAATCGGAGA |
| Primer 2 forward | CAGAACCGCTGGGATTACGAC |
| Primer 2 reverse | CTGTCCACTTCGGTCCACTG |
Statistical Analysis
Statistics were performed using Student's paired, 2-tailed T-test. P values less than 0.05 were considered to be significant.
Results
Expression of Wild-Type and Mutant CDKN1C Constructs In Human Islets
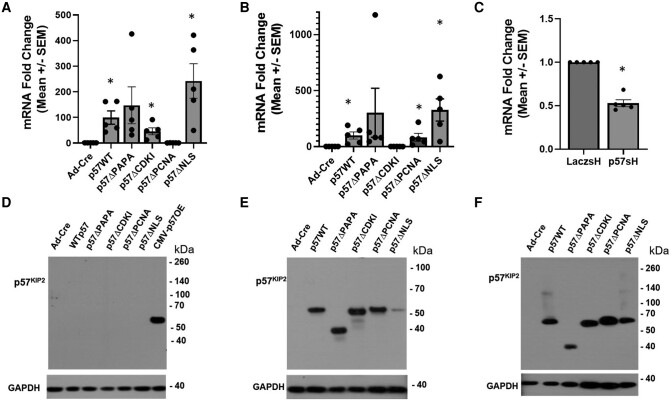
Dispersed human islets were transduced with either control adenoviruses (AdCre or Laczsh) or adenoviruses expressing the p57KIP2 WT or mutant constructs: Ad.p57WT, Ad.p57ΔPAPA, Ad.p57ΔCDKI, Ad.p57ΔPCNA, Ad.p57ΔNLS (Fig. 1B), or Ad.p57sh for 96 hours. Gene expression analysis by RT-PCR demonstrated that each p57KIP2 construct was expressed in human islets at expected levels (Fig. 3A and 3B). Note that the CDKN1C primers (primer pair 1) used in Fig. 3A overlapped with the deleted proliferating cell nuclear antigen (PCNA) region (Fig. 2) and interfered with measurement of the Ad.p57ΔPCNA construct by RT-PCR. Thus, in order to quantify expression of the PCNA mutant, a second primer pair was designed (primer pair 2) (Fig. 2) remote from the PCNA region but close to, overlapping with—and interfering with—assessment of the CDKI domain. Using this second primer pair, expression of the Ad.p57ΔPCNA in human islets was readily detected, but detection of the Ad.p57ΔCDKI construct was lost (Fig. 3B). Taken together, Fig. 3A and 3B demonstrate effective expression of all 5 CDKN1C constructs in human islets. Finally, transduction of human islets with Ad.p57sh construct demonstrated an approximate 50% reduction in CDKN1C expression as compared to control Ad.Laczsh-transduced islets, confirming that this virus effectively attenuates CDKN1C gene expression (Fig. 3C).
Figure 3.
Determination of mRNA and protein expression resulting from RIP1-CDKN1C adenoviral constructs in human islets. (A) Real-time polymerase chain reaction or CDKN1C RNA expression using primer pair 1 in Fig. 2. CDKN1C was overexpressed in human islets transfected with mutant adenoviruses (n = 5). Primer pair 1 overlapped with PCNA domain and interfered with quantitation of the PCNA mutant. (B) The same as panel A except primer pair 2, which avoided the PCNA region but overlapped with CDKI domain and thus interfered quantitation of the CDKI region. (C) Reduced expression of CDKN1C in human islets transfected with Ad.p57sh adenovirus as compared to control islets transduced with a LacZ short hairpin RNA control adenovirus. (D) Immunoblots of HepG2 hepatoma cells transduced with the constructs indicated, all but the last driven by the RIP1 promoters, with the final driven by the cytomegalovirus promoter. p57KIP2 was only observed in the last lane and was absent from the others, supporting the beta cell-specificity of the RIP1 promoter for subsequent studies. Note that p57KIP2 appears at the expected Mr. (E, F) Overexpression of the same adenoviral constructs as in (D) in the Ins1 rat insulinoma line and in human islets, driven by the RIP1 promotor. All of the constructs are expressed abundantly and generate a p57KIP2 protein of the anticipated Mr. Glyceraldehyde 3 phosphate dehydrogenase used as loading control. Immunoblots shown are representative of 3 islet or cell line preps. All quantitative polymerase chain reaction experiments were performed in 5 different human islet preparations. All immunoblots were performed in at least 3 different human islet preparations. Bars indicate mean ± SEM; * = P < .05 vs control. Abbreviations: CDKI, cyclin-dependent kinase inhibitory domain; Mr, molecular mass; PCNA, proliferating cell nuclear antigen domain.
