Abstract
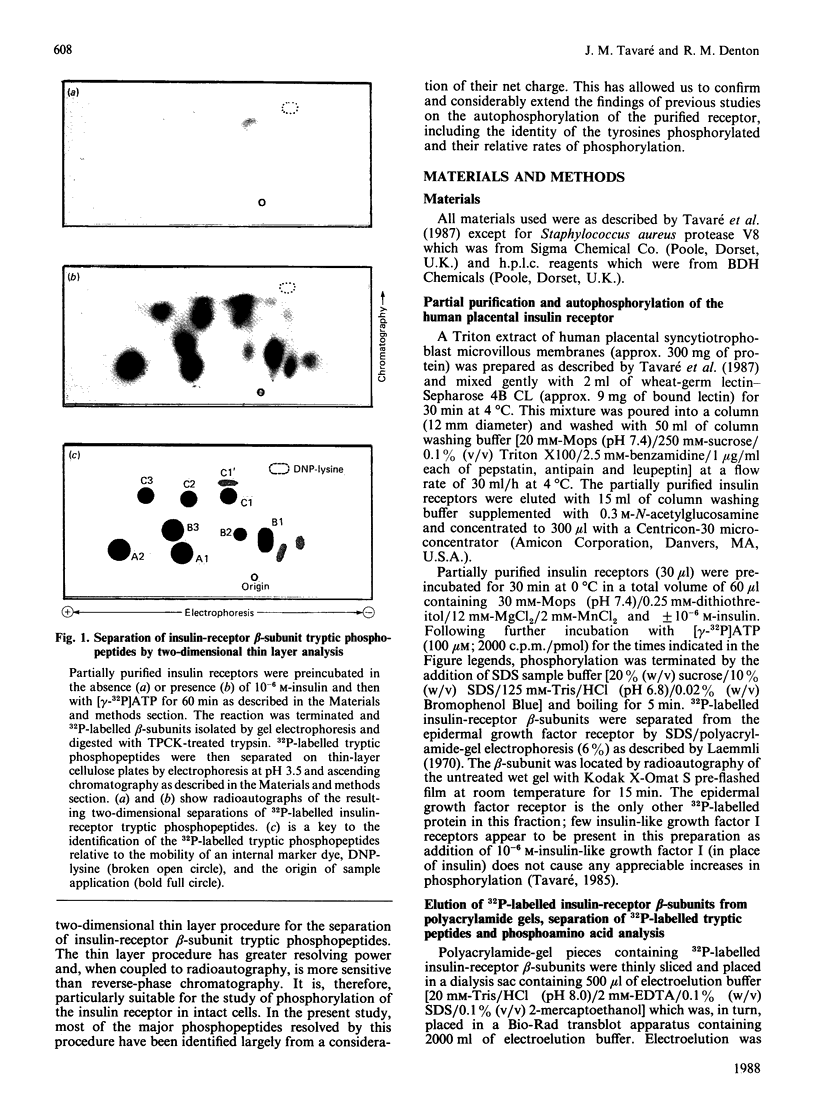
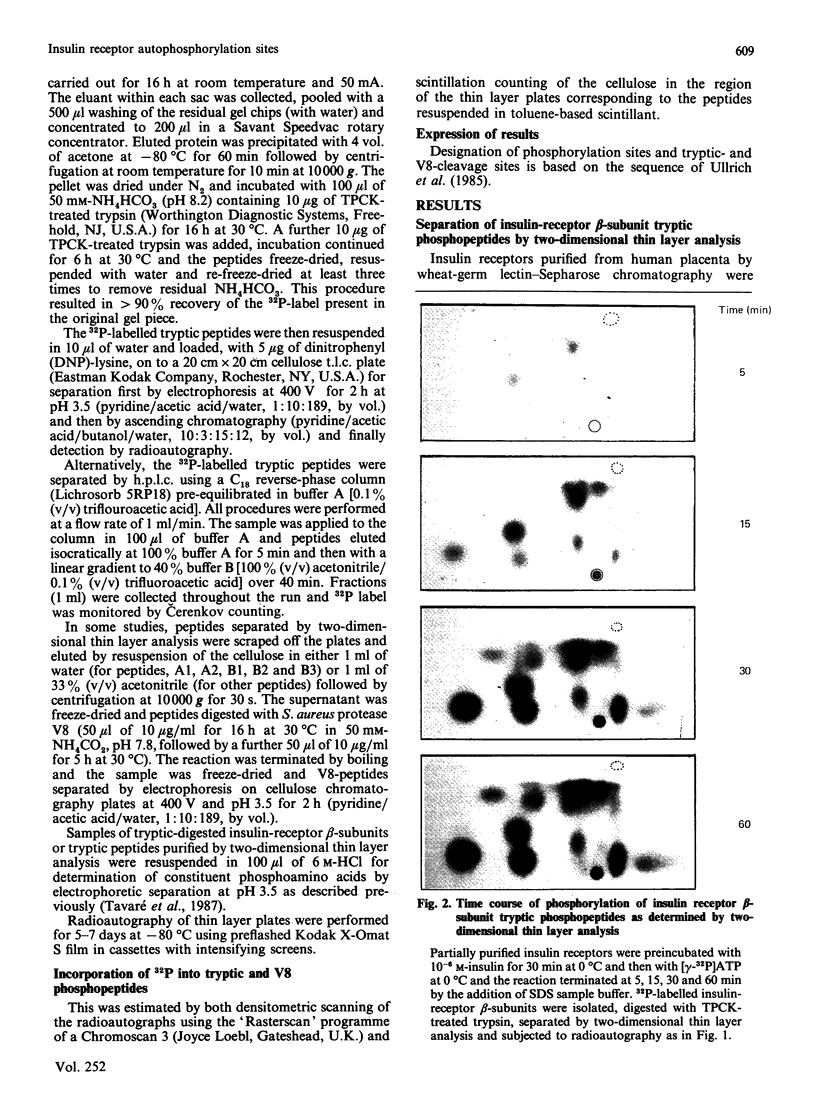
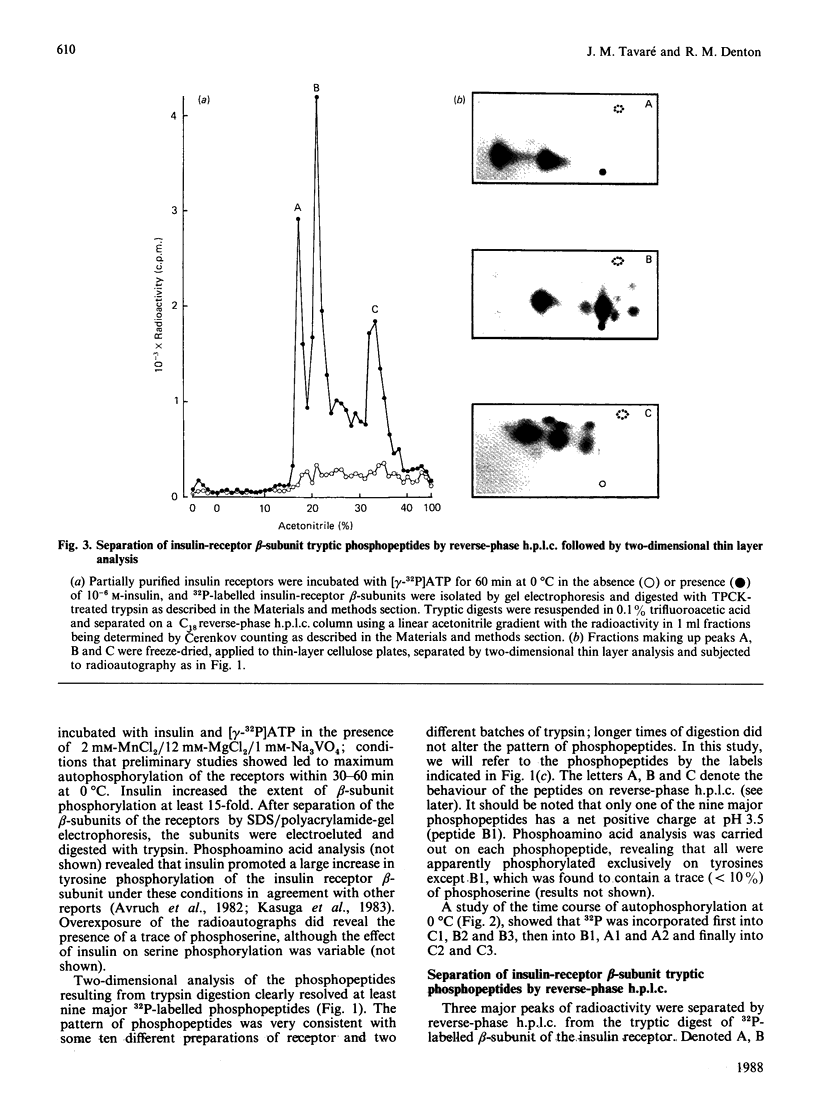
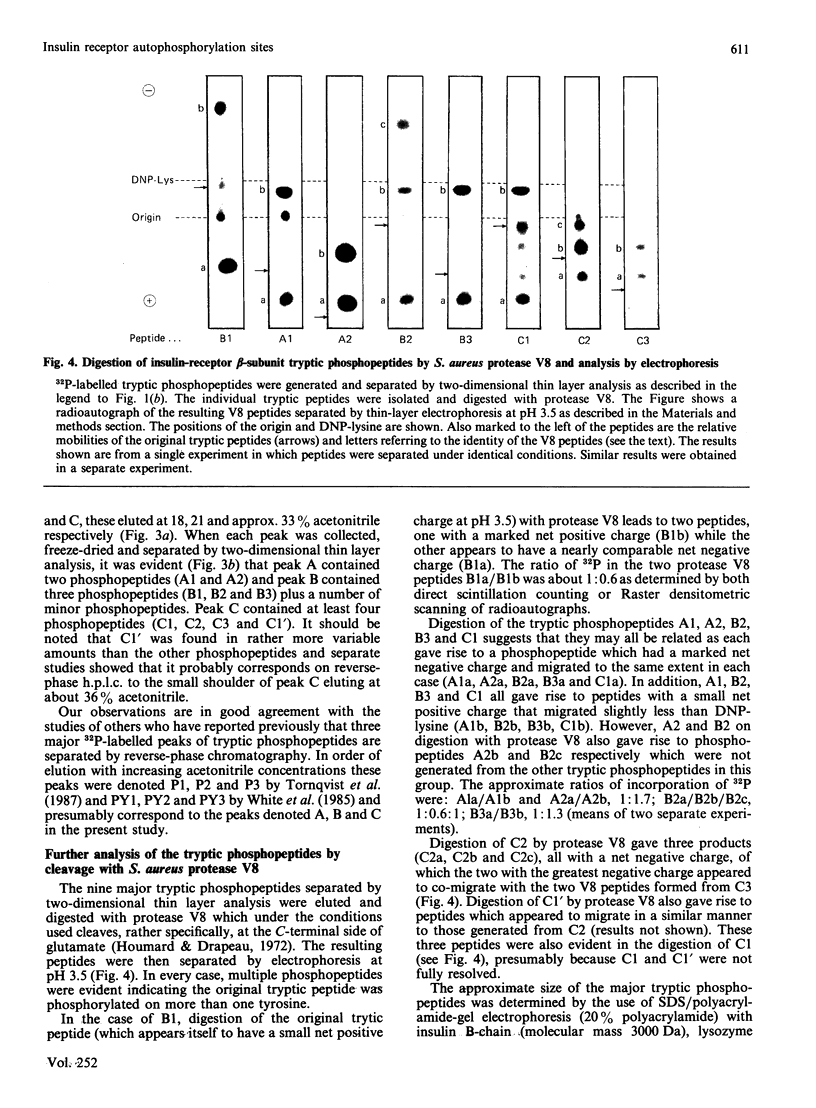
1. A partially purified preparation of human placental insulin receptors was incubated with [gamma-32P]ATP in the presence or absence of insulin. The 32P-labelled insulin-receptor beta-subunits were then isolated, cleaved with trypsin followed by protease V8 and the [32P]phosphopeptides generated were analysed by thin layer electrophoresis and chromatography. This approach revealed that insulin stimulates autophosphorylation of the insulin-receptor beta-subunit in vitro on at least seven tyrosine residues distributed among three distinct domains. 2. One domain (domain 2), containing tyrosine residues 1146, 1150 and 1151 was the most rapidly phosphorylated and could be recovered as mono-, di- and triphosphorylated peptides cleaved by trypsin at Arg-1143 and either Lys-1153 or Lys-1156. Multiple phosphorylation of this domain appears to partially inhibit the cleavage at Lys-1153 by trypsin. 3. In a second domain (domain 3) containing two phosphorylated tyrosine residues at positions 1316 and 1322 the tyrosines were phosphorylated more slowly than those in domain 2. This domain is close to the C-terminus of the beta-subunit polypeptide chain. 4. At least two further tyrosine residues appeared to be phosphorylated after those in domains 2 and 3. These residues probably residue within a domain lying in close proximity to the inner face of the plasma membrane containing tyrosines 953, 960 and 972, but conclusive evidence is still required. 