Abstract
Addiction, characterized by compulsive drug-seeking behavior and impaired self-control, remains a significant public health concern. Understanding the neurobiology of addiction is crucial for identifying novel therapeutic targets and further developing effective treatments. Recently, the apelin/APJ system, an emerging signaling pathway, has attracted attention for its involvement in various neuropsychiatric disorders. The cross-talk between the apelin/APJ system and hypothalamic mu opioid signaling, as well as its heterodimerization with kappa opioid receptors (KORs), supports the potential relevance of this system to addiction. Moreover, several protective effects of apelin against various addictive substances, including methamphetamine, morphine, and alcohol, underscore the need for further investigation into its role in substance use disorder. Understanding the contribution of the apelin/APJ system in addiction may offer valuable insights into the underlying neurobiology and pave the way for novel therapeutic interventions in substance use disorders. This review provides a concise overview of the apelin/APJ system, emphasizing its physiological roles and highlighting its relevance to addiction research.
Keywords: Apelin, APJ, Addiction, Substance use disorder, Drug abuse
Introduction
Addiction can be delineated as a persistent and recurrent disorder, displaying an inherent compulsion to actively pursue and consume a particular substance, coupled with a diminished ability to exert control over intake. Notably, the emergence of a detrimental emotional state becomes apparent upon the obstruction of access to said substance.1 The understanding of the neurobiology underlying addiction has advanced through the investigations of animal models and, more recently, by means of brain imaging investigations conducted on individuals affected by addiction. The activation of brain reward systems occurs upon the administration of drugs of abuse, and extensive research conducted on drug addiction has predominantly elucidated the neurocircuitry associated with the reward process.2
Drug addiction disrupts multiple brain regions and circuits, each of which is likely to contribute differentially to the intricate phenotype observed in addicted individuals. Although much focus has been put into the role of opioid system,3 dopaminergic signaling,4 and glutamatergic neurotransmission,5 in drug reward and development of addiction, emerging signaling systems with multiple implications in the central nervous system (CNS) have gathered attention. In this review, we aimed to gather evidence on the potential involvement of a relatively new system called apelin/APJ in addiction. We first provide an overview of the system along with its various neuroprotective roles. Based on the protective effects of apelin against drugs of abuse and its interplay with opioid receptors (OPRs), we further raise hypotheses on how apelin/APJ signaling might be a potential system worth exploring in the context of addiction.
An overview of the apelin/APJ system
APJ, discovered by O’Dowd in 1993, is a G protein-coupled receptor (GPCR) derived from the human gene.6 Initially characterized as an orphan GPCR, APJ is composed of 380 amino acids and exhibits a hydrophobic region with a homology of 40% to 50% to the angiotensin type I receptor.7 Later in 1998, Tatemoto et al successfully isolated and purified the endogenous ligand of APJ from bovine gastric secretions. They subsequently named this ligand “apelin”.8
Apelin is expressed from the APLN gene and is initially synthesized as a 77-amino acid pre-propeptide.9 Proteolytic cleavage of the pre-proapelin molecule by specific proteases leads to the generation of various biologically active apelin peptide forms, such as apelin-13, apelin-12, apelin-17, and apelin-36s. Apelin-13 and apelin-36 are considered to be the most prevalent and biologically active fragments among the different apelin peptide forms.10,11
Both apelin and APJ mRNA exhibit broad expression patterns in various organs and tissues, encompassing the CNS, cardiovascular system, gastrointestinal tract, lung, kidney, and placenta.12,13 Apelin is mostly known for its pivotal physiological roles in cardiovascular physiology by regulating heart contractility, mediating vasorelaxation and facilitating neoangiogenesis.14,15 Furthermore, apelin is involved in bone physiology, modulation of immune system function, maintenance of body fluid homeostasis, and energy metabolism.16,17 The extensive involvement of apelin/APJ system in different physiological functions of the body has made it a potential target in developing drugs, particularly gathering attention as an emerging therapeutic target for neurological diseases.18
Role of apelin/APJ system in various neuropsychiatric disorders
The intricate interplay between molecular signaling pathways and the complex manifestations of neuropsychiatric disorders has captivated the attention of researchers worldwide. Among the fascinating avenues of investigation in recent years is the apelin/APJ system, that has emerged as a potential key player in the pathophysiology of numerous neuropsychiatric disorders.19 This system has increasingly garnered attention for its involvement in diverse aspects of brain function and behavior. In this section, we explore the connections between the apelin/APJ system and several neuropsychiatric disorders, shedding light on its potential as a novel therapeutic target and offering fresh insights into the neurobiology underlying these debilitating conditions.
