Abstract
The RTS,S/AS02A malaria vaccine is based on the Plasmodium falciparum circumsporozoite protein (PfCSP), which is O-fucosylated on the sporozoite surface. We determined whether RTS,S/AS02A-induced immunoglobulin G (IgG) antibodies recognize vaccine-like nonfucosylated PfCSP better than native-like fucosylated PfCSP. Similar to previous vaccine trials, RTS,S/AS02A vaccination induced high anti-PfCSP IgG levels associated with malaria protection. IgG recognition of nonfucosylated and fucosylated PfCSP was equivalent, suggesting that PfCSP fucosylation does not affect antibody recognition.
Clinical Trials Registration. NCT00197041.
Keywords: RTS,S; antibodies; immunogenicity; Plasmodium falciparum; circumsporozoite protein (PfCSP); fucosylation; malaria
The RTS,S/AS02A malaria vaccine, in which the Plamodium falciparum circumsporozoite protein is not fucosylated, induces IgG antibodies that equally recognize nonfucosylated and native-like fucosylated target antigens, suggesting that posttranslational modification by O-fucosylation does not significantly affect antibody-antigen binding.
RTS,S/AS01E is the first malaria vaccine recommended for widespread use by the World Health Organization in African children. The vaccine has a favorable safety profile and reduces episodes of both clinical and severe malaria in children [1]. Data from the phase 3 trial showed that vaccine efficacy against clinical malaria is modest 12 months after a 3-dose primary vaccination, estimated at 55.8% (97.5% confidence interval [CI], 50.6%–60.4%) in children aged 5–17 months [1], and 31.3% (97.5% CI, 23.6%–38.3%) in infants aged 6–12 weeks [2], and the duration of protection wanes over time [1, 2]. The development of a more efficacious vaccine remains a high priority for malaria control and elimination. Multiple factors may contribute to the suboptimal efficacy of adjuvanted recombinant protein subunit vaccines like RTS,S/AS01E, and the absence of native glycosylation residues may be one important element [3]. The vaccine, produced by GlaxoSmithKline Biologicals (Belgium), contains the hepatitis B virus surface antigen (HBsAg) genetically fused to a fragment of the Plasmodium falciparum circumsporozoite protein (PfCSP) including the last 18 NANP repeats of the central domain and its C-terminal end, and is formulated as virus-like particles with a liposome-based adjuvant (AS01E) [4]. We have previously shown that the avidity of antibodies to the C-terminus of PfCSP correlates with protection against malaria following vaccination with RTS,S/AS01E [5].
The PfCSP C-terminus comprises a domain with homology to the thrombospondin type-1 repeat superfamily (TSR), which in its native form contains an O-fucosylation motif [6], and a fucose monosaccharide modification was recently identified on PfCSP from salivary gland sporozoites [7]. O-fucosylation is a simple posttranslational modification that facilitates protein folding and trafficking and may affect antigenicity [6]. Recent evidence suggests that O-fucosylation of TSR domains in P. falciparum proteins is important for liver infection and may also be relevant in the mosquito stages of the life cycle [8]. Moreover, the glycosylation profile on P. falciparum surface proteins such as PfCSP has the potential to influence parasite-specific humoral and cellular immune responses. We, therefore, hypothesized that the antibodies induced by the RTS,S vaccine, in which PfCSP is not fucosylated, might not properly recognize the native fucosylated PfCSP antigen on the sporozoite, which could compromise the overall protective efficacy. To test this hypothesis, we used immune sera from young children enrolled in the pivotal Mozambique phase 2b trial of the RTS,S vaccine formulated with a previous version of the adjuvant (AS02A) [9], and compared the levels of vaccine-induced immunoglobulin G (IgG) that bound to fucosylated versus nonfucosylated PfCSP.
METHODS
Study Participants
Participants in this phase 2b clinical trial of the malaria vaccine candidate RTS,S/AS02A in the Manhiça District, southern Mozambique (ClinicalTrials.gov registry No. NCT00197041) were children aged 1–4 years at first vaccination. The samples analyzed in this study were from children in the Manhiça cohort 1 (n = 708) (Supplementary Figure 1). The clinical trial definitions, study design and population, procedures, and interventions have been described elsewhere [9].