Confirmation of Cell Type-Specific Expression of Constructs at the Protein Level
In order to selectively express their p57KIP2 cargoes in beta cells and to avoid expression in other islet cell types, all of the constructs shown in Figs. 1B, 3A, and 3B employ a RIP1-MiniCMV promoter (21). To assess tissue specificity of the RIP1 promoter, we explored expression of the adenoviruses in HepG2 human liver-derived cells. As expected, no detectable expression was observed (Fig. 3D). In contrast, the same Ad.p57WT construct driven by the generalized CMV promoter was easily detected in HepG2 cells at the expected molecular weight (MW) (Fig. 3D). In contrast to the failure of p57KIP2 expression in immunoblots in HepG2 cells, all 5 RIP1-driven CDKN1C constructs effectively expressed their respective p57KIP2 mutant constructs in both rat INS1 insulinoma cells and human islet extracts (Fig. 3E and 3F). Note that all were readily and equally detected using a p57KIP2 antibody directed to the C-terminus of p57KIP2 denoted by the red asterisk in Fig. 1B. These results confirm the beta cell specificity and efficacy of the RIP1 promoter construct employed for INS1 cells and human beta cells.
Figure 3E and 3F make 2 additional points. First, they demonstrate the detection of WT p57KIP2 at its expected MW of 57 kDa in INS1 cells and human islets transfected with Ad.p57WT. This MW is the same as observed by Matsuoka and Elledge in their original 1995 CDKN1C cloning work (2) and for which they assigned the name “p57.” Surprisingly, the predicted MW of p57KIP2, based on its 316 amino acids, is only 32 177 kDa, making the point that “p57” migrates anomalously on SDS-PAGE, perhaps reflecting unusual charge or 3D structural features that impede its migration. Second, Fig. 3E and 3F reveal that Ad.p57ΔCDKI, Ad.p57ΔPCNA, and Ad.p57ΔNLS constructs generate proteins that migrate close to the MW of the WT Ad.p57WT product. In contrast, the Ad.p57ΔPAPA construct generates a product with an apparent MW of ∼38 000, confirming effective deletion of the PAPA domain. Interestingly, the Ad.p57ΔPAPA mutant product contains 35 fewer amino acids (281 amino acids) than the Ad.p57WT product (316 amino acids) and thus should have an MW of approximately 29 000 kDa, as compared to its apparent MW of ∼38 000 kDa. This suggests that domains other than the PAPA domain contribute to the anomalously slow migration of WT p57KIP2 on SDS-PAGE.
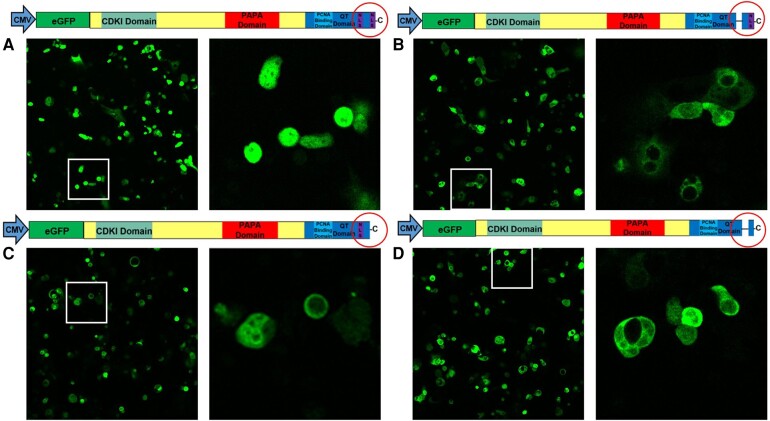
Deletion of the Putative NLS at KRKR 278–281 Sequence Prevents Nuclear Access in Human Beta Cells
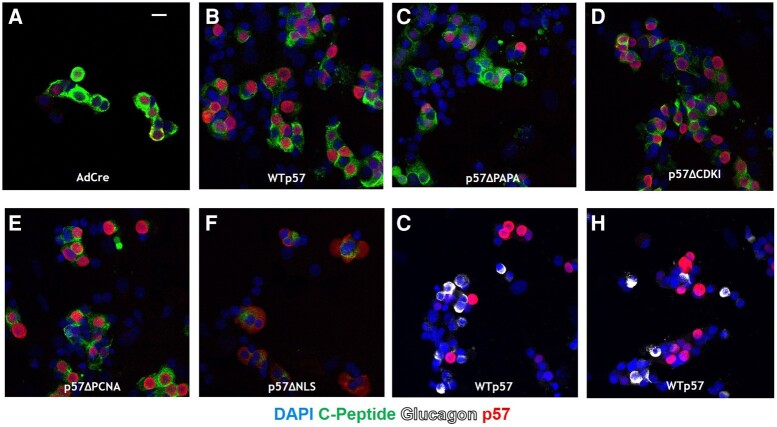
While predicted by Matsuoka et al to serve as an NLS for p57KIP2, the KRKR 278–281 sequence has never been confirmed to be functionally important for nuclear access in any cell type, including human beta cells. Accordingly, we used immunocytochemistry and laser confocal microscopy to visualize subcellular localization of RIP1-driven CDKN1C deletion mutant constructs in human beta cells. As reported previously (19-21), Fig. 4A demonstrates that p57KIP2 is present in approximately half of normal human beta cells transduced with a control (Ad.Cre) adenovirus. Transduction of human islets with Ad.p57WT, Ad.p57ΔPAPA, Ad.p57ΔCDKI, and Ad.p57ΔPCNA mutants leads to overexpression of p57KIP2 products, with clear nuclear localization in human beta cells (Fig. 4B–E). (Note that laser intensity is greater in Fig. 4A as compared the other panels in Fig. 4: equivalent laser intensity in Fig. 4B–F rendered the images too bright to visualize p57KIP2 cellular detail). In striking contrast to the other constructs, in human islets transduced with Ad.p57ΔNLS in which amino acids 278–281 are deleted, p57KIP2 is unable to translocate into the nucleus and remains strongly cytoplasmic (Fig. 4F). This documents that amino acids 278–281 are indeed required for nuclear access by p57KIP2.
