Abstract
Background
Laparoscopic cholecystectomy is used to manage symptomatic gallstones. There is considerable controversy regarding whether it should be done as day‐surgery or as an overnight stay surgery with regards to patient safety.
Objectives
To assess the impact of day‐surgery versus overnight stay laparoscopic cholecystectomy on patient‐oriented outcomes such as mortality, severe adverse events, and quality of life.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded, and mRCT until September 2012.
Selection criteria
We included randomised clinical trials comparing day‐surgery versus overnight stay surgery for laparoscopic cholecystectomy, irrespective of language or publication status.
Data collection and analysis
Two authors independently assessed trials for inclusion and independently extracted the data. We analysed the data with both the fixed‐effect and the random‐effects models using Review Manager 5 analysis. We calculated the risk ratio (RR), mean difference (MD), or standardised mean difference (SMD) with 95% confidence intervals (CI) based on intention‐to‐treat or available case analysis.
Main results
We identified a total of six trials at high risk of bias involving 492 participants undergoing day‐case laparoscopic cholecystectomy (n = 239) versus overnight stay laparoscopic cholecystectomy (n = 253) for symptomatic gallstones. The number of participants in each trial ranged from 28 to 150. The proportion of women in the trials varied between 74% and 84%. The mean or median age in the trials varied between 40 and 47 years.
With regards to primary outcomes, only one trial reported short‐term mortality. However, the trial stated that there were no deaths in either of the groups. We inferred from the other outcomes that there was no short‐term mortality in the remaining trials. Long‐term mortality was not reported in any of the trials. There was no significant difference in the rate of serious adverse events between the two groups (4 trials; 391 participants; 7/191 (weighted rate 1.6%) in the day‐surgery group versus 1/200 (0.5%) in the overnight stay surgery group; rate ratio 3.24; 95% CI 0.74 to 14.09). There was no significant difference in quality of life between the two groups (4 trials; 333 participants; SMD ‐0.11; 95% CI ‐0.33 to 0.10).
There was no significant difference between the two groups regarding the secondary outcomes of our review: pain (3 trials; 175 participants; MD 0.02 cm visual analogue scale score; 95% CI ‐0.69 to 0.73); time to return to activity (2 trials, 217 participants; MD ‐0.55 days; 95% CI ‐2.18 to 1.08); and return to work (1 trial, 74 participants; MD ‐2.00 days; 95% CI ‐10.34 to 6.34). No significant difference was seen in hospital readmission rate (5 trials; 464 participants; 6/225 (weighted rate 0.5%) in the day‐surgery group versus 5/239 (2.1%) in the overnight stay surgery group (rate ratio 1.25; 95% CI 0.43 to 3.63) or in the proportion of people requiring hospital readmissions (3 trials; 290 participants; 5/136 (weighted proportion 3.5%) in the day‐surgery group versus 5/154 (3.2%) in the overnight stay surgery group; RR 1.09; 95% CI 0.33 to 3.60). No significant difference was seen in the proportion of failed discharge (failure to be discharged as planned) between the two groups (5 trials; 419 participants; 42/205 (weighted proportion 19.3%) in the day‐surgery group versus 43/214 (20.1%) in the overnight stay surgery group; RR 0.96; 95% CI 0.65 to 1.41). For all outcomes except pain, the accrued information was far less than the diversity‐adjusted required information size to exclude random errors.
Authors' conclusions
Day‐surgery appears just as safe as overnight stay surgery in laparoscopic cholecystectomy. Day‐surgery does not seem to result in improvement in any patient‐oriented outcomes such as return to normal activity or earlier return to work. The randomised clinical trials backing these statements are weakened by risks of systematic errors (bias) and risks of random errors (play of chance). More randomised clinical trials are needed to assess the impact of day‐surgery laparoscopic cholecystectomy on the quality of life as well as other outcomes of patients.
Keywords: Adult; Female; Humans; Male; Middle Aged; Hospitalization; Ambulatory Surgical Procedures; Ambulatory Surgical Procedures/adverse effects; Cholecystectomy, Laparoscopic; Cholecystectomy, Laparoscopic/adverse effects; Gallstones; Gallstones/surgery; Length of Stay; Patient Readmission; Patient Readmission/statistics & numerical data; Randomized Controlled Trials as Topic
Plain language summary
Day‐surgery versus overnight stay surgery for laparoscopic cholecystectomy
This review compares same‐day discharge (day‐surgery) with overnight stay after keyhole removal of the gallbladder (laparoscopic cholecystectomy) for various conditions affecting the gallbladder but mainly for gallstones causing pain.
Stones that develop in the gallbladder can cause pain in the upper abdomen. This condition is treated by surgical removal of the gallbladder through keyhole surgery, a procedure that is known as laparoscopic cholecystectomy. This procedure may involve the person staying in hospital overnight, but increasingly it is possible to perform the operation and allow them to go home on the same day ('day‐surgery'). There is some controversy over whether performing laparoscopic cholecystectomy as day‐surgery is safe.