Initial investigations have demonstrated that apelin-13 exhibits a dose-dependent capability to mitigate brain injuries and post-ischemic cerebral edema. These effects are believed to be achieved through the inhibition of apoptosis.20 Subsequent studies have provided additional evidence supporting the protective role of various isoforms of apelin, such as apelin-36, apelin-17, and apelin-12, in the context of ischemic stroke. These findings further underscore the significance of the apelin/APJ system in the pathophysiology of ischemic stroke.21 The neuroprotective properties of apelin extend into its impact on neurodegenerative diseases. Studies have shown that apelin-13 has the ability to alleviate the dopaminergic neurotoxicity induced by 6-hydroxydopamine (6-OHDA), a neurotoxin known for selectively damaging dopaminergic neurons in the animal model of Parkinson’s disease (PD).22 Apelin could also significantly improve cognitive and motor impairments in PD rats.23,24 Other studies have pointed out the neuroprotective effects of apelin in animal models of Alzheimer’s disease (AD). An illustrative example is the potential of apelin-13 to ameliorate autophagy and apoptosis by modulating the mTOR signaling pathway. This mechanism has been shown to contribute to the improvement of working and spatial memory impairments induced by amyloid-beta (Aβ), a hallmark feature of AD,25 suggesting its potential as a promising target for AD prevention and treatment.26
Besides its impact on neurodegenerative neurological diseases, apelin exerts several behavioral and cognitive effects on mental disorders. Preliminary studies have indicated the presence of apelin and APJ receptor mRNA in brain regions such as the hypothalamus, amygdala, and dentate gyrus, implying that the apelin/APJ system might be involved in the regulation of emotional behaviors.27,28 In line with this, recent studies have underscored the role of apelin in the amelioration of stress-induced depression-like phenotypes,29 as well as in reversing the memory impairments following chronic stress.30
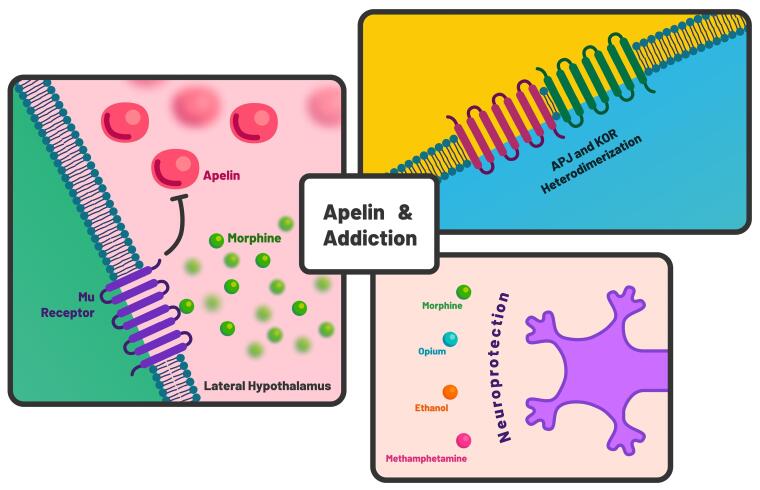
Given the substantial involvement of the apelin/APJ system in neurological and psychiatric disorders, it is not surprising that its role may extend to the neurobiology of addiction and the underlying mechanisms related to drug response. In the subsequent sections, we highlight current research studies that underscore the potential of the apelin/APJ system within the context of addiction (Figure 1).
Figure 1.
An overview of the potential of apelin/APJ system in addiction. Interaction of mu receptor and apelin in lateral hypothalamus, as well as the heterodimerization of APJ and kappa opioid receptor (KOR), along with neuroprotective effects of apelin against drugs of abuse contribute to its potential involvement in addiction medicine
Protective effects of apelin against drugs of abuse
Besides its protective properties in various neurological disorders, several studies have suggested that apelin possesses cytoprotective effects against drugs of abuse. Foroughi and colleagues showed that apelin-13 decreased the apoptotic response post-methamphetamine exposure in PC12 cells by increasing cell viability and reducing apoptotic rates. Moreover, apelin could reduce autophagy as well as intracellular reactive oxygen species (ROS) in methamphetamine-exposed cells.31 These findings indicate that apelin could potentially ameliorate drug-induced neurotoxicity via the decrease in oxidative damage, apoptosis, and autophagy cell death. Therefore, apelin could be considered a neuroprotective agent against neurodegeneration caused by psychostimulant drugs.
