Neonatal BCG vaccination is associated with a reduction in all-cause mortality. This study reports altered cytokine responses to heterologous pathogens and TLR ligands in BCG-vaccinated neonates compared to non-BCG-vaccinated controls, consistent with modulation of innate immunity.
Keywords: Bacille Calmette-Guérin, immunisation, heterologous, nonspecific, trained immunity
Abstract
Background
BCG vaccination is associated with a reduction in all-cause infant mortality in high-mortality settings. The underlying mechanisms remain uncertain, but long-term modulation of the innate immune response (trained immunity) may be involved.
Methods
Whole-blood specimens, collected 7 days after randomization from 212 neonates enrolled in a randomized trial of neonatal BCG vaccination, were stimulated with killed pathogens and Toll-like receptor (TLR) ligands to interrogate cytokine responses.
Results
BCG-vaccinated infants had increased production of interleukin 6 (IL-6) in unstimulated samples and decreased production of interleukin 1 receptor antagonist, IL-6, and IL-10 and the chemokines macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and monocyte chemoattractant protein 1 (MCP-1) following stimulation with peptidoglycan (TLR2) and R848 (TLR7/8). BCG-vaccinated infants also had decreased MCP-1 responses following stimulation with heterologous pathogens. Sex and maternal BCG vaccination status interacted with neonatal BCG vaccination.
Conclusions
Neonatal BCG vaccination influences cytokine responses to TLR ligands and heterologous pathogens. This effect is characterized by decreased antiinflammatory cytokine and chemokine responses in the context of higher levels of IL-6 in unstimulated samples. This supports the hypothesis that BCG vaccination modulates the innate immune system. Further research is warranted to determine whether there is an association between these findings and the beneficial nonspecific (heterologous) effects of BCG vaccine on all-cause mortality.
Substantial evidence supports the concept that BCG vaccine reduces all-cause infant mortality in high-mortality settings [1–4]. The recent World Health Organization Special Advisory Group of Experts on Immunization systematic review of the nonspecific (heterologous) effects of BCG showed that neonatal BCG vaccination is associated with a 30% reduction in all-cause mortality (relative risk, 0.70; 95% confidence interval, .49–1.01) [5]. Epidemiological studies also suggest that BCG vaccine given at birth protects against the development of allergic disease [6, 7] and pneumonia [8].
Randomized controlled trials of BCG vaccination at birth report a reduction in neonatal mortality attributable to infection [1, 5]. Evidence suggests that the benefit of BCG vaccination is conferred as early as 7 days of life [4]. Clinical and demographic factors that influence vaccine responses include sex [1, 9], coadministration and timing of other neonatal vaccines [10], and maternal BCG vaccination [11].
The need for further research into the immunological mechanisms underlying the heterologous effects of BCG vaccine has been highlighted [12]. In animal models, BCG vaccine improves survival against a wide range of pathogens [13]. Multiple pathways of innate, cellular, and adaptive immunity are proposed to underpin BCG vaccine’s protective effect [14]. In a study done in West Africa, BCG vaccination altered neonatal cytokine responses following stimulation with Toll-like receptor (TLR) ligands [15]. Similarly, BCG vaccination influences cytokine responses to heterologous bacteria in peripheral blood mononuclear cells (PBMCs) from adults [16]. It is proposed that this results from induction of so-called trained immunity (ie, immune memory in the innate immune system) through epigenetic reprogramming of monocytes [16].
Our study aimed to investigate whether BCG vaccination influences neonatal cytokine responses to TLR ligands and clinically significant bacterial and fungal pathogens and, thus, induces heterologous innate immune effects.
METHODS
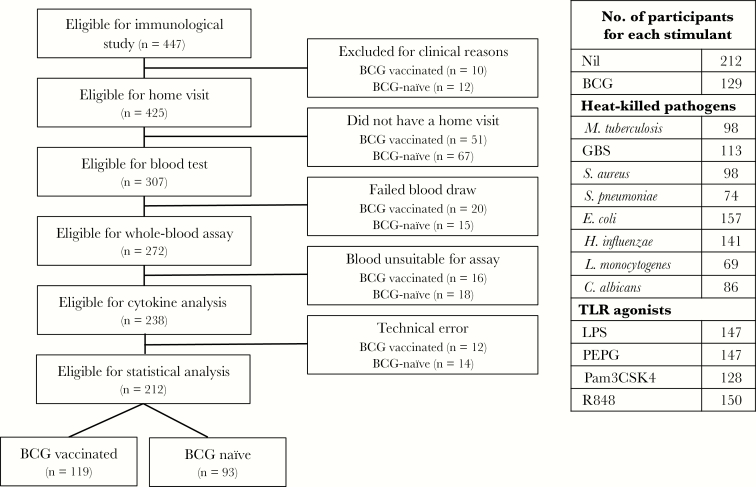
Participants were a subset of infants from the Melbourne Infant Study: BCG for the Prevention of Allergy and Infection (MIS BAIR), a randomized controlled trial of BCG vaccine in 1272 neonates, with the primary outcomes of allergic sensitization, eczema, and lower respiratory tract infection (clinical trials registration NCT01906853). Participants were randomized 1:1 to undergo vaccination with BCG-Denmark (0.05 mL) intradermally within 10 days of birth or to receive no BCG vaccine. All participants also received a birth dose of hepatitis B vaccine as per Australian guidelines. A subset of 447 participants recruited over an 18-month period (January 2014–June 2015) had blood samples collected for immunological analyses (Figure 1). Exclusion criteria for infants in the immunological study were suspected perinatal sepsis, no receipt of hepatitis B vaccine, or receipt of blood products.
Figure 1.
Flow chart of eligible participants and relevant exclusions. Table shows number of individual stimulations done for each antigen. C. albicans, Candida albicans; GBS, group B streptococcus; E. coli, Escherichia coli; H. influenzae, Haemophilus influenzae type B; L. monocytogenes, Listeria monocytogenes; LPS, lipopolysaccharide; M. tuberculosis, Mycobacterium tuberculosis; Pam3CSK4, (S)-(2,3-bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4OH, trihydrochloride; PEPG, peptidoglycan; R848, resiquimod; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae serotype 15C.
Written informed consent was obtained from the parents of all participants. The study was approved by the human ethics research committees of the Royal Children’s Hospital Melbourne (human research ethics committee [HREC] authorization 33025) and Mercy Hospital for Women (HREC authorization R12/28).