5. The two-dimensional thin-layer analysis employed in this study to investigate insulin-receptor phosphorylation has several advantages over previous methods based on reverse-phase chromatography. It allows greater resolution of 32P-labelled tryptic peptides and, when coupled to radioautography, is considerably more sensitive. The approach can be readily adapted to study phosphorylation of the insulin receptor within intact cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avruch J., Nemenoff R. A., Blackshear P. J., Pierce M. W., Osathanondh R. Insulin-stimulated tyrosine phosphorylation of the insulin receptor in detergent extracts of human placental membranes. Comparison to epidermal growth factor-stimulated phosphorylation. J Biol Chem. 1982 Dec 25;257(24):15162–15166. [PubMed] [Google Scholar]
- Chou C. K., Dull T. J., Russell D. S., Gherzi R., Lebwohl D., Ullrich A., Rosen O. M. Human insulin receptors mutated at the ATP-binding site lack protein tyrosine kinase activity and fail to mediate postreceptor effects of insulin. J Biol Chem. 1987 Feb 5;262(4):1842–1847. [PubMed] [Google Scholar]
- Denton R. M. Early events in insulin actions. Adv Cyclic Nucleotide Protein Phosphorylation Res. 1986;20:293–341. [PubMed] [Google Scholar]
- Ebina Y., Araki E., Taira M., Shimada F., Mori M., Craik C. S., Siddle K., Pierce S. B., Roth R. A., Rutter W. J. Replacement of lysine residue 1030 in the putative ATP-binding region of the insulin receptor abolishes insulin- and antibody-stimulated glucose uptake and receptor kinase activity. Proc Natl Acad Sci U S A. 1987 Feb;84(3):704–708. doi: 10.1073/pnas.84.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Ellis L., Jarnagin K., Edery M., Graf L., Clauser E., Ou J. H., Masiarz F., Kan Y. W., Goldfine I. D. The human insulin receptor cDNA: the structural basis for hormone-activated transmembrane signalling. Cell. 1985 Apr;40(4):747–758. doi: 10.1016/0092-8674(85)90334-4. [DOI] [PubMed] [Google Scholar]
- Ellis L., Clauser E., Morgan D. O., Edery M., Roth R. A., Rutter W. J. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986 Jun 6;45(5):721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Ellis L., Morgan D. O., Clauser E., Edery M., Jong S. M., Wang L. H., Roth R. A., Rutter W. J. Mechanisms of receptor-mediated transmembrane communication. Cold Spring Harb Symp Quant Biol. 1986;51(Pt 2):773–784. doi: 10.1101/sqb.1986.051.01.090. [DOI] [PubMed] [Google Scholar]
- Goren H. J., White M. F., Kahn C. R. Separate domains of the insulin receptor contain sites of autophosphorylation and tyrosine kinase activity. Biochemistry. 1987 Apr 21;26(8):2374–2382. doi: 10.1021/bi00382a044. [DOI] [PubMed] [Google Scholar]
- Herrera R., Rosen O. M. Autophosphorylation of the insulin receptor in vitro. Designation of phosphorylation sites and correlation with receptor kinase activation. J Biol Chem. 1986 Sep 15;261(26):11980–11985. [PubMed] [Google Scholar]