Alterations in apelin expression have also been reported following morphine dependence. Yildiz et al demonstrated that morphine-dependent rats exhibited a slight increase in apelin gene expression in the hippocampus compared to the control group, suggesting that apelin expression might have been altered to compensate for the neurodegeneration caused by morphine addiction.32 Similar observations were reported in another study where morphine-dependent rats exhibited significant increases in serum apelin levels compared to their healthy counterparts.33
Several studies have also pointed out the neuroprotective effects of apelin on alcohol neurotoxicity. For instance, it has been demonstrated that apelin-13 could significantly alleviate the cognitive impairment and anxiety-like behavior associated with fetal alcohol spectrum disorder in pups of rats exposed to ethanol.34 Moreover, apelin could significantly increase the brain-derived neurotrophic factor (BDNF) and decrease necrotic cell death induced by alcohol neurotoxicity.34 In another study, apelin-13 could alleviate ethanol-induced-hippocampal neurotoxicity through anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms.35
The protective effects of apelin in addicted individuals extend beyond its preventive actions against neurotoxicity. For instance, while Tavanai et al reported that opium-addicted patients with acute myocardial infarction did not exhibit altered serum levels of apelin compared to non-addicted patients, they suggested that apelin is associated with the protection of cardiomyocytes in addicted patients.36 An overview of studies on the protective effects of apelin against drugs of abuse is presented in Table 1. Overall, these findings suggest that apelin might provide clinical utility against the cytotoxicity of abused substances. However, there is a paucity of studies in this field, particularly on other psychostimulant drugs, including cocaine, which warrants further research.
Table 1. Protective effects of apelin against drugs of abuse .
| Drug | Type of species | Apelin isoform (dose) | Cytoprotective effects | Reference |
| Methamphetamine | PC12 cells of rat | Apelin-13 (4 mM) | Reduction of oxidative damage, apoptosis, and autophagy cell death | 31 |
| Morphine | Male Wistar rats | Hippocampal apelin | Increased expression of apelin in compensation for morphine-induced neurodegeneration | 32 |
| Morphine | Male Wistar rats | Serum apelin | Significant increase in serum apelin levels of morphine-dependent rats | 33 |
| Opium | Acute myocardial infarction patients | Serum apelin | Cardioprotective role of apelin in opium addicted patients | 36 |
| Ethanol | Wistar rats | Apelin-13 (25 and 50 µg) | Amelioration of cognitive impairment and anxiety-like behavior. Increase in BDNF expression and decrease in necrotic cell death | 34 |
| Ethanol | Wistar rats | Apelin-13 (25 and 50 µg) | Alleviation of spatial memory impairment through anti-oxidative, anti-inflammatory, and anti-apoptotic mechanisms | 35 |
The interplay between APJ and opioid receptors: insights into the potential involvement of apelin/APJ system in addiction
OPRs represent a well-established class of GPCRs that participate in numerous physiological processes within the body. These receptors consist of three primary subtypes: mu, delta, and kappa. Each subtype plays a distinct role in mediating the effects of endogenous opioids and exogenous opioid drugs, thereby influencing pain perception and other related physiological responses, including addictive behavior.37 There are several findings indicative of the interplay between the apelin/APJ system and opioid signaling, suggesting that this system might be involved in the neurobiology of addiction. Herein, we focus on two opioid-related signalings associated with the apelinergic system that strengthen the possibilities of its involvement in addiction.
Hypothalamic mu receptor signaling and apelin in hedonic homeostasis
The lateral hypothalamus is involved in the regulation of motivated behaviors and serves as a crucial player in modulating reward processes.38 This hypothalamic region comprises intrinsic neuronal populations that establish connections with reward-related regions, including the ventral tegmental area and nucleus accumbens.39-41 The activation of lateral hypothalamus neurons contributes significantly to the modulation of the hedonic properties associated with both natural rewarding stimuli and substances of abuse.42 Research findings have indicated that lateral hypothalamus neurons mediate certain aspects of the rewarding effects observed with morphine.43,44 The mu opioid receptor (MOR) is responsible for generating rewarding effects through direct activation by morphine, as well as indirect activation by various other drugs of abuse, such as tetrahydrocannabinol, nicotine, cocaine, and alcohol.45-48 Additionally, the MOR can be stimulated by naturally occurring endogenous opioids, leading to rewarding experiences.49 As a result, this receptor is consistently engaged throughout episodes of drug intake, and excessive stimulation of the MOR probably plays a role in the disruption of reward systems observed in individuals who are dependent on drugs.