Participants were visited in their home at a mean duration (±SD) of 7 ± 4 days after randomization. Blood specimens were collected via a closed loop system into tubes containing sodium-heparin (S-monovette) and pretested for endotoxin. Blood samples were kept at room temperature, protected from direct sunlight, and stimulated in the laboratory for a mean duration of 4 hours ± 24 minutes after collection. Laboratory personnel were masked to the participants’ BCG vaccination status.
Whole-blood specimens, diluted 1:1 with Roswell Park Memorial Institute (RPMI) 1640 medium (Glutamax supplement and HEPES; Gibco), were added to preprepared stimulation assay strips (Microlon 600 Greiner-One 8-well strips). The assay strips were made using a validated robotic system, to maximize standardization of stimulant volume, and were stored at –80°C until addition of blood. The final stimulant concentrations were as follows: BCG Denmark, 75 μg/mL; killed Mycobacterium tuberculosis, 1.0 × 106 colony-forming units (CFU)/mL; 7 heat-killed bacteria (Escherichia coli, 1.0 × 106 CFU/mL; Haemophilus influenzae type B, 1.0 × 106 CFU/mL; Staphylococcus aureus, 1.0 × 107 CFU/mL; group B streptococcus [GBS; Streptococcus agalactiae], 1.0 × 107 CFU/mL; Streptococcus pneumoniae serotype 15C 1.0 × 107 CFU/mL; and Listeria monocytogenes, 1.0 × 107 CFU/mL), 1 fungus (Candida albicans, 1.0 × 106 CFU/mL), and 4 TLR ligands (lipopolysaccharide [LPS], 100 ng/mL; resiquimod [R848], 3.5 μg/mL; peptidoglycan [PEPG], 10 μg/mL; and (S)-(2,3-bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4OH, trihydrochloride [Pam3CSK4], 1 μg/mL; all from InvivoGen), and RPMI 1640 medium. Pathogens were clinical isolates from children with invasive disease at the Royal Children’s Hospital Melbourne and were killed by heat treatment at 70°C for 2 hours. Sterility was confirmed by the absence of growth in culture medium. Concentrations of all stimulants were optimized in preliminary experiments (data not shown). The stimulation assay was incubated at 37°C (5% CO2 in air) for a mean duration (±SD) of 20 ± 2 hours.
Supernatants were analyzed in batches using Bio-Rad human cytokine kits following the manufacturer’s instructions. The mean fluorescence intensity was read for each cytokine, using an xMAP Luminex 200 Bioanalyzer. Each sample was analyzed in 2 separate kits at different dilutions to account for the anticipated dynamic range of the cytokine results indicated by preliminary experiments (data not shown): (1) 1:100 dilution (7-plex): interleukin 1β (IL-1β), IL-1 receptor antagonist (IL-1RA), interleukin 6 (IL-6), interleukin 8 (IL-8), monocyte chemoattractant protein 1 (MCP-1; also known as monocyte chemotactic and activating factor and CCL2), macrophage inflammatory protein 1α/CCL3 (MIP-1α), and MIP-1β (CCL4); and (2) 1:4 dilution (5-plex): interferon γ (IFN-γ), interleukin 10 (IL-10), tumor necrosis factor α (TNF-α), macrophage migration inhibitory factor (MIF), and monokine induced by IFN-γ/CXCL9 (MIG).
Statistical analysis was done using Stata, version 13.1. Cytokine levels that were below the lower limit of detection were assigned a value of half the lowest detectable value (either the lowest standard on the curve or the lowest extrapolated value below). There were no instances when cytokine results were greater than the upper limit of detection. Prior to analysis, cytokine data were log transformed.
The effect of (1) BCG vaccination, (2) reported maternal BCG vaccination status, and (3) timing of BCG vaccination (early, <48 hours after birth; late, ≥48 hours after birth) on cytokine production in response to each stimulant was investigated using logistic regression, with the log-transformed value of the unstimulated (RPMI 1640 medium only) cytokine values as a covariate. Results were expressed as geometric mean ratios (GMRs) with 95% CIs. Because IFN-γ values were not normally distributed, IFN-γ production was also analyzed using nonparametric methods (ie, quantile regression). One participant was an extreme outlier for all cytokines in unstimulated samples, but use of the unstimulated value as a covariate successfully corrected for this in stimulated cytokine results; because this could not be done for the analysis of unstimulated cytokine results, this participant was excluded in that analysis. Results of cytokine/stimulant pairs are presented as GMRs (95% CIs) without emphasis on tests of statistical significance [17]. Subgroup effects (ie, sex, mode of delivery, and maternal BCG vaccination status) were investigated where there was statistical evidence of an interaction between BCG vaccination and the subgroup variable. Sensitivity analyses were performed to investigate the effect of excluding values assigned at the lower end of the standard curve and values extrapolated by xMAP software.
RESULTS
Demographic features are outlined in Table 1. There was a high degree of standardization in the laboratory procedures (Supplementary Table 1). Our data exhibited the marked interindividual variability in cytokine responses in both stimulated and unstimulated samples, characteristic of cytokine data (Supplementary Figure 1).
Table 1.
Baseline Characteristics of Study Participants
| Characteristic | BCG-vaccinated, No. (%) | BCG-naïve No. (%) | Overall, No. (%) |
|---|---|---|---|
| Overall | 119 (56.1) | 93 (43.9) | 212 (100) |
| Sex | |||
| Male | 58 (48.7) | 43 (46.2) | 101 (47.6) |
| Female | 61 (51.3) | 50 (53.8) | 111 (52.3) |
| Gestational age, wk, mean ± SD | 39.3 ± 1.5 | 39.2 ± 1.3 | 39.3 ± 0.1 |
| Birth weight, g, mean ± SD | 3388 ± 487.3 | 3333 ± 492.7 | 3364 ± 489.3 |
| Mode of delivery | |||
| Vaginal birth | 64 (53.8) | 62 (66.7) | 126 (59.4) |
| Cesarean section | 55 (46.2) | 31 (33.3) | 86 (40.6) |
| Last trimester vaccinationa | |||
| Yes | 59 (49.6) | 43 (46.2) | 102 (48.1) |
| No | 58 (48.7) | 49 (52.8) | 107 (50.5) |
| Not reported | 2 (1.7) | 1 (1.1) | 3 (1.4) |
| Feeding | |||
| Breast feeding | 82 (69.2) | 67 (71.7) | 149 (70.2) |
| Formula feeding | 3 (2.4) | 7 (7.6) | 10 (4.8) |
| Combination | 29 (24.2) | 18 (19.6) | 47 (22.2) |
| Not reported | 5 (4.2) | 1 (1.1) | 6 (2.8) |
| Infant vitamin D supplementation | |||
| Yes | 64 (53.4) | 48 (51.8) | 112 (52.8) |
| No | 55 (46.6) | 45 (48.2) | 100 (47.2) |
| Day of BCG vaccination | |||
| Mean ± SD | 2.1 ± 1.9 | … | … |
| Median (IQR) | 2 (0–6) | … | … |
| Interval from randomization to blood collection, d, mean ± SD | 6.9 ± 2.2 | … | … |
| Timing of hepatitis B vaccination in relation to BCG vaccination, h | |||
| ≤24 | 82 (69.0) | … | … |
| 24–48 | 12 (10.1) | … | … |
| >48 | 17 (14.3) | … | … |
| Not recorded | 8 (6.3) | … | … |
| Maternal BCG vaccinations status | |||
| Yes | 24 (20.2) | 31 (33.3) | 55 (25.9) |
| No | 90 (75.6) | 54 (58.1) | 144 (67.9) |
| Not reported | 5 (4.2) | 8 (8.6) | 13 (6.2) |
Data are no. (%) of participants, unless otherwise indicated.