Ethics
The clinical trial research protocols were approved by the National Mozambican Ethics Review Committee, the Hospital Clínic of Barcelona Ethics Review Committee in Spain, and the PATH-Malaria Vaccine Initiative Research Ethics Committee, and all parents or legal guardians of the participants provided written informed consent.
Antigens
The recombinant full-length PfCSP was expressed using the Pichia pastoris expression system, and the TSR C-terminal domain of PfCSP was expressed in Escherichia coli. Both PfCSP TSR and full-length PfCSP fucosylated domains were produced using the human protein O-fucosyltransferase 2 (huPoFUT2). The details of the antigens production, purification and sequences can be found in Supplementary Materials and Methods and Supplementary Figure 2.
Antibody Assays
An in-house multiplex bead-based antibody assay was performed to assess IgG responses against fucosylated and nonfucosylated versions of the PfCSP full-length and the C-terminal TSR domain (Supplementary Materials and Methods and Supplementary Figure 2). The assay applied the xMAP technology (Luminex Corporation), previously standardized and optimized [10]. Briefly, antigens were coupled to magnetic beads, multiplexed, and incubated with 50 μL of the test, negative control, or positive control serum samples. Next, beads were washed and incubated with a biotinylated secondary antibody, washed again, and incubated with streptavidin-R-phycoerythrin. Finally, using a Luminex xMAP 100/200 analyzer, at least 50 microspheres per analyte were acquired per well. The IgG levels were measured as median fluorescence intensity [10] and transformed to levels in arbitrary units using the modelled calibration curves from serial dilutions of a positive reference sample (Supplementary Figure 3).
Statistical Analysis
Antibody levels were compared with boxplots showing geometric means, medians, and interquartile ranges. Fold-changes in IgG level geometric means between time points or between vaccination groups were estimated by calculating average differences in log10-transformed measurements. The 95% CI and P values were obtained from 2-tailed 2-sample t tests for comparisons between vaccination groups and 2-tailed paired t tests for comparisons between time points. Through exponentiation of the log10-transformed level differences and their corresponding 95% CI, we obtained the desired IgG level fold-change estimates.
To study the relationship between the levels of IgG binding to fucosylated and nonfucosylated antigens, we used scatter plots to capture the bivariate distribution, acknowledging that the closer the points to the diagonal, the closer the relationship to that of perfect identity. We also calculated the Pearson correlation coefficients to assess the strength of this relationship. Statistics and plots were always conducted or shown in log10-transformed levels or scales to stabilize the variance.
RESULTS
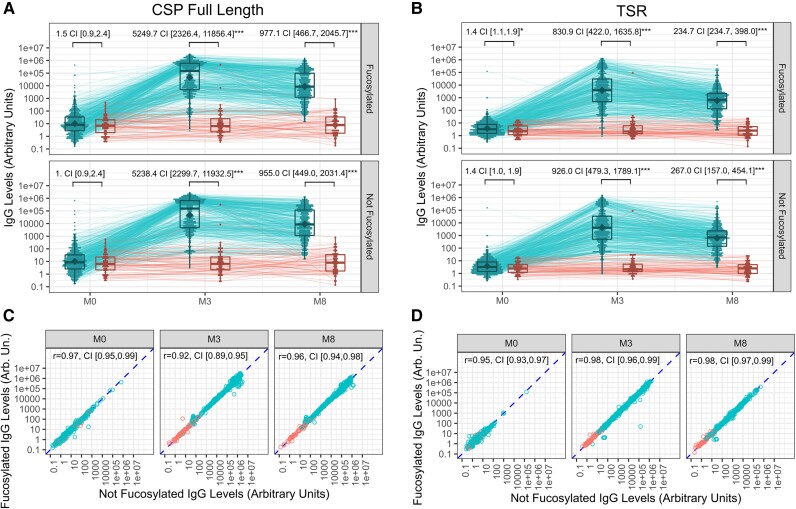
The baseline characteristics comparing vaccination groups (Supplementary Table 1) and prevaccination antibody levels (month 0) were similar for both fucosylated and nonfucosylated versions of PfCSP full-length (Figure 1A) and C-terminus TSR domain-only antigens (Figure 1B). Primary vaccination (month 3) anti-PfCSP IgG reactivity was strong, with fold-increases in geometric mean levels >1000 for RTS,S vaccinees as compared to the comparator group. These data are well aligned with previous estimates using enzyme-linked immunosorbent assay (ELISA) from a larger