Figure 4.
Subcellular localization of p57KIP2 in human islets transfected with Ad.p57WT, ad.p57ΔPAPA, Ad.p57ΔCDKI, Ad.p57ΔPCNA, and Ad.p57ΔNLS. (A) Dispersed normal human islets transduced with a control adenovirus expressing Cre recombinase display well delineated nuclear p57KIP2. (B-E) Wild-type p57KIP2 and all of the mutants shown localize to the nucleus of human beta cells. (F) In contrast, the putative ΔNLS mutant lacking amino acids 278–281 is unable to translocate into the nucleus, indicating that amino acids 278–281 are required for nuclear import. Note that laser intensity is reduced in panels B to F where p57KIP2 constructs are overexpressed in order to reduce brightness, as compared to panel A, to allow visualization of p57KIP2 in all experiments. (G, H) Glucagon positive cells in dispersed human islets are not transduced with Ad.p57WT driven by the RIP1 promotor, demonstrating specificity of the promotor for beta cells. Abbreviations: CDKI, cyclin-dependent kinase inhibitory domain; NLS, nuclear localization sequence; PCNA, proliferating cell nuclear antigen domain.
p57KIP2 Contains a Second NLS at RKRLR 312-316
Recently, Stampone et al identified a patient with BWS in whom CDKN1C contained a c.946C > T variant that resulted in a p.Arg316-Trp substitution in at the C-terminus of p57KIP2 (24). They demonstrated that this mutant p57KIP2 is unable to access the nucleus in HEK and HepG2 cells and concluded that the RKRLR 312–316 sequence serves as an authentic NLS for p57KIP2 (Fig. 5). In the current context, these studies raise questions as to the relative importance of the 2 NLSs, whether they function independently or in concert, and how they function in the context of the human beta cell.
Figure 5.
Amino acid sequence of p57KIP2 suggesting the existence of 2 nuclear localization sequences (red ovals) at amino acid positions 278–281 and 312–316. These 2 sequences are comprised of the basic amino acids lysine and arginine, separated by a linker region, suggesting the presence of a classical bipartite nuclear localization sequence.
With these considerations in mind, we generated adenoviruses containing CDKN1C constructs encoding p57WT, p57ΔNLS1 (deletion of amino acids 278–281), p57ΔNLS2 (deletion of amino acids 312–316), and p57ΔNLS1 + 2 (deletion of both amino acids 278–281 and 312–316), all driven by a CMV promoter and containing enhanced GFP (eGFP) at the amino-terminus of each construct (Fig. 6). These were transfected into HEK-293A cells, and live cells were visualized with confocal microscopy. As shown in Fig. 6A, for the p57WT construct with both putative NLS sequences intact, p57KIP2-eGFP localized strongly and exclusively to the nucleus. In contrast, when either or both of the 2 putative NLS is sequences were deleted, the p57KIP2-eGFP products were constrained almost exclusively to the cytoplasm (Fig. 6B–D).
Figure 6.
Documentation of a functional bipartite NLS in p57KIP2 in HEK cells. Plasmids containing N-terminal GFP driven by the CMV promoter were transfected into HEK-293A cells, followed by live-cell imaging by confocal microscopy. The constructs are shown over their respective panels. (A) Enhanced GFP is localized to the cell nucleus when cells transfected with p57WT plasmid. (B-D). In contrast, enhanced GFP constructs are unable to translocate into the cell nucleus and are stranded in the cytoplasm when NLSΔ1 (AA 278–281), NLSΔ2 (AA 312–316), or both (NLSΔ1 + 2) are deleted. Thus, both “halves” of the putative bipartite NLS are required for nuclear entry. Images shown are representative of 3 individual experiments. Abbreviations: HEK, human embryonic kidney; NLS, nuclear localization sequence.