Abbreviation: IQR, interquartile range.
aBoostrix (diphtheria-tetanus-acellular pertussis) or influenza vaccine in the last trimester, in line with local recommendations.
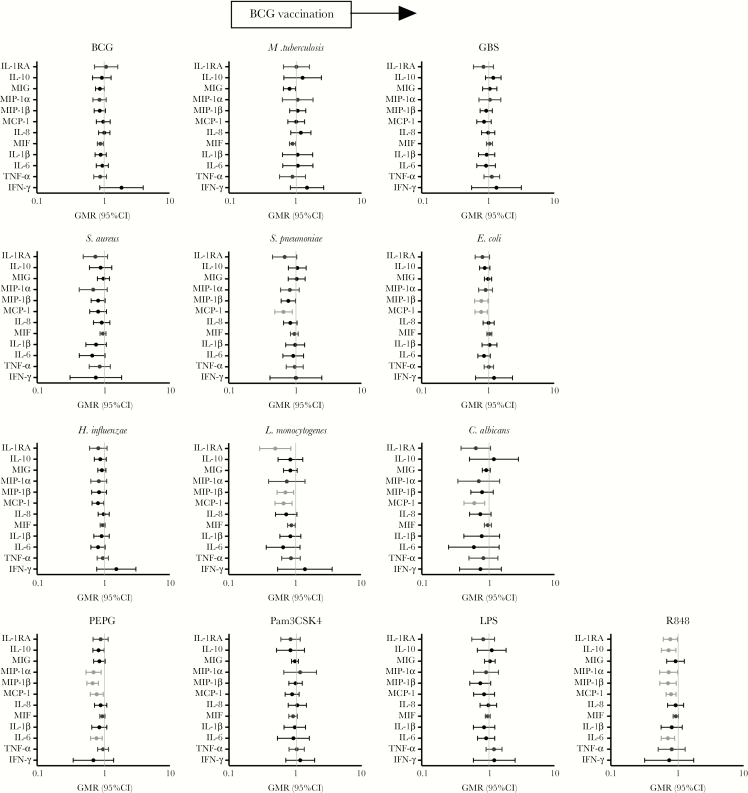
In unstimulated samples (ie, those exposed to RPMI 1640 medium), BCG-vaccinated infants showed increased production of IL-6, IL-1RA, and IL-1β, but the evidence that this was a real effect was weak for IL-1RA and IL-1β (Table 2). For TLR agonist responses, chemokine and cytokine production was unchanged by BCG in response to Pam3CSK4 and LPS. However, responses to stimulation with the TLR ligands PEPG and R848 were decreased for most cytokines in BCG-vaccinated infants as compared to BCG-naïve. This effect was strongest for the cytokines IL-6 and IL-10 and the chemokines MIP-1α, MIP-1β, and MCP-1 (Figure 2 and Supplementary Tables 2 and 3).
Table 2.
Geometric Mean Ratio (GMR) for Unstimulated Cytokines
| Cytokine | GMR (95% CI) | P |
|---|---|---|
| IFN-γ | 1.0 (.8–1.2) | .85 |
| TNF-α | 1.0 (.9–1.2) | .80 |
| IL-1β | 1.6 (.8–3.0) | .18 |
| IL-6 | 2.0 (1.1–3.7) | .03 |
| MIF | 1.0 (.9–1.2) | .90 |
| MIG | 0.9 (.8–1.1) | .42 |
| MCP-1 | 1.2 (.8–1.6) | .41 |
| IL-8 | 1.0 (.4–2.1) | .94 |
| MIP-1α | 0.8 (.5–1.4) | .50 |
| MIP-1β | 1.0 (.7–1.4) | 1.00 |
| IL-1RA | 1.9 (.9–3.8) | .08 |
| IL-10 | 1.0 (1.0–1.1) | .55 |
Data are for Roswell Park Memorial Institute 1640 (RPMI) medium stimulation. Univariate analysis was for the effect of BCG vaccination.
Abbreviations: CI, confidence interval; IFN-γ, interferon γ; IL-1RA, interleukin 1 receptor antagonist; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; MCP-1, monocyte chemoattractant protein 1/CCL2; MIF, macrophage migration inhibitory factor; MIG, monokine induced by interferon γ (CXCL9); MIP-1α/β, macrophage inflammatory protein 1 CCL3/4; TNF-α, tumor necrosis factor α.
Figure 2.
The effect of BCG vaccination on cytokine expression. Significant results P < .05 are depicted in pale gray. A geometric mean ratio (GMR) of >1.0 indicates cytokine levels were higher in BCG-vaccinated infants, compared with BCG-naïve infants. C. albicans, Candida albicans; CI, confidence interval; E. coli, Escherichia coli; GBS, group B streptococcus; H. influenzae, Haemophilus influenzae type B; IFN-γ, interferon γ; IL-1RA, interleukin 1 receptor antagonist; IL-1β, interleukin 1β; IL-6, interleukin 6; IL-8, interleukin 8; IL-10, interleukin 10; L. monocytogenes, Listeria monocytogenes; LPS, lipopolysaccharide; MCP-1, monocyte chemoattractant protein 1/CCL2; MIF, macrophage migration inhibitory factor; MIG, monokine induced by interferon γ/CXCL9; MIP-1 α/β, macrophage inflammatory protein 1 CCL3/CCL4; M. tuberculosis, Mycobacterium tuberculosis; Pam3CSK4, (S)-(2,3-bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4OH, trihydrochloride; PEPG, peptidoglycan; R848, resiquimod; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae serotype 15C; TNF-α, tumor necrosis factor α.