It has been demonstrated that chronic stimulation of MORs modifies transcriptional activity within the lateral hypothalamus. Befort et al reported that chronic activation of MOR in the lateral hypothalamus resulted in the downregulation of apelin gene, indicating the potential role of apelin in opioid signaling and hedonic homeostasis.50 The discovery of decreased apelin transcript levels in the lateral hypothalamus of animals dependent on morphine has two significant implications. Firstly, this observation suggests the potential involvement of apelin peptide in maintaining hedonic homeostasis. Findings of two other studies indicating altered levels of hypothalamic and serum apelin in morphine-dependent rat support this notion.32,33 Secondly, there is evidence demonstrating the interaction between endogenous opioids of the brain and various peptides, including cholecystokinin and neuropeptide FF.51 Apelin is now identified as a novel opioid-related neuropeptide and the interaction between this peptide and MOR signaling in the lateral hypothalamus, suggests that it could be involved in the reward system and development of addiction.
Heterodimerization of kappa opioid receptor and APJ: a lead for further investigation
The kappa opioid receptor (KOR) and its endogenous ligands, dynorphins, exhibit widespread expression in the CNS.52 Upon activation, KOR exerts significant influence on a range of physiological and pharmacological responses, including analgesia, dysphoria, and attenuation of drug-seeking behavior in individuals with substance use disorders.53 KOR agonists have the ability to inhibit the rewarding effects associated with morphine and psychostimulants like cocaine54-56; hence, due to their ability to attenuate the rewarding effects of addictive substances, KOR agonists hold promise as potential therapeutic agents for the treatment of drug abuse. Furthermore, the use of KOR antagonists, such as norbinaltorphimine (nor-BNI) and 5’-guanidinonaltrindole (5’-GNTI) has demonstrated efficacy in alleviating depressive and anxiety-related disorders, which often arise as common withdrawal symptoms and can contribute to relapse in drug use.57 By targeting the KOR system, these antagonists have shown potential in mitigating these adverse effects and reducing the likelihood of relapse in individuals recovering from substance abuse.57
The current understanding of GPCRs has evolved to recognize their existence and functional activity as dimers. It is now widely accepted that GPCRs can form dimers, and emerging evidence suggests the physiological relevance and significance of GPCR heterodimers. Studies have revealed that human APJ forms a heterodimer with KOR resulting in increased protein kinase C and decreased protein kinase A activity.58 Notably, the apelin/APJ and KOR share similar signaling pathways, most possibly due to their heterodimerization, in various critical cellular processes, including cell proliferation, pain regulation, blood pressure maintenance, and gastrointestinal transit (for review, see 59). For instance, activation of APJ results in pain modulation through enhanced dynorphins/KOR signaling.60 Given the close interaction of downstream signaling pathways and the heterodimerization of these two receptors, it is not far-fetched to imagine that the apelin/APJ system may play a role in KOR-mediated actions within the neurobiology of addiction. The extensive distribution of APJ and KOR in neuroanatomical regions involved in the reward system, including the nucleus accumbens, strengthens this hypothesis.61,62 Indeed, further research is required to elucidate the intricate cross-talk between these systems and their implications in the neurobiology of addiction.
Concluding remarks and future perspectives
Overall, growing evidence suggests that the apelin/APJ system may have implications for addiction, given its protective effects against drugs of abuse and its interplay with OPRs. The exploration of the apelin/APJ system in addiction opens up exciting avenues for future research. Understanding these mechanisms will not only provide valuable insights into the neurobiology of addiction but may also pave the way for the development of novel therapeutic strategies. Based on the existing evidence on the interplay between the apelin/APJ system and OPRs, further studies are needed to unravel the significance of these interactions in the development of addictive behaviors.
Citation: Saboori Amleshi R, Soltaninejad M, Ilaghi M. Potential involvement of apelin/APJ system in addiction and neuroprotection against drugs of abuse. Addict Health. 2024;16(3):198–204. doi:10.34172/ahj.1479
Funding Statement
The authors received no financial support for the current study.