longitudinal study of the same clinical trial [11] and with other RTS,S clinical trials measuring anti-NANP IgG [12]. Anti-TSR IgG responses were somewhat weaker than the full-length PfCSP IgG responses, with increase estimates between vaccination groups at month 3 that were 5- to 6-fold lower for TSR. Also, as expected, PfCSP full-length and TSR antibody levels waned between month 3 and month 8 (reductions by 5- and 3.5-fold, respectively), closely replicating previous estimates of IgG decay rates [11]. Contrary to our expectation, we observed nearly identical responses when we compared IgG levels bound to fucosylated versus nonfucosylated PfCSP full-length and TSR antigens. Figure 1C and 1D shows anti-PfCSP fucosylated versus not fucosylated IgG levels, yielding a bivariate distribution that approached perfect correlation.
Figure 1.
RTS,S/AS02A induces strong IgG responses that equally recognize fucosylated and nonfucosylated Plasmodium falciparum CSP and C-terminal TSR antigen constructs. A and B, Levels of IgG antibodies binding to fucosylated (y-axis) and nonfucosylated (x-axis) PfCSP full-length (A) and C-terminus TSR (B) at different time points (month 0, 3, and 8). Boxplots represent the geometric mean (diamond), median (central lines), quartiles (box limits), and estimated range (whiskers extending 1.5 times the interquartile range). IgG levels from the same individual across time points are linked with thin lines (longitudinal trajectories). Vaccination groups were compared through a 2-sample t test on the log10-transformed levels and statistical significance indicated by asterisks (*P < .05; **P < .01, ***P < .001). C and D, Scatter plots comparing IgG antibody levels binding to the fucosylated antigen (y-axis) versus those binding to the nonfucosylated equivalent (x-axis). The comparison is repeated for all time points (month 0, 3, and 8) for PfCSP full-length (C) and TSR (D). The Pearson correlation coefficient is computed across all observations. Abbreviations: AU, arbitrary unit; CI, 95% confidence interval; CSP, circumsporozoite protein; IgG, immunoglobulin G; PfCSP, Plasmodium falciparum circumsporozoite protein; TSR, thrombospondin type-1 repeat.
Next, we examined the children's characteristics affecting increases in IgG antibody levels reacting to fucosylated PfCSP constructs relative to prevaccination. We observed no significant differences in the rise of IgG geometric means between pre- and postvaccination time points against the fucosylated PfCSP proteins between age groups or sexes in the RTS,S vaccinees. However, we observed a lower increase of IgG to fucosylated TSR in older children (2–4 years) compared to younger (1–2 years) after RTS,S vaccination (month 3) (P = .02; Table 1). As expected, higher increases in anti-PfCSP full-length and TSR IgG levels were associated with protection from disease. Thus, RTS,S-vaccinated children who did not develop clinical malaria within the first year of follow-up had significantly higher anti-PfCSP IgG increases from month 0 to month 3 (P = .042; Table 1) as well as a trend towards higher anti-TSR IgG increases (P = .2). Differences were larger and more clearly significant for anti-PfCSP IgG level increases from month 0 to month 8 for both full-length (P = .0004) and TSR (P = .005) (Table 1). Associations between IgG levels against nonfucosylated PfCSP full-length and TSR antigens with age, sex, and clinical malaria were identical to the results reported for fucosylated antigens (Supplementary Table 2).
Table 1.