In response to stimulation with BCG, M. tuberculosis and the heterologous bacteria S. aureus and GBS, there was no difference in cytokine production between BCG-vaccinated and BCG-naïve infants. Conversely, responses to stimulation with S. pneumoniae, E. coli, H. influenzae, and particularly the intracellular organisms L. monocytogenes and C. albicans were decreased for most cytokines in BCG-vaccinated infants as compared to BCG-naïve infants (Figure 2 and Supplementary Tables 2 and 3). The chemokines and cytokines for which production was most consistently decreased were IL-1RA, MCP-1, and MIP-1β.
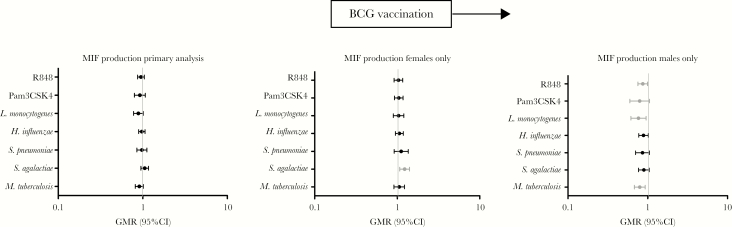
Sex independently influenced cytokine responses in our study (data not shown). There was a significant interaction between sex and BCG vaccination for production of MIF in response to stimulation with L. monocytogenes, H. influenzae, GBS, M. tuberculosis, and R848 (Supplementary Table 4). Subset analyses of males and females separately showed that there were generally lower MIF responses in BCG-vaccinated males, compared with BCG-naïve males, and higher responses in BCG-naïve females, compared with BCG-naïve females. BCG-vaccinated females had higher MIF production in response to stimulation with GBS, whereas BCG-vaccinated males had lower MIF production in response to stimulation with the intracellular stimulants R848, L. monocytogenes, and M. tuberculosis (Figure 3) as compared to BCG-naïve controls of the same sex.
Figure 3.
Interaction and subgroup analysis for the effect of sex on BCG vaccination. Macrophage migration inhibitory factor (MIF) production for cytokine/stimulant pairs where a significant interaction between sex and BCG vaccination was seen are shown for all participants (left), BCG-vaccinated females versus BCG-naïve females (middle), and BCG-vaccinated males versus BCG-naïve males. CI, confidence interval; GMR, geometric mean ratio; H. influenzae, Haemophilus influenzae type B; L. monocytogenes, Listeria monocytogenes; M. tuberculosis, Mycobacterium tuberculosis; Pam3CSK4, (S)-(2,3-bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4OH, trihydrochloride; R848, resiquimod; S. pneumoniae, Streptococcus pneumoniae serotype 15C.
Mode of delivery independently influenced cytokine responses in our population (data not shown), but there was minimal evidence of an interaction between BCG vaccination and mode of delivery on cytokine responses (Supplementary Table 4). Despite randomization of participants to BCG vaccination, attrition from the immunological study led to differences in the distribution of sex and mode of delivery between the 2 groups (Table 1). A multivariate regression model including these 2 variables and the log-transformed unstimulated cytokine yielded the same results as the primary analysis and excluded confounding as a contributor to the primary results.
Infants whose mothers had a history of BCG vaccination had increased production of IL-1β, IL-1RA, IL-6, and MIP-1α in response to Pam3CSK4, IL-6, and MIP-1α in response to H. influenzae and increased production of MIP-1β in response to PEPG. Conversely, infants whose mothers were not vaccinated with BCG had higher production of TNF-α and IL-1β in response to R848 and higher IL-10 production in response to GBS, independent of infant BCG vaccination status (Supplementary Table 2).
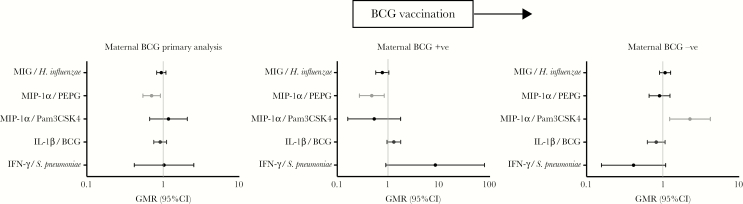
There was evidence of an interaction between maternal BCG vaccination status and infant BCG vaccination status for 5 cytokine/stimulant pairs (IFN-γ/S. pneumoniae, IL-1β/BCG, MIP-1α/PEPG, MIP-1α/Pam3CSK4, and MIG/H. influenzae; Supplementary Table 4). Separate analyses in infants of mothers who were vaccinated with BCG and those whose mothers were not vaccinated with BCG showed that in the former, BCG-vaccinated infants had higher IFN-γ production in response to stimulation with S. pneumoniae and MIP-1α in response to PEPG. Conversely for BCG-vaccinated infants whose mothers were not vaccinated with BCG, MIP-1α expression was increased in response to Pam3CSK4 (Figure 4).
Figure 4.
Interaction and subgroup analysis for the effect of maternal BCG vaccination status on neonatal BCG vaccination. Cytokine responses for cytokine/stimulant pairs where a significant interaction between maternal BCG vaccination status and neonatal BCG vaccination was seen are shown for all participants (left), in participants whose mothers were vaccinated with BCG (BCG-vaccinated infants vs BCG-naïve infants; middle), and participants whose mothers were not vaccinated with BCG (BCG-vaccinated infants vs BCG-naïve infants; right). CI, confidence interval; GMR, geometric mean ratio; H. influenzae, Haemophilus influenzae type B; IFN-γ, interferon γ; IL-1β, interleukin 1β; MIG, monokine induced by interferon γ/CXCL9; MIP-1α, macrophage inflammatory protein 1α CCL3; Pam3CSK4, (S)-(2,3-bis(palmitoyloxy)-(2-RS)-propyl)-N-palmitoyl-(R)-Cys-(S)-Ser-(S)-Lys4OH, trihydrochloride; PEPG, peptidoglycan; S. pneumoniae, Streptococcus pneumoniae serotype 15C.
Infants were categorized into those who were vaccinated with BCG early (<48 hours after birth) and those who were vaccinated late (≥48 hours after birth; Supplementary Figure 2A and 2B). The median interval between BCG vaccination and blood sampling was similar between both groups (7 days [range, 0–11 days] in the BCG-vaccinated group and 8 days [range, 3–11 days] in the BCG-naïve group; Supplementary Figure 2C).