Footnotes
Authors’ Contribution
Conceptualization: Mehran Ilaghi.
Investigation: Reza Saboori Amleshi, Masoud Soltaninejad, Mehran Ilaghi.
Methodology: Reza Saboori Amleshi, Masoud Soltaninejad, Mehran Ilaghi.
Project administration: Mehran Ilaghi.
Supervision: Mehran Ilaghi.
Validation: Mehran Ilaghi.
Visualization: Reza Saboori Amleshi.
Writing–original draft: Reza Saboori Amleshi, Masoud Soltaninejad, Mehran Ilaghi.
Writing–review & editing: Mehran Ilaghi.
Competing Interests
The authors declare that no competing and financial interests exist.
Consent for Publication
Not applicable.
Data Availability Statement
Not applicable.
Ethical Approval
All study protocols were conducted under the approval of the Ethics Committee of Kerman University of Medical Sciences and in accordance with relevant guidelines and regulations.
References
- 1.Koob GF, Volkow ND. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry. 2016;3(8):760–73. doi: 10.1016/s2215-0366(16)00104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belzeaux R, Lalanne L, Kieffer BL, Lutz PE. Focusing on the opioid system for addiction biomarker discovery. Trends Mol Med. 2018;24(2):206–20. doi: 10.1016/j.molmed.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 4.Wise RA, Jordan CJ. Dopamine, behavior, and addiction. J Biomed Sci. 2021;28(1):83. doi: 10.1186/s12929-021-00779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tzschentke TM, Schmidt WJ. Glutamatergic mechanisms in addiction. Mol Psychiatry. 2003;8(4):373–82. doi: 10.1038/sj.mp.4001269. [DOI] [PubMed] [Google Scholar]
- 6.Falcão-Pires I, Leite-Moreira AF. Apelin: a novel neurohumoral modulator of the cardiovascular system Pathophysiologic importance and potential use as a therapeutic target. Rev Port Cardiol. 2005;24(10):1263–76. [PubMed] [Google Scholar]
- 7.Gurzu B, Petrescu BC, Costuleanu M, Petrescu G. Interactions between apelin and angiotensin II on rat portal vein. J Renin Angiotensin Aldosterone Syst. 2006;7(4):212–6. doi: 10.3317/jraas.2006.040. [DOI] [PubMed] [Google Scholar]
- 8.Tatemoto K, Hosoya M, Habata Y, Fujii R, Kakegawa T, Zou MX, et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem Biophys Res Commun. 1998;251(2):471–6. doi: 10.1006/bbrc.1998.9489. [DOI] [PubMed] [Google Scholar]
- 9.Charles CJ. Putative role for apelin in pressure/volume homeostasis and cardiovascular disease. Cardiovasc Hematol Agents Med Chem. 2007;5(1):1–10. doi: 10.2174/187152507779315804. [DOI] [PubMed] [Google Scholar]
- 10.Ladeiras-Lopes R, Ferreira-Martins J, Leite-Moreira AF. The apelinergic system: the role played in human physiology and pathology and potential therapeutic applications. Arq Bras Cardiol. 2008;90(5):343–9. doi: 10.1590/s0066-782x2008000500012. [DOI] [PubMed] [Google Scholar]
- 11.Zhen EY, Higgs RE, Gutierrez JA. Pyroglutamyl apelin-13 identified as the major apelin isoform in human plasma. Anal Biochem. 2013;442(1):1–9. doi: 10.1016/j.ab.2013.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Kleinz MJ, Davenport AP. Emerging roles of apelin in biology and medicine. Pharmacol Ther. 2005;107(2):198–211. doi: 10.1016/j.pharmthera.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 13.Wang G, Anini Y, Wei W, Qi X, O’Carroll AM, Mochizuki T, et al. Apelin, a new enteric peptide: localization in the gastrointestinal tract, ontogeny, and stimulation of gastric cell proliferation and of cholecystokinin secretion. Endocrinology. 2004;145(3):1342–8. doi: 10.1210/en.2003-1116. [DOI] [PubMed] [Google Scholar]
- 14.Folino A, Montarolo PG, Samaja M, Rastaldo R. Effects of apelin on the cardiovascular system. Heart Fail Rev. 2015;20(4):505–18. doi: 10.1007/s10741-015-9475-x. [DOI] [PubMed] [Google Scholar]