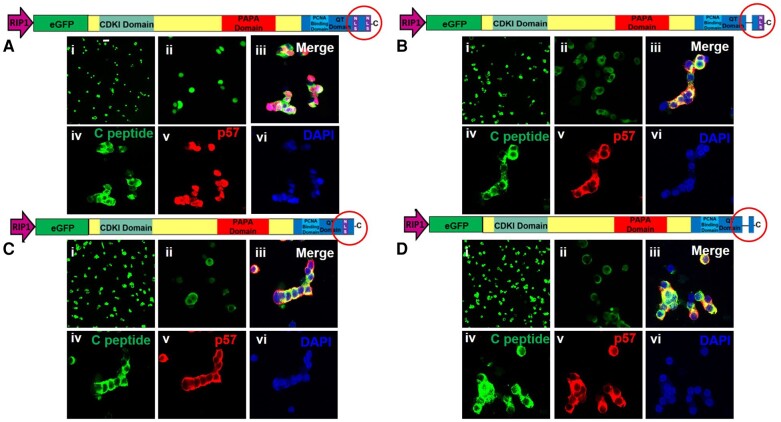
These studies were repeated using the same constructs with 3 modifications. First, they were performed in human islets. Second, adenoviruses containing these constructs were prepared in which the RIP1-mini-CMV promoter was substituted for the CMV promoter so that events confined to beta cells could be observed. And finally, we used both eGFP (live cells) and p57KIP2 immunohistochemistry (same cells, fixed) as dual readouts. As shown in Fig. 7, confocal microscopy of live islet cells demonstrates that the eGFP-p57WT construct effectively targets the nuclear compartment (Fig. 7Ai-ii) as does WT p57KIP2 itself (Fig. 7Aiii-vi). In contrast, none of the single or dual NLS deletion mutants encoded by Ad.p57ΔNLS1, Ad.p57ΔNLS2, or Ad.p57ΔNLS1 + 2 were able to access the nucleus and instead remained cytoplasmic, both as assessed by eGFP (Fig. 7Bi-ii, Ci-ii, Di-ii) and p57KIP2 immunocytochemistry (Fig. 7Aiii-vi, Biii-vi, Ciii-vi, Diii-vi).
Figure 7.
Documentation of a functional bipartite NLS in p57KIP2 in human beta cells. (A-D) Dispersed human islet cells were transfected with Ad.p57WT, Ad.p57ΔNLS1, Ad.p57ΔNLS2, and Ad.p57ΔNLS1 + 2 as indicated in the constructs above each panel. All constructs were driven by the RIP1 promotor. In each panel, photos (i) and (ii) show imaging of enhanced GFP in live cells at 20 × and 63 × magnification, respectively. In each panel, photos (iii)-(vi) show confocal microscopy of dispersed islets cells, transduced with the constructs shown, then fixed and immunolabeled for C-peptide, p57KIP2 and 4′,6-diamidino-2-phenylindole. (A.i-ii) enhanced GFP localized to the nucleus when both “halves” of the NLS are present. (B.i-ii), (C.i-ii), and (D.i-ii) Loss of either or both “halves” of the NLS results in cytoplasmic localization of GFP. (Aiii-vi), (Biii-vi), (Ciii-vi), and (Diii-vi) Wild-type p57KIP2 is uniformly nuclear, whereas constructs in which either or both “halves” of the NLS are deleted are unable to enter the nucleus and remain in the cytoplasm of beta cells. Abbreviations: NLS, nuclear localization sequence.
Discussion
These studies make several novel and important points. First, they demonstrate for the first time that p57KIP2 contains a classical bipartite nuclear localization signal and that both “halves” of the NLS are each necessary for nuclear localization of p57KIP2. Second, they demonstrate that this classical bipartite NLS is not only necessary but also sufficient to translocate p57KIP2 to the nucleus. Third, this bipartite NLS is also necessary and sufficient to translocate a heterologous cargo, in this case eGFP, to the nucleus. Fourth, these events are described for the first time in human pancreatic beta cells. And fifth, they are directly relevant in etiologic terms to a variety of human diseases.