Compared with those in the late BCG vaccination group, infants in the early BCG vaccination group had significantly higher cytokine and chemokine production following stimulation with all stimulants except for BCG and M. tuberculosis (Supplementary Table 5).
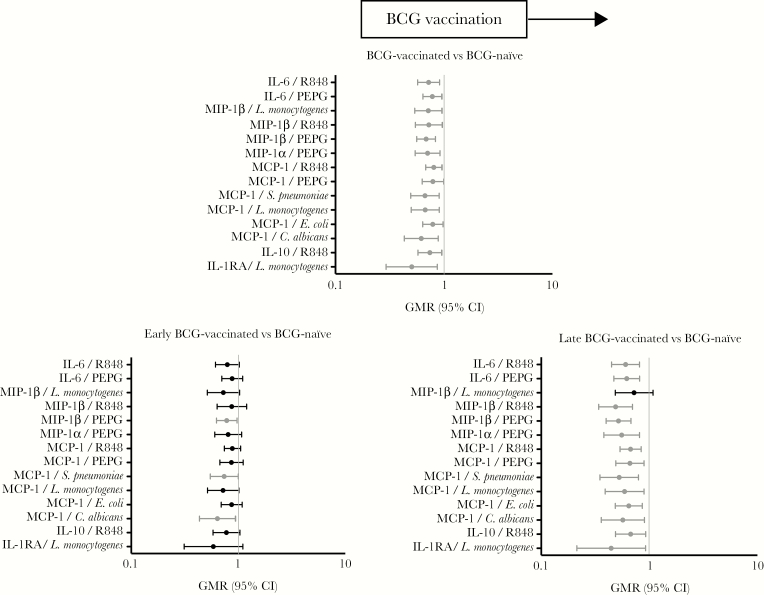
To determine how the timing of BCG vaccination influenced the results of the primary analysis, we compared cytokine production in the early BCG-vaccinated group to that in the BCG-naïve group and production in the late BCG-vaccinated group to that in the BCG-naïve group (Supplementary Table 5). The influence of BCG vaccination on cytokine production (ie, that overall cytokine production was decreased in BCG-vaccinated infants following heterologous stimulation) was more marked in the late BCG-vaccinated group than in the early BCG-vaccinated group when each was compared to the BCG-naïve group. (Figure 5)
Figure 5.
Effect of the timing of BCG on the overall effect of BCG shown by the primary analysis. Cytokine responses for cytokine/stimulant pairs where a significant effect of BCG vaccination was seen are shown for all participants (top), for infants who received BCG < 48 hours of birth (early) vs controls (bottom left), for participants who received BCG ≥48 hours from birth (late) vs controls (bottom right). C. albicans, Candida albicans; CI, confidence interval; GMR, geometric mean ratio; E. coli, Escherichia coli; IL-1RA, interleukin 1 receptor antagonist; IL-10, interleukin 10; L. monocytogenes, Listeria monocytogenes; MCP-1, monocyte chemoattractant protein 1/CCL2; MIP-1 α/β, macrophage inflammatory protein 1 (CCL3/CCL4); PEPG, peptidoglycan; R848, resiquimod; S. pneumoniae, Streptococcus pneumoniae serotype 15C.
Sensitivity analyses were performed for the primary outcome—the effect of BCG vaccination on cytokine responses—to investigate the influence of (1) the assigned values at the lower end of the standard curve and (2) the values extrapolated by Luminex xMAP software at both the upper and lower ends of the standard curve (Supplementary Table 6). Overall, the results of the primary analysis were preserved across sensitivity analyses. Notable differences were seen only for production of IL-1RA in response to R848, MIP-1β in response to R848, and IFN-γ in response to several stimulants, including M. tuberculosis (Supplementary Table 6).
DISCUSSION
This study adds to the immunological evidence for the heterologous effects of BCG vaccination by finding differential cytokine production between BCG-vaccinated infants and BCG-naïve infants following in vitro stimulation with heterologous stimulants. This is the first study to investigate the heterologous effects of BCG in the first week of life, the time from which a reduction in all-cause mortality associated with BCG-vaccination is reported [1].
The pattern of cytokine production observed in our study was consistent with findings of previous studies in neonates [18, 19]. IL-10 production was high relative to that of IFN-γ for all stimulants [19]. IL-6 production was relatively high as compared to that for proinflammatory cytokines, such as TNF-α and IL-1β [20]. In addition, there was robust chemokine production in response to TLR ligand stimulation, particularly R848 [21]. These patterns were observed despite considerable interindividual variability, both in unstimulated samples and following stimulation [22].
In our study, compared with BCG-naïve infants, BCG-vaccinated infants had reduced production of multiple cytokines and chemokines in response to stimulation with the TLR ligands PEPG (TLR2/[Nod]-like receptor 1/2) and R848 (TLR7/8). PEPG and R848 are potent activators of neonatal innate immunity when compared to Pam3CSK4 (TLR2) and LPS (TLR4) [23, 24]. R848 induces neonatal antigen-presenting cells, leading to T-helper cell type 1 polarization and production of interleukin 12p70 and TNF-α at levels equivalent to those for adults [24]. Excess stimulation of TLR7/8 with R848 is associated with neutrophil-mediated lung injury following RSV infection and an increased incidence of viral-associated wheeze in mouse models [25, 26]. PEPG has endotoxic properties and potentiates LPS signaling, augmenting its pathogenicity in sepsis syndromes [27–29]. The observed differential production of MCP-1 following stimulation with several heterologous bacteria between BCG-vaccinated and BCG-naïve infants is interesting in light of MCP-1’s pivotal role in the control of mycobacterial disease. Elevated levels of MCP-1 are associated with proinflammatory diseases, including asthma, multiple sclerosis, and malignancy [30].
The modified neonatal cytokine profile induced by BCG vaccination in our study, characterized by reduced antiinflammatory cytokine (IL-10 and IL-1RA) and IL-6 responses following stimulation with heterologous bacteria, suggests a proinflammatory bias in BCG-vaccinated infants in the context of bacterial challenge. A concomitant downregulation in production of several chemokines, including MCP-1, could reduce leukocyte recruitment and associated tissue damage during infection. Overall, these effects might result in a less severe sepsis response and contribute to the reduction in mortality reported in BCG-vaccinated infants in high-mortality settings.