- 15.Masri B, van den Berghe L, Sorli C, Knibiehler B, Audigier Y. [Apelin signalisation and vascular physiopathology] J Soc Biol. 2009;203(2):171–9. doi: 10.1051/jbio/2009021.[French]. [DOI] [PubMed] [Google Scholar]
- 16.Nishida M, Hamaoka K. The apelin-APJ system: its role in renal physiology and potential therapeutic applications for renal disease. OA Nephrol. 2013;1(7):1–5. [Google Scholar]
- 17.Hu G, Wang Z, Zhang R, Sun W, Chen X. The role of apelin/apelin receptor in energy metabolism and water homeostasis: a comprehensive narrative review. Front Physiol. 2021;12:632886. doi: 10.3389/fphys.2021.632886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li A, Zhao Q, Chen L, Li Z. Apelin/APJ system: an emerging therapeutic target for neurological diseases. Mol Biol Rep. 2023;50(2):1639–53. doi: 10.1007/s11033-022-08075-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Jiang W, Sun W, Guo W, Xia B, Shen X, et al. Neuroprotective roles of apelin-13 in neurological diseases. Neurochem Res. 2023;48(6):1648–62. doi: 10.1007/s11064-023-03869-0. [DOI] [PubMed] [Google Scholar]
- 20.Khaksari M, Aboutaleb N, Nasirinezhad F, Vakili A, Madjd Z. Apelin-13 protects the brain against ischemic reperfusion injury and cerebral edema in a transient model of focal cerebral ischemia. J Mol Neurosci. 2012;48(1):201–8. doi: 10.1007/s12031-012-9808-3. [DOI] [PubMed] [Google Scholar]
- 21.Zhou JX, Shuai NN, Wang B, Jin X, Kuang X, Tian SW. Neuroprotective gain of apelin/APJ system. Neuropeptides. 2021;87:102131. doi: 10.1016/j.npep.2021.102131. [DOI] [PubMed] [Google Scholar]
- 22.Pouresmaeili-Babaki E, Esmaeili-Mahani S, Abbasnejad M, Ravan H. Protective effect of neuropeptide apelin-13 on 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y dopaminergic cells: involvement of its antioxidant and antiapoptotic properties. Rejuvenation Res. 2018;21(2):162–7. doi: 10.1089/rej.2017.1951. [DOI] [PubMed] [Google Scholar]
- 23.Haghparast E, Sheibani V, Abbasnejad M, Esmaeili-Mahani S. Apelin-13 attenuates motor impairments and prevents the changes in synaptic plasticity-related molecules in the striatum of Parkinsonism rats. Peptides. 2019;117:170091. doi: 10.1016/j.peptides.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Haghparast E, Esmaeili-Mahani S, Abbasnejad M, Sheibani V. Apelin-13 ameliorates cognitive impairments in 6-hydroxydopamine-induced substantia nigra lesion in rats. Neuropeptides. 2018;68:28–35. doi: 10.1016/j.npep.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Aminyavari S, Zahmatkesh M, Farahmandfar M, Khodagholi F, Dargahi L, Zarrindast MR. Protective role of apelin-13 on amyloid β25-35-induced memory deficit; involvement of autophagy and apoptosis process. Prog Neuropsychopharmacol Biol Psychiatry. 2019;89:322–34. doi: 10.1016/j.pnpbp.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 26.Masoumi J, Abbasloui M, Parvan R, Mohammadnejad D, Pavon-Djavid G, Barzegari A, et al. Apelin, a promising target for Alzheimer disease prevention and treatment. Neuropeptides. 2018;70:76–86. doi: 10.1016/j.npep.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 27.Reaux A, Gallatz K, Palkovits M, Llorens-Cortes C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience. 2002;113(3):653–62. doi: 10.1016/s0306-4522(02)00192-6. [DOI] [PubMed] [Google Scholar]
- 28.Lee DK, Cheng R, Nguyen T, Fan T, Kariyawasam AP, Liu Y, et al. Characterization of apelin, the ligand for the APJ receptor. J Neurochem. 2000;74(1):34–41. doi: 10.1046/j.1471-4159.2000.0740034.x. [DOI] [PubMed] [Google Scholar]
- 29.Dai TT, Wang B, Xiao ZY, You Y, Tian SW. Apelin-13 upregulates BDNF against chronic stress-induced depression-like phenotypes by ameliorating HPA axis and hippocampal glucocorticoid receptor dysfunctions. Neuroscience. 2018;390:151–9. doi: 10.1016/j.neuroscience.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 30.Tian SW, Xu F, Gui SJ. Apelin-13 reverses memory impairment and depression-like behavior in chronic social defeat stressed rats. Peptides. 2018;108:1–6. doi: 10.1016/j.peptides.2018.08.009. [DOI] [PubMed] [Google Scholar]