Factors Affecting Increases in IgG Antibody Levels Reacting to Fucosylated PfCSP Constructs
| Compared Conditions | Month 3/Month 0 | Month 8/Month 0 | ||
|---|---|---|---|---|
| Fold Difference (95% CI) | P Value | Fold Difference (95% CI) | P Value | |
| Antigen PfCSP full length | ||||
| Increase ratio estimate | 5079.1 (3833.1–6730.01) | <.0001*** | 829.2 (631.9–1088.2) | <.0001*** |
| Old/young | 0.65 (.34–1.25) | .20 | 0.61 (.32–1.14) | .12 |
| Female/male | 1.04 (.59–1.83) | .89 | 0.83 (.48–1.43) | .48 |
| P. falciparum not infected/infected | 1.96 (1.02–3.75) | .042* | 3.07 (1.64–5.73) | .0004** |
| Antigen PfCSP TSR | ||||
| Increase ratio estimate | 778.4 (625.7–968.3) | <.0001*** | 153.3 (128.0–183.5) | < .0001*** |
| Old/young | 0.56 (.34–.92) | .02* | 0.7 (.46–1.06) | .09 |
| Female/male | 1.1 (.71–1.7) | .68 | 0.85 (.59–1.21) | .36 |
| P. falciparum not infected/infected | 1.39 (.84–2.31) | .2 | 1.8 (1.18–2.72) | .005* |
Associations are reported as ratio estimates of month 3/month 0 or month 8/month 0 between groups of interest (compared conditions).
Abbreviations: CI, confidence interval; PfCSP, Plasmodium falciparum circumsporozoite protein; TSR, thrombospondin type-1 repeat.
* P < .05; **P < .01, ***P < .001.
To determine whether malaria endemicity could influence the binding of vaccine or naturally induced IgG to fucosylated versus nonfucosylated PfCSP antigens, another set of samples (n = 124) from Ilha Josina, a trial site of higher transmission intensity (cohort 2) [9], was also tested. Again, no difference was noted in recognition of fucosylated compared to nonfucosylated PfCSP antigens (Supplementary Figure 4), suggesting that the binding is independent of malaria exposure.
DISCUSSION
To our knowledge, no previous study has directly tested whether RTS,S vaccine-induced IgG antibodies bind less to a native-like fucosylated PfCSP (present on P. falciparum sporozoites) [7] than to nonfucosylated PfCSP (as present in the RTS,S vaccine). Of note, while our results show that RTS,S-induced antibodies recognize similarly fucosylated and nonfucosylated PfCSP constructs, we observed the same phenomenon in prevaccination and comparator postvaccination samples. This suggests that even IgG induced by natural exposure, presumably to fucosylated PfCSP on sporozoites, efficiently recognized the target antigen epitopes regardless of fucosylation.
Because our assay was multiplexed and tested the same sample for each individual against 2 slightly different PfCSP presentations, potential differences in the binding of any relatively important subpopulation of polyclonal IgG would have implied a divergence in the paired antigen-specific IgG levels, which was not observed.
Nevertheless, we cannot rule out a potential benefit of an alternative RTS,S vaccine containing fucosylated PfCSP. To establish this, fucosylated and nonfucosylated PfCSP-based vaccines should be tested head-to-head to compare overall immunogenicity (humoral and cellular responses) and vaccine efficacy. The concentration and quality of immunoglobulins might differ when induced by a fucosylated PfCSP, particularly IgG subclasses, their avidity, and their neutralizing and nonneutralizing functional capacities. It is also possible that a fucosylated PfCSP protein-based vaccine could lead to broader glycan-dependent T helper and cytotoxic cell responses, as it can be presented on both MHC-I and MHC-II molecules, and the resulting complex can be recognized by T cells. Recent advances in human immunodeficiency virus (HIV-1) [13] and hepatitis C virus (HCV) [14] vaccine development have been stimulated by a better understanding of the HIV-1 envelope spike and HCV E1 and E2 glycan composition and their interactions with the human immune responses. These advances highlight increasing evidence that glycans are important antigenic determinants in immune responses to various pathogens. Their relevance for prophylactic and therapeutic vaccine design and development is worth exploring in more depth.