Jensen et al also investigated the immunological nonspecific effects of BCG by measuring cytokine responses in whole-blood specimens [15]. In low-birth-weight infants in Guinea-Bissau, compared with BCG-naïve infants, BCG-vaccinated infants had increased IL-1β and TNF-α production in unstimulated samples. Consistent with this, we found increased IL-6 and IL-1RA (but not TNF-α) production in unstimulated samples. Also consistent, we found no effect of BCG vaccination on cytokine production following stimulation with LPS. In Jensen et al’s study, BCG-vaccinated infants had increased IL-1β, IL-6, and TNF-α production in response to stimulation with Pam3CSK4, whereas in our study BCG vaccination had no effect on cytokine responses to Pam3CSK4. Several factors that influence cytokine production differed between the two studies. These include ethnicity, geography [31], mean age at sampling (30 days in Guinea-Bissau vs 8.9 days in our study) [32], maternal HIV status [33], weight, gestational age, underlying health, hepatitis B vaccination status, maternal BCG vaccination status, blood sampling method, and anticoagulant. Jensen et al’s conclusion that BCG vaccination results in a stronger proinflammatory profile is consistent with our findings, albeit through different mechanisms.
Kleinnijenhuis et al also found a proinflammatory response to heterologous bacteria induced by BCG vaccination. In this study, in adults, increased IFN-γ, TNF-α and IL-1β production in PBMCs was observed following stimulation with heat-killed M. tuberculosis, S. aureus and C. albicans [16]. More recently, a similar study in infants born in the United Kingdom showed increased IL-6 production in PBMCs stimulated with C. albicans, S. aureus, and Pam3CSK4 [34]. The authors’ attributed these changes to epigenetic reprogramming that resulted in so-called trained immunity or innate immune memory.
Our finding that maternal BCG vaccination influenced the production of cytokines in infants is interesting. Latent maternal M. tuberculosis infection is associated with increased production of proinflammatory cytokines in response to mycobacterial stimulants in BCG-vaccinated infants [35]. Moreover, in a recently reported trial from Denmark, BCG-vaccinated infants had a decreased risk of infectious illnesses requiring hospitalization during the first 3 months of life if their mother had a history of BCG vaccination [36]. Potential mechanisms for the potentiating effect of maternal BCG vaccination include transplacentally acquired maternal antibodies [37] and transgenerational epigenetic changes in immune responses, such has been reported in lower organisms [38, 39]. Understanding how maternal and infant mycobacterial exposures influence the neonatal response to BCG warrants further investigation.
It is well recognized that vaccine responses are affected by sex [40]. Although BCG vaccination alone did not affect the production of MIF in our study, there was a significant interaction between BCG vaccination and sex for MIF production in response to several heterologous antigens. MIF is produced both constitutively and in response to bacterial infection or stress [41]. MIF has been implicated in severe sepsis, malaria, and chronic inflammatory diseases [42, 43]. The high baseline MIF levels among infants in our study are consistent with previous findings [44]. Given its pivotal role in the control of sepsis, the sex-differential effect of BCG vaccination on MIF production in our study is noteworthy.
The effect of the timing of BCG vaccination on the vaccine response is complex. In our study, when compared to late BCG vaccination, early BCG vaccination was associated with increased production of multiple cytokines in response to most stimulants. We had inadequate power for subgroup analysis of the effect of age on BCG vaccination, and instead we explored the influence of timing of BCG vaccination on the results of our primary analysis. We found that the effect of BCG was more pronounced with older age at vaccination. Interpretation of this finding is difficult because the analysis is potentially confounded by the older age of infants at the time of blood sampling. Furthermore, all infants in the late BCG vaccination group received hepatitis B vaccination prior to BCG vaccination, while some of those in the early BCG vaccination group received hepatitis B vaccination on the same day as or after BCG vaccination.
The strengths of our study include our ability to randomize term infants, because BCG is not routinely given in Australia, and the early timing of our blood sampling. Further strengths are the highly standardized and controlled protocols that were used for blood sample collection, handling of samples and laboratory assays [45], as well as the consideration given to the effect of assigned values outside the limit of detection of the assay and values that have been extrapolated by the xMAP software. There is no agreement on the method for standardizing or reporting these parameters; methods described in the literature are highly variable. The findings in our study were preserved across sensitivity analyses, which confirmed the validity of our use of assigned variables. Finally, our study, like others, found that in neonates certain cytokines have high unstimulated (ie, baseline) levels, while others remain largely undetectable in the absence of stimulation [44]. To account for the variability between individuals in unstimulated levels of immunological markers [22], we included the unstimulated value for each cytokine as a covariate in the primary analysis.
A potential limitation is the coadministration of hepatitis B vaccine as part of the Australian immunization schedule. The results of our study should be interpreted as BCG vaccination and hepatitis B vaccination, compared with hepatitis B vaccination alone. BCG has been associated with effects on hepatitis B virus antibody production in studies in the Gambia [46] and Australia [47] but not in South Africa [48]. The effect of hepatitis B vaccines on innate immunity has not been well studied [49], and there have been no studies investigating potential interactions with BCG. In our study, the vast majority of participants randomized to BCG vaccination received BCG and hepatitis B vaccines within 48 hours of each other. We were therefore unable to stratify our results by the timing of hepatitis B vaccination. A further limitation is the possibility of spurious statistically significant results arising from multiple subset analyses with reduced sample size. To mitigate this possibility, we restricted subset analyses to cytokine/stimulant pairs for which there was an interaction of BCG with the covariate of interest. In addition, to aid interpretation and avoid undue emphasis on tests of statistical significance or, conversely, to avoid discarding results that were not significant owing to type 2 error, we presented all our analyses with confidence intervals [50].
Based on our data, future studies on the innate immune response to BCG should focus on interrogation of chemokine-producing cells and pathways activated by R848 and PEPG, including TLR7/8, TLR2, RIG, and NOD. Furthermore, studies in high-mortality settings would be required to confirm any association between patterns of innate immune modulation in vitro and clinical outcomes following infection.
In conclusion, our study provides evidence of differential cytokine responses to heterologous antigens between BCG-vaccinated and BCG-naïve term infants. The responses can be broadly described as inhibition of antiinflammatory cytokine production, as well as downregulation of chemokine responses. Both sex and maternal BCG vaccination status interacted with BCG vaccination. This study supports the concept that BCG modifies the neonatal innate immune response.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Financial support. This work was supported by the Australian National Health and Medical Research Council (NHMRC) (grants GNT1051228 and GNT1099680), Murdoch Children’s Research Institute (Infection and Immunity Theme Grant), the University of Melbourne (Human Rights Scholarship to B. F.), the European Society of Paediatric Infectious Diseases (ESPID) (fellowship to B. F.), the European Research Council (ERC) (Consolidator Grant 310372 to M. G. N.), and the Netherlands Organization for Scientific Research (Spinoza Grant to M. G. N.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: 9th World Congress of the World Society for Paediatric Infectious Diseases, Rio De Janeiro, Brazil, 18–21 November 2015; 35th Annual Meeting of the European Society for Paediatric Infectious Diseases, Madrid, Spain, 23–27 May 2017.