In addition to BWS, cases of which can be caused directly by CDKN1C mutations, CDKN1C is important in the pathogenesis of the focal variant of congenital hyperinsulinism (FoCHI); Russel Silver syndrome (RSS); and intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia, and genital anomalies (IMAGe) syndrome. Congenital hyperinsulinism (CHI) is the most common cause of persistent hypoglycemia in infancy (1). Individuals with CHI are unable to suppress pancreatic beta cell insulin secretion, which leads to hypoglycemia. Clinically, CHI presents with poor feeding, lethargy, jitteriness, hypotonia, macrosomia, and/or seizures. It is diagnosed by the presence of hypoglycemia accompanied by inappropriately elevated serum insulin levels. There are 2 principal subtypes of CHI: diffuse and focal CHI (FoCHI). Diffuse CHI is most often due to biallelic recessive or monoallelic dominant inactivating mutations in ABCC8 or KCNJ11, genes encoding the key components of the beta cell ATP-sensitive potassium channel (1, 25, 26). In contrast, FoCHI is characterized by a single focus of beta cell hyperplasia within the pancreas (19, 27). FoCHI is associated with paternal transmission of a recessive mutation in ABCC8 or KCNJ11 coupled with somatic loss of maternal chromosome 11p15, including CDKN1C. Consequently, beta cells in FoCHI lack p57 expression (19). Since p57KIP2 is a critical cell cycle inhibitor for beta cells, its loss or inactivation is believed to permit unrestrained beta cell proliferation (19, 28). In mice, and presumably humans, loss of both alleles of Cdkn1c causes embryonic lethality (15). Accordingly, the focal nature of beta cell expansion in FoCHI, as compared to diffuse CHI, reflects the fact that FoCHI and BWS are most commonly mosaic syndromes (5, 6, 29, 30).
In contrast to the pancreatic endocrine and somatic cellular overgrowth seen in BWS and FoCHI, mutations that enhance CDKN1C/p57KIP2 expression are associated with undergrowth syndromes, most notably RSS. Intrauterine growth restriction, poor postnatal growth, body asymmetry, and typical craniofacial features, including a triangular-shaped face and broad forehead, characterize RSS (9, 31, 32). The majority of cases of RSS are caused by DNA hypomethylation in the ICR1 on the paternal chromosome 11p15.5. RSS may also result from maternal uniparental disomy or a maternal duplication of chromosome 11p15.5. These events lead to enhanced expression of the CDKN1C gene in affected tissues (31). Finally, some instances of RSS result from activating mutations in CDKN1C/p57KIP2 (31, 33, 34).
Gain-of-function point mutations in CDKN1C/p57KIP2 are associated with another pediatric endocrine syndrome, a rare undergrowth disorder defined by IMAGe (35). All reported IMAGe syndrome-associated point mutations are localized to the region of the gene encoding either the PCNA binding domain or the putative NLS at amino acids 278–281 (Fig. 1A) (9, 36-39).
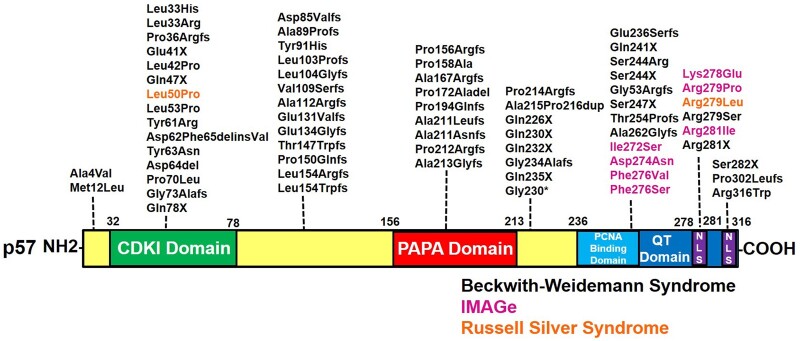
Figure 8 summarizes nucleotide and amino acid alterations in p57KIP2 that have been associated with these disorders, summarized from prior reports (3, 4, 9, 24, 31, 37, 40). Borges et al has shown that IMAGe syndrome-causing mutations in the PCNA binding domain result in p57KIP2 stabilization (40). Similar findings were also seen in a subject with RSS associated with a gain of function mutation in the PCNA binding domain of CDKN1C (31). In contrast to the localization of the IMAGe/RSS causing mutations, variants causing BWS have been identified throughout the gene sequence. In a patient cohort examined by Romanelli et al, 15 out of 25 patients with BWS had mutations in CDKN1C that altered the QT domain while maintaining an intact CDKI domain (3). More recently, Stampone et al identified a novel BWS-causing mutation in the terminal amino acid of CDKN1C, which we now demonstrate to be one half of a bipartite NLS (24). Collectively, these findings suggest that the unique structural domains of CDKN1C reflect differing but fundamental functional roles in beta cell biology and in human disease.
Figure 8.
A summary of disease-associated variants in CDKN1C/p57KIP2 that lead to Beckwith-Wiedemann syndrome, Russel Silver syndrome, and IMAGe, from references (3, 4, 9, 24, 31, 37). Pathologic variants are dispersed throughout each of the distinct domains. Note that multiple sequence-changing variants that alter the basic residues in the 2 NLS regions have been identified. Abbreviations: del, deletion; fs, frame shift; IMAGe, intrauterine growth restriction, metaphyseal dysplasia, adrenal hypoplasia and genital anomalies; NLS, nuclear localization sequence; X, stop.