Limitations of our study include the lack of data on IgG subclasses, which have different affinities for cellular Fc receptors, thus mediating different antiparasite effector functions, and functional antibody measures. In addition, we used PfCSP, recombinantly expressed in P. pastoris instead of native PfCSP, which might replicate better the complexity of the sporozoite surface. However, obtaining sufficient quantities of native PfCSP poses significant technical challenges and the potential divergent outcomes remain uncertain, considering our robust data that show a similar recognition of fucosylated and nonfucosyltated protein. In contrast, recombinant PfCSP can be readily overexpressed in P. pastoris, its production has been optimized, and it is well characterized [15], possibly mirroring native PfCSP conformation.
In conclusion, our data suggest that posttranslational modification by O-fucosylation in PfCSP, absent in the RTS,S vaccine, does not affect antibody-antigen binding both for IgG induced by vaccination (not fucosylated) and natural infections (fucosylated). This negative result does not weigh in favor of the hypothesis that a fucosylated PfCSP-based vaccine could lead to improved vaccine efficacy. However, this result alone is insufficient to rule it out and further experiments are required.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Chenjerai Jairoce, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique.
Dídac Macià, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Barcelona, Spain.
Jorge P Torres-Yaguana, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain.
Leonie Mayer, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Université Claude Bernard Lyon 1, Université de Lyon, Villeurbanne, France.
Marta Vidal, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain.
Rebeca Santano, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain.
Ramón Hurtado-Guerrero, Institute of Biocomputation and Physics of Complex Systems, University of Zaragoza, Zaragoza, Spain; Copenhagen Center for Glycomics, Department of Cellular and Molecular Medicine, University of Copenhagen, Copenhagen, Denmark; Fundación Agencia Aragonesa para la Investigación y el Desarrollo, Zaragoza, Spain.
Karine Reiter, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
David L Narum, Laboratory of Malaria Immunology and Vaccinology, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Borja Lopez-Gutierrez, Emerging Pathogens Institute, Department of Infectious Diseases and Immunology, College of Veterinary Medicine, University of Florida, Gainesville, Florida, USA.
Timothy Hamerly, Emerging Pathogens Institute, Department of Infectious Diseases and Immunology, College of Veterinary Medicine, University of Florida, Gainesville, Florida, USA.
Jahit Sacarlal, Centro de Investigação em Saúde de Manhiça, Maputo, Mozambique.
Ruth Aguilar, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain.
Rhoel R Dinglasan, Emerging Pathogens Institute, Department of Infectious Diseases and Immunology, College of Veterinary Medicine, University of Florida, Gainesville, Florida, USA.
Gemma Moncunill, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Barcelona, Spain.
Luis Izquierdo, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Barcelona, Spain.
Carlota Dobaño, Barcelona Institute for Global Health (ISGlobal), Hospital Clínic-Universitat de Barcelona, Barcelona, Catalonia, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas (CIBERINFEC), Barcelona, Spain.
Notes
Acknowledgments. We thank the study volunteers and their families for their participation, the clinical, field, and laboratory teams, and their research institutions. The authors acknowledge James M. Rini, University of Toronto, for providing key reagents.
Author contributions. R. R. D., G. M., L. I., and C. D. contributed conceptualization. C. J., J. P. T.-Y., L. M. and M. V. performed experimental work. R. A., C. D., G. M. supervised laboratory work. D. M. and R. S. performed data curation and analysis. J. S. coordinated the clinical trial. C. J., D. M., R. A., G. M., L. I., and C. D. wrote the original draft. R. H.-G. A., K. R., D. L. N., B. L.-G., T. H., and R. R. D. performed antigen expression and modification. All authors critically read, revised, and approved the final manuscript.
Disclaimer. The content and interpretation are solely the responsibility of the investigators and do not necessarily represent the official views of the funders. GSK was provided with the opportunity to review a draft of this manuscript.
Data availability. The datasets generated and/or analyzed in the current investigation are available from the corresponding author upon reasonable request.