Contributor Information
Bridget Freyne, Infectious Diseases and Microbiology Group, Parkville, Australia; Department of Paediatrics, Parkville, Australia.
Susan Donath, Clinical Epidemiology and Biostatistics Unit, Murdoch Children’s Research Institute, Parkville, Australia; Department of Paediatrics, Parkville, Australia.
Susan Germano, Infectious Diseases and Microbiology Group, Parkville, Australia.
Kaya Gardiner, Infectious Diseases and Microbiology Group, Parkville, Australia.
Dan Casalaz, Department of Paediatrics, Mercy Hospital for Women, Heidelberg, Australia.
Roy M Robins-Browne, Infectious Diseases and Microbiology Group, Parkville, Australia; Department of Microbiology and Immunology, The University of Melbourne, Parkville, Australia.
Nelly Amenyogbe, Department of Experimental Medicine, University of British Columbia, Vancouver, Canada; Division of Infectious Diseases, Department of Pediatrics, University of British Columbia, Vancouver, Canada.
Nicole L Messina, Infectious Diseases and Microbiology Group, Parkville, Australia; Department of Paediatrics, Parkville, Australia.
Mihai G Netea, Department of Internal Medicine, Radboud Institute for Molecular Life Sciences, Nijmegen, The Netherlands; Radboud Center for Infectious Diseases, Radboud University Medical Center, Nijmegen, The Netherlands.
Katie L Flanagan, School of Medicine, University of Tasmania, Launceston Australia; Department of Immunology and Pathology, Monash University, Clayton, Australia.
Tobias Kollmann, Department of Experimental Medicine, University of British Columbia, Vancouver, Canada; Division of Infectious Diseases, Department of Pediatrics, University of British Columbia, Vancouver, Canada.
Nigel Curtis, Infectious Diseases and Microbiology Group, Parkville, Australia; Department of Paediatrics, Parkville, Australia; Infectious Diseases Unit, The Royal Children’s Hospital Melbourne, Parkville, Australia.
References
- 1. Aaby P, Roth A, Ravn H, et al. Randomized trial of BCG vaccination at birth to low-birth-weight children: beneficial nonspecific effects in the neonatal period?J Infect Dis 2011; 204:245–52. [DOI] [PubMed] [Google Scholar]
- 2. Shann F. The nonspecific effects of vaccines and the expanded program on immunization. J Infect Dis 2011; 204:182–4. [DOI] [PubMed] [Google Scholar]
- 3. Pollard AJ, Finn A, Curtis N. Non-specific effects of vaccines: plausible and potentially important, but implications uncertain. Arch Dis Child 2017; 102:1077–81. [DOI] [PubMed] [Google Scholar]
- 4. Kristensen I, Aaby P, Jensen H. Routine vaccinations and child survival: follow up study in Guinea-Bissau, West Africa. BMJ (Clinical research ed) 2000; 321:1435–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Higgins JP, Soares-Weiser K, Lopez-Lopez JA, et al. Association of BCG, DTP, and measles containing vaccines with childhood mortality: systematic review. BMJ (Clinical research ed) 2016; 355:i5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnoldussen DL, Linehan M, Sheikh A. BCG vaccination and allergy: a systematic review and meta-analysis. J Allergy Clin Immunol 2011; 127:246–53, 53 e1-21. [DOI] [PubMed] [Google Scholar]
- 7. Freyne B, Curtis N. Does neonatal BCG vaccination prevent allergic disease in later life?Arch Dis Child 2014; 99:182–4. [DOI] [PubMed] [Google Scholar]
- 8. Hollm-Delgado MG, Stuart EA, Black RE. Acute lower respiratory infection among Bacille Calmette-Guérin (BCG)-vaccinated children. Pediatrics 2014; 133:e73–81. [DOI] [PubMed] [Google Scholar]
- 9. Aaby P, Jensen H, Rodrigues A, et al. Divergent female-male mortality ratios associated with different routine vaccinations among female-male twin pairs. Int J Epidemiol 2004; 33: 367–73. [DOI] [PubMed] [Google Scholar]
- 10. Shann F. The non-specific effects of vaccines. Arch Dis Child 2010; 95:662–7. [DOI] [PubMed] [Google Scholar]
- 11. Mawa PA, Webb EL, Filali-Mouhim A, et al. Maternal BCG scar is associated with increased infant proinflammatory immune responses. Vaccine 2017; 35:273–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kandasamy R, Voysey M, McQuaid F, et al. Non-specific immunological effects of selected routine childhood immunisations: systematic review. BMJ (Clinical research ed) 2016; 355:i5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: intervention studies in animal models of infectious diseases. Trans R Soc Trop Med Hyg 2015; 109:52–61. [DOI] [PubMed] [Google Scholar]
- 14. Freyne B, Marchant A, Curtis N. BCG-associated heterologous immunity, a historical perspective: experimental models and immunological mechanisms. Trans R Soc Trop Med Hyg 2015; 109:46–51. [DOI] [PubMed] [Google Scholar]
- 15. Jensen KJ, Larsen N, Biering-Sørensen S, et al. Heterologous immunological effects of early BCG vaccination in low-birth-weight infants in Guinea-Bissau: a randomized-controlled trial. J Infect Dis 2015; 211:956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kleinnijenhuis J, Quintin J, Preijers F, et al. Bacille Calmette-Guerin induces NOD2-dependent nonspecific protection from reinfection via epigenetic reprogramming of monocytes. Proc Natl Acad Sci USA 2012; 109:17537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. J Anaesthesiol Clin Pharmacol 2016; 32:421–3.28096569 [Google Scholar]
- 18. Kollmann TR, Crabtree J, Rein-Weston A, et al. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol 2009; 183:7150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kollmann TR, Levy O, Montgomery RR, Goriely S. Innate immune function by Toll-like receptors: distinct responses in newborns and the elderly. Immunity 2012; 37:771–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Levy O. Innate immunity of the newborn: basic mechanisms and clinical correlates. Nat Rev Immunol 2007; 7:379–90. [DOI] [PubMed] [Google Scholar]