- 31.Foroughi K, Khaksari M, Rahmati M, Bitaraf FS, Shayannia A. Apelin-13 protects PC12 cells against methamphetamine-induced oxidative stress, autophagy and apoptosis. Neurochem Res. 2019;44(9):2103–12. doi: 10.1007/s11064-019-02847-9. [DOI] [PubMed] [Google Scholar]
- 32.Yildiz I, Çimen YA, Eroğlu Güneş C, Özkürkçüler A, Kurar E, Kutlu S. Effect of morphine dependency on apelinergic system in rat hippocampus. Acta Physiol. 2022;234(Suppl 724):45. [Google Scholar]
- 33.Zarrinkalam E, Heidarianpour A. The effect of different training modes on serum apelin and pain threshold in morphine-dependent rats. Avicenna J Neuro Psycho Physiology. 2015;2(3):60–5. doi: 10.17795/ajnpp-34440. [DOI] [Google Scholar]
- 34.Mohseni F, Khaksari M, Rafaiee R, Rahimi K, Norouzi P, Garmabi B. Apelin-13 improves anxiety and cognition via hippocampal increases BDNF expression and reduction cell death in neonatal alcohol exposed rats. Int J Pept Res Ther. 2021;27(2):1351–62. doi: 10.1007/s10989-021-10173-4. [DOI] [Google Scholar]
- 35.Mohseni F, Garmabi B, Khaksari M. Apelin-13 attenuates spatial memory impairment by anti-oxidative, anti-apoptosis, and anti-inflammatory mechanism against ethanol neurotoxicity in the neonatal rat hippocampus. Neuropeptides. 2021;87:102130. doi: 10.1016/j.npep.2021.102130. [DOI] [PubMed] [Google Scholar]
- 36.Tavanai A, Asadikaram G, Masoumi M. Opium addiction is associated with increased damage to cardiomyocytes: protective roles played by apelins. Iran Heart J. 2020;21(3):6–14. [Google Scholar]
- 37.Darcq E, Kieffer BL. Opioid receptors: drivers to addiction? Nat Rev Neurosci. 2018;19(8):499–514. doi: 10.1038/s41583-018-0028-x. [DOI] [PubMed] [Google Scholar]
- 38.Stuber GD, Wise RA. Lateral hypothalamic circuits for feeding and reward. Nat Neurosci. 2016;19(2):198–205. doi: 10.1038/nn.4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tyree SM, de Lecea L. Lateral hypothalamic control of the ventral tegmental area: reward evaluation and the driving of motivated behavior. Front Syst Neurosci. 2017;11:50. doi: 10.3389/fnsys.2017.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nieh EH, Vander Weele CM, Matthews GA, Presbrey KN, Wichmann R, Leppla CA, et al. Inhibitory input from the lateral hypothalamus to the ventral tegmental area disinhibits dopamine neurons and promotes behavioral activation. Neuron. 2016;90(6):1286–98. doi: 10.1016/j.neuron.2016.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Castro DC, Cole SL, Berridge KC. Lateral hypothalamus, nucleus accumbens, and ventral pallidum roles in eating and hunger: interactions between homeostatic and reward circuitry. Front Syst Neurosci. 2015;9:90. doi: 10.3389/fnsys.2015.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DiLeone RJ, Georgescu D, Nestler EJ. Lateral hypothalamic neuropeptides in reward and drug addiction. Life Sci. 2003;73(6):759–68. doi: 10.1016/s0024-3205(03)00408-9. [DOI] [PubMed] [Google Scholar]
- 43.Harris GC, Wimmer M, Randall-Thompson JF, Aston-Jones G. Lateral hypothalamic orexin neurons are critically involved in learning to associate an environment with morphine reward. Behav Brain Res. 2007;183(1):43–51. doi: 10.1016/j.bbr.2007.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ghozland S, Matthes HW, Simonin F, Filliol D, Kieffer BL, Maldonado R. Motivational effects of cannabinoids are mediated by mu-opioid and kappa-opioid receptors. J Neurosci. 2002;22(3):1146–54. doi: 10.1523/jneurosci.22-03-01146.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Berrendero F, Kieffer BL, Maldonado R. Attenuation of nicotine-induced antinociception, rewarding effects, and dependence in mu-opioid receptor knock-out mice. J Neurosci. 