Financial support. This work was supported by the Secretaria d'Universitats i Recerca del Departament d'Empresa i Coneixement de la Generalitat de Catalunya cofunded by the European Social Fund (grant number 2019 FI B 00986 AGAUR-FI scholarship to C. J.); the European Commission (L. M. registered on Erasmus+Mundus Joint Master Degree Leading International Vaccinology Education, cofunded by the Education, Audiovisual and Culture Executive Agency); Fondation Mérieux (scholarship to L. M.); Ministry of Science and Innovation, Spain (grant number PID2019-110810RB-I00/AEI/10.13039/501100011033 to L. I.); European Social Fund (grant number RYC2020-029886-I/AEI/10.13039/501100011033 to G. M.); PATH Malaria Vaccine Initiative (support for Mozambican phase 2b clinical trial); GlaxoSmithKline Biologicals S.A (sponsor of the study, NCT00197041, during which samples were collected); and Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases. Core funding for Centro de Investigação em Saúde de Manhiça is provided by the Agencia Española de Cooperación Internacional para el Desarrollo. ISGlobal is supported by the Spanish Ministry of Science and Innovation through the Centro de Excelencia Severo Ochoa 2019-2023 Program (grant number CEX2018-000806-S), and the Generalitat de Catalunya through the CERCA Program. This work is part of the ISGlobal Program on the Molecular Mechanisms of Malaria, partially supported by the Fundación Ramón Areces.
References
- 1. RTS,S Clinical Trials Partnership; Agnandji ST, Lell B, Soulanoudjingar SS, et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N Engl J Med 2011; 365:1863–75. [DOI] [PubMed] [Google Scholar]
- 2. RTS,S Clinical Trials Partnership; Agnandji ST, Lell B, et al. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med 2012; 367:2284–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goddard-Borger ED, Boddey JA. Implications of Plasmodium glycosylation on vaccine efficacy and design. Future Microbiol 2018; 13:609–12. [DOI] [PubMed] [Google Scholar]
- 4. Kaslow DC, Biernaux S. RTS,S: toward a first landmark on the malaria vaccine technology roadmap. Vaccine 2015; 33:7425–32. [DOI] [PubMed] [Google Scholar]
- 5. Dobaño C, Sanz H, Sorgho H, et al. Concentration and avidity of antibodies to different circumsporozoite epitopes correlate with RTS,S/AS01E malaria vaccine efficacy. Nat Commun 2019; 10:2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Holdener BC, Haltiwanger RS. Protein O-fucosylation: structure and function. Curr Opin Struct Biol 2019; 56:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Swearingen KE, Lindner SE, Shi L, et al. Interrogating the Plasmodium sporozoite surface: identification of surface-exposed proteins and demonstration of glycosylation on CSP and TRAP by mass spectrometry-based proteomics. PLoS Pathog 2016; 12:e1005606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lopaticki S, Yang ASP, John A, et al. Protein O-fucosylation in Plasmodium falciparum ensures efficient infection of mosquito and vertebrate hosts. Nat Commun 2017; 8:561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Alonso PL, Sacarlal J, Aponte JJ, et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: randomised controlled trial. Lancet 2004; 364:1411–20. [DOI] [PubMed] [Google Scholar]
- 10. Ubillos I, Aguilar R, Sanz H, et al. Analysis of factors affecting the variability of a quantitative suspension bead array assay measuring IgG to multiple Plasmodium antigens. PLoS One 2018; 13:e0199278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Aide P, Dobã No C, Sacarlal J, et al. Four year immunogenicity of the RTS,S/AS02 A malaria vaccine in Mozambican children during a phase IIb trial. Vaccine 2011; 29:6059–67. [DOI] [PubMed] [Google Scholar]
- 12. White MT, Verity R, Griffin JT, et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect Dis 2015; 15:1450–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Crispin M, Ward AB, Wilson IA. Structure and immune recognition of the HIV glycan shield. Annu Rev Biophys 2018; 47:499–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sepulveda-Crespo D, Resino S, Martinez I. Hepatitis C virus vaccine design: focus on the humoral immune response. J Biomed Sci 2020; 27:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Herrera R, Anderson C, Kumar K, et al. Reversible conformational change in the Plasmodium falciparum circumsporozoite protein masks its adhesion domains. Infect Immun 2015; 83:3771–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.