Our interest in CDKN1C and p57KIP2 was piqued by their recurring association with human insulinomas. Among 22 sets of pure human beta cells on which we have reported RNA sequencing, CDKN1C is among the most highly expressed cell cycle inhibitor (21), and its expression is recurrently and markedly reduced in the 26 human insulinomas on which we have performed RNA sequencing (21). Silencing CDKN1C in human beta cells induces proliferation (21, 28). In parallel, human beta cell regenerative drugs in the DYRK1A inhibitor class, such as harmine, repress CDKN1C expression in human beta cells (41, 42), contributing to their proliferation.
These studies and their predecessors beg the question as to the specific functional roles of p57KIP2 in the human beta cell. As noted earlier, p57KIP2 is widely viewed simply as a cell cycle inhibitor, based principally on its homology with the 2 other CIP-KIP family members, p21CIP1 and p27KIP1. However, several features of p57KIP2 make this an oversimplification: p57KIP2 likely has additional important biology in general and in human beta cells in particular. First, if it is “just another CIP/KIP cell cycle inhibitor,” why is it expressed principally in embryonic life? Second, why is it twice the size of the other 2 CIP/KIP members (Fig. 1A)? Third, why does it contain unique domains like the PAPA domain not present in p21CIP1 and p27KIP1? Fourth, why is it reproducibly reported to be expressed in only 40% of beta cells (19, 20), and what is the functional difference between p57KIP2+ and p57KIP2− beta cells? Fifth, if it is essential to normal beta cell biology, why is it absent in rodent beta cells? These questions are not addressed here but are of particular interest for future studies, for example through single cell and tissue transcriptomic approaches.
In conclusion, we have identified a previously unrecognized canonical bipartite NLS in the p57KIP2 protein and shown how substitutions, deletion, frameshifts, and other variants in the CDKN1C gene can lead to clinical phenotypes in rare but important human diseases. We note from examining the sequences of other members of the CIP/KIP family, p21CIP1 and p27KIP1 (Fig. 9), that they too likely employ previously unrecognized functional bipartite NLSs (43, 44). Future structure-function studies will be important in elucidating the biology of the PAPA, QT, PCNA, and CDKI domains in human beta cell biology. As noted, these studies will need to be performed in human islets, as rodent beta cells do not express cdkn1c.
Figure 9.
Amino acid sequences of the CIP/KIP family reveals classical bipartite NLS sequences, shown in bold underline, in each member, similar to the motifs in p57KIP2. Abbreviations: NLS, nuclear localization sequence.
Acknowledgments
We thank Bonnie and Joel Bergstein, Lonnie and Thomas Schwartz, and the Martha and Fred Farkouh families for their support of our work. We thank the Einstein-Sinai Diabetes Research Center Pilot and Feasibility Program, National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIH/NIDDK) Grants P-30 DK 020541, R01 DK105015-05-A1, and R-01 DK129196 for their support of this project. We thank the NIH/NIDDK Integrated Islet Distribution Program, Prodo Laboratories, and Drs. Patrick MacDonald at The Alberta Diabetes Institute, Piotr Witkowski at the University of Chicago, and Tatsuya Kin at the University of Alberta for providing human cadaveric islets.
Contributor Information
Lauryn Choleva, Division of Pediatric Endocrinology and Diabetes, Department of Pediatrics, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Peng Wang, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Hongtao Liu, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Olivia Wood, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Luca Lambertini, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Donald K Scott, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Esra Karakose, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Andrew F Stewart, Diabetes, Obesity and Metabolism Institute, Icahn School of Medicine at Mount Sinai, New York, NY 10029, USA.
Funding
Support for this work provided by the Einstein-Sinai Diabetes Research Center Pilot and Feasibility Program and NIH/NIDDK grant P-30 DK 020541.
Author Contributions
L.C., P.W., H.L., and O.W. performed experiments. L.C., P.W., and A.F.S. drafted the manuscript. All authors provided intellectual analysis and approved the manuscript.
Disclosures
L.C., H.L., O.W., L.L., D.K.S., and E.K. have nothing to disclose. A.F.S. and P.W. are inventors on patents filed by the Icahn School of Medicine at Mount Sinai.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Duality of Interest
A.F.S. and P.W. are inventors on patents filed by the Icahn School of Medicine at Mount Sinai. The other authors declare that there is no duality of interest associated with this manuscript.