- Houmard J., Drapeau G. R. Staphylococcal protease: a proteolytic enzyme specific for glutamoyl bonds. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3506–3509. doi: 10.1073/pnas.69.12.3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Cuatrecasas P. Phosphorylation of receptors for insulin and insulin-like growth factor I. Effects of hormones and phorbol esters. J Biol Chem. 1986 Jan 15;261(2):934–939. [PubMed] [Google Scholar]
- Kasuga M., Fujita-Yamaguchi Y., Blithe D. L., Kahn C. R. Tyrosine-specific protein kinase activity is associated with the purified insulin receptor. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2137–2141. doi: 10.1073/pnas.80.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Morgan D. O., Roth R. A. Acute insulin action requires insulin receptor kinase activity: introduction of an inhibitory monoclonal antibody into mammalian cells blocks the rapid effects of insulin. Proc Natl Acad Sci U S A. 1987 Jan;84(1):41–45. doi: 10.1073/pnas.84.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponzio G., Dolais-Kitabgi J., Louvard D., Gautier N., Rossi B. Insulin and rabbit anti-insulin receptor antibodies stimulate additively the intrinsic receptor kinase activity. EMBO J. 1987 Feb;6(2):333–340. doi: 10.1002/j.1460-2075.1987.tb04759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen O. M., Herrera R., Olowe Y., Petruzzelli L. M., Cobb M. H. Phosphorylation activates the insulin receptor tyrosine protein kinase. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3237–3240. doi: 10.1073/pnas.80.11.3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtmauer L., Rosen O. M. Phosphorylation of synthetic insulin receptor peptides by the insulin receptor kinase and evidence that the preferred sequence containing Tyr-1150 is phosphorylated in vivo. J Biol Chem. 1986 Jul 25;261(21):10000–10005. [PubMed] [Google Scholar]
- Tavaré J. M., Diggle T. A., Denton R. M. Epidermal growth factor, but not insulin, stimulates tyrosine phosphorylation of an endogenous protein of Mr 95,000 in triton extracts of human placental syncytiotrophoblast membranes. Biochem J. 1987 Jun 15;244(3):769–774. doi: 10.1042/bj2440769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tornqvist H. E., Pierce M. W., Frackelton A. R., Nemenoff R. A., Avruch J. Identification of insulin receptor tyrosine residues autophosphorylated in vitro. J Biol Chem. 1987 Jul 25;262(21):10212–10219. [PubMed] [Google Scholar]
- Ullrich A., Bell J. R., Chen E. Y., Herrera R., Petruzzelli L. M., Dull T. J., Gray A., Coussens L., Liao Y. C., Tsubokawa M. Human insulin receptor and its relationship to the tyrosine kinase family of oncogenes. 1985 Feb 28-Mar 6Nature. 313(6005):756–761. doi: 10.1038/313756a0. [DOI] [PubMed] [Google Scholar]
- White M. F., Takayama S., Kahn C. R. Differences in the sites of phosphorylation of the insulin receptor in vivo and in vitro. J Biol Chem. 1985 Aug 5;260(16):9470–9478. [PubMed] [Google Scholar]
- Yu K. T., Czech M. P. Tyrosine phosphorylation of the insulin receptor beta subunit activates the receptor-associated tyrosine kinase activity. J Biol Chem. 1984 Apr 25;259(8):5277–5286. [PubMed] [Google Scholar]