- 21. van Haren SD, Ganapathi L, Bergelson I, et al. In vitro cytokine induction by TLR-activating vaccine adjuvants in human blood varies by age and adjuvant. Cytokine 2016; 83:99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li Y, Oosting M, Deelen P, et al. Inter-individual variability and genetic influences on cytokine responses to bacteria and fungi. Nat Med 2016; 22:952–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Levy O, Suter EE, Miller RL, Wessels MR. Unique efficacy of Toll-like receptor 8 agonists in activating human neonatal antigen-presenting cells. Blood 2006; 108:1284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Levy O, Zarember KA, Roy RM, Cywes C, Godowski PJ, Wessels MR. Selective impairment of TLR-mediated innate immunity in human newborns: neonatal blood plasma reduces monocyte TNF-alpha induction by bacterial lipopeptides, lipopolysaccharide, and imiquimod, but preserves the response to R-848. J Immunol 2004; 173:4627–34. [DOI] [PubMed] [Google Scholar]
- 25. Johnson TR, Rao S, Seder RA, Chen M, Graham BS. TLR9 agonist, but not TLR7/8, functions as an adjuvant to diminish FI-RSV vaccine-enhanced disease, while either agonist used as therapy during primary RSV infection increases disease severity. Vaccine 2009; 27:3045–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tang FS, Van Ly D, Spann K, et al. Differential neutrophil activation in viral infections: Enhanced TLR-7/8-mediated CXCL8 release in asthma. Respirology 2016; 21:172–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hadley JS, Wang JE, Foster SJ, Thiemermann C, Hinds CJ. Peptidoglycan of Staphylococcus aureus upregulates monocyte expression of CD14, Toll-like receptor 2 (TLR2), and TLR4 in human blood: possible implications for priming of lipopolysaccharide signaling. Infect Immun 2005; 73:7613–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 2003; 20:402–14. [DOI] [PubMed] [Google Scholar]
- 29. Wang JE, Jørgensen PF, Ellingsen EA, et al. Peptidoglycan primes for LPS-induced release of proinflammatory cytokines in whole human blood. Shock 2001; 16:178–82. [DOI] [PubMed] [Google Scholar]
- 30. Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractant protein-1 (MCP-1): an overview. J Interferon Cytokine Res 2009; 29:313–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Smolen KK, Ruck CE, Fortuno ES 3rd, et al. Pattern recognition receptor-mediated cytokine response in infants across 4 continents. J Allergy Clin Immunol 2014; 133:818–26 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Levy O, Wynn JL. A prime time for trained immunity: innate immune memory in newborns and infants. Neonatology 2014; 105:136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reikie BA, Adams RC, Leligdowicz A, et al. Altered innate immune development in HIV-exposed uninfected infants. J Acquir Immune Defic Syndr 2014; 66:245–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Smith SG, Kleinnijenhuis J, Netea MG, Dockrell HM. Whole blood profiling of bacillus calmette-guérin-induced trained innate immunity in infants identifies epidermal growth factor, IL-6, platelet-derived growth factor-AB/BB, and natural killer cell activation. Front Immunol 2017; 8:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mawa PA, Nkurunungi G, Egesa M, et al. The impact of maternal infection with Mycobacterium tuberculosis on the infant response to bacille Calmette-Guerin immunization. Philos Trans R Soc Lond B Biol Sci 2015; 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Stensballe LG, Sørup S, Aaby P, et al. BCG vaccination at birth and early childhood hospitalisation: a randomised clinical multicentre trial. Arch Dis Child 2017; 102:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Costello AM, Kumar A, Narayan V, et al. Does antibody to mycobacterial antigens, including lipoarabinomannan, limit dissemination in childhood tuberculosis?Trans R Soc Trop Med Hyg 1992; 86:686–92. [DOI] [PubMed] [Google Scholar]
- 38. Beemelmanns A, Roth O. Grandparental immune priming in the pipefish Syngnathus typhle. BMC Evol Biol 2017; 17:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Green TJ, Helbig K, Speck P, Raftos DA. Primed for success: Oyster parents treated with poly(I:C) produce offspring with enhanced protection against Ostreid herpesvirus type I infection. Mol Immunol 2016; 78:113–20. [DOI] [PubMed] [Google Scholar]
- 40. Flanagan KL, Klein SL, Skakkebaek NE, et al. Sex differences in the vaccine-specific and non-targeted effects of vaccines. Vaccine 2011; 29:2349–54. [DOI] [PubMed] [Google Scholar]
- 41. Calandra T. Macrophage migration inhibitory factor and host innate immune responses to microbes. Scand J Infect Dis 2003; 35:573–6. [DOI] [PubMed] [Google Scholar]
- 42. Clark I, Awburn M. Migration inhibitory factor in the cerebral and systemic endothelium in sepsis and malaria. Crit Care Med 2002; 30:S263–7. [DOI] [PubMed] [Google Scholar]
- 43. Renner P, Roger T, Calandra T. Macrophage migration inhibitory factor: gene polymorphisms and susceptibility to inflammatory diseases. Clin Infect Dis 2005; 41(Suppl 7):S513–9. [DOI] [PubMed] [Google Scholar]
- 44. Roger T, Schneider A, Weier M, et al. High expression levels of macrophage migration inhibitory factor sustain the innate immune responses of neonates. Proc Natl Acad Sci USA 2016; 113:E997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Blimkie D, Fortuno ES 3rd, Yan H, et al. Variables to be controlled in the assessment of blood innate immune responses to Toll-like receptor stimulation. J Immunol Methods 2011; 366:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ota MO, Vekemans J, Schlegel-Haueter SE, et al. Influence of Mycobacterium bovis bacillus Calmette-Guerin on antibody and cytokine responses to human neonatal vaccination. J Immunol 2002; 168:919–25. [DOI] [PubMed] [Google Scholar]
- 47. Ritz N, Mui M, Balloch A, Curtis N. Non-specific effect of Bacille Calmette-Guérin vaccine on the immune response to routine immunisations. Vaccine 2013; 31:3098–103. [DOI] [PubMed] [Google Scholar]
- 48. Hesseling AC, Blakney AK, Jones CE, et al. Delayed BCG immunization does not alter antibody responses to EPI vaccines in HIV-exposed and -unexposed South African infants. Vaccine 2016; 34:3702–9. [DOI] [PubMed] [Google Scholar]
- 49. Said ZN, Abdelwahab KS. Induced immunity against hepatitis B virus. World J Hepatol 2015; 7:1660–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Halsey LG, Curran-Everett D, Vowler SL, Drummond GB. The fickle P value generates irreproducible results. Nat Methods 2015; 12:179–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.