References
- 1. Kalish JM, Boodhansingh KE, Bhatti TR, et al. Congenital hyperinsulinism in children with paternal 11p uniparental isodisomy and Beckwith-Wiedemann syndrome. J Med Genet. 2016;53(1):53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Matsuoka S, Edwards MC, Bai C, et al. P57(Kip2), a structurally distinct member of the P21(Cip1) Cdk inhibitor family, is a candidate tumor-suppressor gene. Genes Dev. 1995;9(6):650‐662. [DOI] [PubMed] [Google Scholar]
- 3. Romanelli V, Belinchón A, Benito-Sanz S, et al. CDKN1C (p57(Kip2)) analysis in Beckwith-Wiedemann syndrome (BWS) patients: genotype-phenotype correlations, novel mutations, and polymorphisms. Am J Med Genet A. 2010;152A(6):1390‐1397. [DOI] [PubMed] [Google Scholar]
- 4. Brioude F, Netchine I, Praz F, et al. Mutations of the imprinted CDKN1C gene as a cause of the overgrowth Beckwith-Wiedemann syndrome: clinical spectrum and functional characterization. Hum Mutat. 2015;36(9):894‐902. [DOI] [PubMed] [Google Scholar]
- 5. Hatada I, Ohashi H, Fukushima Y, et al. An imprinted gene p57KIP2 is mutated in Beckwith-Wiedemann syndrome. Nat Genet. 1996;14(2):171‐173. [DOI] [PubMed] [Google Scholar]
- 6. Pateras IS, Apostolopoulou K, Niforou K, Kotsinas A, Gorgoulis VG. p57KIP2: “kip”ing the cell under control. Mol Cancer Res. 2009;7(12):1902‐1919. [DOI] [PubMed] [Google Scholar]
- 7. Lam WW, Hatada I, Ohishi S, et al. Analysis of germline CDKN1C (p57KIP2) mutations in familial and sporadic Beckwith-Wiedemann syndrome (BWS) provides a novel genotype-phenotype correlation. J Med Genet. 1999;36(7):518‐523. [PMC free article] [PubMed] [Google Scholar]
- 8. Lee MH, Reynisdottir I, Massague J. Cloning of p57KIP2, a cyclin-dependent kinase inhibitor with unique domain structure and tissue distribution. Genes Dev. 1995;9(6):639‐649. [DOI] [PubMed] [Google Scholar]
- 9. Eggermann T, Binder G, Brioude F, et al. CDKN1C mutations: two sides of the same coin. Trends Mol Med. 2014;20(11):614‐622. [DOI] [PubMed] [Google Scholar]
- 10. Creff J, Besson A. Functional versatility of the CDK inhibitor p57(Kip2). Front Cell Dev Biol. 2020;8:584590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monk D, Mackay DJG, Eggermann T, Maher ER, Riccio A. Genomic imprinting disorders: lessons on how genome, epigenome and environment interact. Nat Rev Genet. 2019;20(4):235‐248. [DOI] [PubMed] [Google Scholar]
- 12. Abukhdeir AM, Park BH. P21 and p27: roles in carcinogenesis and drug resistance. Expert Rev Mol Med. 2008;10:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Besson A, Dowdy SF, Roberts JM. CDK inhibitors: cell cycle regulators and beyond. Dev Cell. 2008;14(2):159‐169. [DOI] [PubMed] [Google Scholar]
- 14. Westbury J, Watkins M, Ferguson-Smith AC, Smith J. Dynamic temporal and spatial regulation of the cdk inhibitor p57kip2 during embryo morphogenesis. Mech Dev. 2001;109(1):83‐89. [DOI] [PubMed] [Google Scholar]
- 15. Zhang P, Liégeois NJ, Wong C, et al. Altered cell differentiation and proliferation in mice lacking p57KIP2 indicates a role in Beckwith-Wiedemann syndrome. Nature. 1997;387(6629):151‐158. [DOI] [PubMed] [Google Scholar]
- 16. Rossi MN, Andresini O, Matteini F, Maione R. Transcriptional regulation of p57(kip2) expression during development, differentiation and disease. Front Biosci (Landmark Ed). 2018;23(1):83‐108. [DOI] [PubMed] [Google Scholar]
- 17. Potikha T, Kassem S, Haber EP, Ariel I, Glaser B. P57(Kip2) (cdkn1c): sequence, splice variants and unique temporal and spatial expression pattern in the rat pancreas. Lab Invest. 2005;85(3):364‐375. [DOI] [PubMed] [Google Scholar]
- 18. Lu JN, Wu T, Zhang B, et al. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun Signal. 2021;19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kassem SA, Ariel I, Thornton PS, et al. P57(KIP2) expression in normal islet cells and in hyperinsulinism of infancy. Diabetes. 2001;50(12):2763‐2769. [DOI] [PubMed] [Google Scholar]
- 20. Fiaschi-Taesch NM, Kleinberger JW, Salim FG, et al. Human pancreatic β-cell G1/S molecule cell cycle atlas. Diabetes. 2013;62(7):2450‐2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang H, Bender A, Wang P, et al. Insights into beta cell regeneration for diabetes via integration of molecular landscapes in human insulinomas. Nat Commun. 2017;8(1):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hohmeier HE, Mulder H, Chen G, Henkel-Rieger R, Prentki M, Newgard CB. Isolation of INS-1-derived cell lines with robust ATP-sensitive K+ channel-dependent and -independent glucose-stimulated insulin secretion. Diabetes. 2000;49(3):424‐430. [DOI] [PubMed] [Google Scholar]