2002;22(24):10935–40. doi: 10.1523/jneurosci.22-24-10935.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berrettini W. Alcohol addiction and the mu-opioid receptor. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:228–33. doi: 10.1016/j.pnpbp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Zubieta JK, Gorelick DA, Stauffer R, Ravert HT, Dannals RF, Frost JJ. Increased mu opioid receptor binding detected by PET in cocaine-dependent men is associated with cocaine craving. Nat Med. 1996;2(11):1225–9. doi: 10.1038/nm1196-1225. [DOI] [PubMed] [Google Scholar]
- 49.Moles A, Kieffer BL, D’Amato FR. Deficit in attachment behavior in mice lacking the mu-opioid receptor gene. Science. 2004;304(5679):1983–6. doi: 10.1126/science.1095943. [DOI] [PubMed] [Google Scholar]
- 50.Befort K, Filliol D, Darcq E, Ghate A, Matifas A, Lardenois A, et al. Gene expression is altered in the lateral hypothalamus upon activation of the mu opioid receptor. Ann N Y Acad Sci. 2008;1129:175–84. doi: 10.1196/annals.1417.028. [DOI] [PubMed] [Google Scholar]
- 51.Mollereau C, Roumy M, Zajac JM. Opioid-modulating peptides: mechanisms of action. Curr Top Med Chem. 2005;5(3):341–55. doi: 10.2174/1568026053544515. [DOI] [PubMed] [Google Scholar]
- 52.Mansour A, Fox CA, Meng F, Akil H, Watson SJ. Kappa 1 receptor mRNA distribution in the rat CNS: comparison to kappa receptor binding and prodynorphin mRNA. Mol Cell Neurosci. 1994;5(2):124–44. doi: 10.1006/mcne.1994.1015. [DOI] [PubMed] [Google Scholar]
- 53.Tejeda HA, Shippenberg TS, Henriksson R. The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell Mol Life Sci. 2012;69(6):857–96. doi: 10.1007/s00018-011-0844-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glick SD, Maisonneuve IM, Raucci J, Archer S. Kappa opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681(1-2):147–52. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- 55.Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology (Berl) 1999;144(4):339–46. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- 56.Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by rhesus monkeys. J Pharmacol Exp Ther. 1997;282(1):44–55. [PubMed] [Google Scholar]
- 57.Khan MI, Sawyer BJ, Akins NS, Le HV. A systematic review on the kappa opioid receptor and its ligands: new directions for the treatment of pain, anxiety, depression, and drug abuse. Eur J Med Chem. 2022;243:114785. doi: 10.1016/j.ejmech.2022.114785. [DOI] [PubMed] [Google Scholar]
- 58.Li Y, Chen J, Bai B, Du H, Liu Y, Liu H. Heterodimerization of human apelin and kappa opioid receptors: roles in signal transduction. Cell Signal. 2012;24(5):991–1001. doi: 10.1016/j.cellsig.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 59.Ilaghi M, Soltanizadeh A, Amiri S, Kohlmeier KA, Shabani M. The apelin/APJ signaling system and cytoprotection: role of its cross-talk with kappa opioid receptor. Eur J Pharmacol. 2022;936:175353. doi: 10.1016/j.ejphar.2022.175353. [DOI] [PubMed] [Google Scholar]
- 60.Lv S, Zhang X, Feng Y, Zhou Y, Cui B, Yang Y, et al. Intravenous administration of pyroglutamyl apelin-13 alleviates murine inflammatory pain via the kappa opioid receptor. Front Neurosci. 2020;14:929. doi: 10.3389/fnins.2020.00929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, et al. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in-situ hybridization study. J Comp Neurol. 1994;350(3):412–38. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 62.Medhurst AD, Jennings CA, Robbins MJ, Davis RP, Ellis C, Winborn KY, et al. Pharmacological and immunohistochemical characterization of the APJ receptor and its endogenous ligand apelin. J Neurochem. 2003;84(5):1162–72. doi: 10.1046/j.1471-4159.2003.01587.x. [DOI] [PubMed] [Google Scholar]