- 23. Wang P, Karakose E, Liu H, et al. Combined inhibition of DYRK1A, SMAD, and trithorax pathways synergizes to induce robust replication in adult human beta cells. Cell Metab. 2019;29(3):638‐652.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Stampone E, Bencivenga D, Barone C, Di Finizio M, Della Ragione F, Borriello A. A Beckwith-Wiedemann-associated CDKN1C mutation allows the identification of a novel nuclear localization signal in human p57(Kip2). Int J Mol Sci. 2021;22(14):7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Snider KE, Becker S, Boyajian L, et al. Genotype and phenotype correlations in 417 children with congenital hyperinsulinism. J Clin Endocrinol Metab. 2013;98(2):E355‐E363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Galcheva S, Demirbilek H, Al-Khawaga S, Hussain K. The genetic and molecular mechanisms of congenital hyperinsulinism. Front Endocrinol (Lausanne). 2019;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sempoux C, Guiot Y, Jaubert F, Rahier J. Focal and diffuse forms of congenital hyperinsulinism: the keys for differential diagnosis. Endocr Pathol. 2004;15(3):241‐246. [DOI] [PubMed] [Google Scholar]
- 28. Ou K, Moss NG, Wang YJ, et al. Targeted demethylation at the CDKN1C/p57 locus induces human beta cell replication. J Clin Invest. 2019;129(1):209‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamada T, Sugiyama G, Higashimoto K, et al. Beckwith-Wiedemann syndrome with asymmetric mosaic of paternal disomy causing hemihyperplasia. Oral Surg Oral Med Oral Pathol Oral Radiol. 2019;127(3):e84‐e88. [DOI] [PubMed] [Google Scholar]
- 30. Giurgea I, Sanlaville D, Fournet JC, et al. Congenital hyperinsulinism and mosaic abnormalities of the ploidy. J Med Genet. 2006;43(3):248‐254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brioude F, Oliver-Petit I, Blaise A, et al. CDKN1C mutation affecting the PCNA-binding domain as a cause of familial Russell Silver syndrome. J Med Genet. 2013;50(12):823‐830. [DOI] [PubMed] [Google Scholar]
- 32. Saal HM, Harbison MD, Netchine I, et al. Silver-Russell syndrome. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews(®) [Internet]. University of Washington; 1993. [PubMed] [Google Scholar]
- 33. Sabir AH, Ryan G, Mohammed Z, et al. Familial russell-silver syndrome like phenotype in the PCNA domain of the CDKN1C gene, a further case. Case Rep Genet. 2019;2019:1398250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Binder G, Ziegler J, Schweizer R, et al. Novel mutation points to a hot spot in CDKN1C causing Silver-Russell syndrome. Clin Epigenetics. 2020;12(1):152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Vilain E, Le Merrer M, Lecointre C, et al. IMAGe, a new clinical association of intrauterine growth retardation, metaphyseal dysplasia, adrenal hypoplasia congenita, and genital anomalies. J Clin Endocrinol Metab. 1999;84(12):4335‐4340. [DOI] [PubMed] [Google Scholar]
- 36. Cabrera-Salcedo C, Kumar P, Hwa V, Dauber A. IMAGe and related undergrowth syndromes: the complex spectrum of gain-of-function CDKN1C mutations. Pediatr Endocrinol Rev. 2017;14(3):289‐297. [DOI] [PubMed] [Google Scholar]
- 37. Arboleda VA, Lee H, Parnaik R, et al. Mutations in the PCNA-binding domain of CDKN1C cause IMAGe syndrome. Nat Genet. 2012;44(7):788‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Milani D, Pezzani L, Tabano S, Miozzo M. Beckwith-Wiedemann and IMAGe syndromes: two very different diseases caused by mutations on the same gene. Appl Clin Genet. 2014;7:169‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schrier Vergano SA, Deardorff MA. IMAGe syndrome. In: Adam, MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews(®) [Internet]. University of Washington; 1993. [PubMed] [Google Scholar]
- 40. Borges KS, Arboleda VA, Vilain E. Mutations in the PCNA-binding site of CDKN1C inhibit cell proliferation by impairing the entry into S phase. Cell Div. 2015;10(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang P, Fiaschi-Taesch NM, Vasavada RC, Scott DK, García-Ocaña A, Stewart AF. Diabetes mellitus–advances and challenges in human β-cell proliferation. Nat Rev Endocrinol. 2015;11(4):201‐212. [DOI] [PubMed] [Google Scholar]
- 42. Ackeifi C, Wang P, Karakose E, et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β cell regeneration. Sci Transl Med. 2020;12(530):eaaw9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Roy A, Banerjee S. P27 and leukemia: cell cycle and beyond. J Cell Physiol. 2015;230(3):504‐509. [DOI] [PubMed] [Google Scholar]
- 44. Kreis NN, Louwen F, Yuan J. Less understood issues: p21(cip1) in mitosis and its therapeutic potential. Oncogene. 2015;34(14):1758‐1767. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.