This review aims to investigate the current literature available and provides an overview of the evidence demonstrated in recent clinical trials on the subject. The review authors identified a total of six trials involving 492 participants. Two hundred and thirty‐nine people underwent planned laparoscopic cholecystectomy as day‐surgery and 253 participants stayed in the hospital overnight after the procedure. All the trials were at high risk of bias (methodological deficiencies that might make it possible to arrive at wrong conclusions by overestimating the benefit or underestimating the harm of the day‐surgery or overnight stay procedure). We looked at outcomes that are considered to be important from the perspective of the participant and also the healthcare provider. These outcomes include death, serious complication, quality of life following procedure, pain, how long it took for people to return to normal activity and to return to work, hospital readmissions, and failed discharges (failure to be discharged as planned). There was no significant difference in the proportion who died or the complication rate between the group who underwent day‐surgery and those who stayed overnight. Quality of life did not differ significantly between the two groups. There was no significant difference in the time taken for people to return to normal activity or to return to work. There was also no significant difference in the hospital readmission or failed discharge rates. The results suggest that day‐surgery is safe for patients. It is important to note that all trials were at risk of bias and the data were sparse, resulting in a considerable chance of arriving at wrong conclusions due to systematic errors (overestimating benefits or underestimating harms of day‐surgery or overnight stay) and random errors (play of chance). More randomised trials are needed to investigate the impact of day‐surgery and overnight stay on the quality of life and other outcomes of people undergoing laparoscopic cholecystectomy.
Summary of findings
Summary of findings for the main comparison. Day‐surgery versus overnight stay surgery for laparoscopic cholecystectomy.
| Day‐surgery versus overnight stay for laparoscopic cholecystectomy | |||||
| Patient or population: patients with laparoscopic cholecystectomy. Settings: secondary or tertiary centre. Intervention: day‐surgery versus overnight stay for laparoscopic cholecystectomy. | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | |
| Assumed risk | Corresponding risk | ||||
| Control | Day‐case versus overnight stay for laparoscopic cholecystectomy | ||||
| Short term mortality | See comment | See comment | Not estimable | 86 (1 study) | ⊕⊕⊝⊝ low1,2,3 |
| Serious adverse events | 5 per 1000 | 16 per 1000 (4 to 70) | Rate Ratio 3.24 (0.74 to 14.09) | 391 (4 studies) | ⊕⊝⊝⊝ very low1,3,4,5 |
| Quality of life | The mean quality of life in the intervention groups was 0.11 standard deviations lower (0.33 lower to 0.1 higher) | SMD ‐0.11 (‐0.33 to 0.1) | 333 (4 studies) | ⊕⊝⊝⊝ very low1,3,4,6,7,8,9 | |
| Pain | The mean pain in the intervention groups was 0.02 cm higher (0.69 lower to 0.73 higher) | 175 (3 studies) | ⊕⊝⊝⊝ very low1,3,4,9,10,11 | ||
| Time to return to activity | The mean time to return to activity in the intervention groups was 0.55 days lower (2.18 lower to 1.08 higher) | 217 (2 studies) | ⊕⊝⊝⊝ very low1,3,4,9,10,12 | ||
| Number of hospital readmissions | 21 per 1000 | 26 per 1000 (9 to 76) | Rate ratio 1.25 (0.43 to 3.63) | 464 (5 studies) | ⊕⊝⊝⊝ very low1,3,4,5 |
| Failed discharge | 201 per 1000 | 193 per 1000 (131 to 283) | RR 0.96 (0.65 to 1.41) | 419 (5 studies) | ⊕⊝⊝⊝ very low1,3,5,6 |
| *The basis for the assumed risk is the average control group risk across studies. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | |||||
1 The methods of randomisation were not clear. 2 Not applicable. 3 Publication bias could not be assessed due to small number of trials. 4 Blinding was not performed. 5 Confidence intervals overlap 1 and 0.75 or 1.25. 6 I² test indicates considerable heterogeneity. 7 There are various ways of measuring quality of life, these methods were not clearly mentioned. 8 Confidence intervals overlap 1 and ‐0.5 or 0.5. 9 Total sample size in both groups below 400. 10 Confidence interval overlaps 0 and minimal important difference. 11 Minimal important difference for pain is 0.9 cm and 1.8 cm (Todd 1996). 12 Minimal important difference for time to return to normal activity is one day.
Background
Description of the condition
About 10% to 15% of the adult western population have gallstones (Jørgensen 1987; NIH 1992; Muhrbeck 1995; Halldestam 2004). Between 1% and 4% become symptomatic each year with an acute inflammation of the gallbladder due to obstruction, a condition known as acute cholecystitis (NIH 1992; Halldestam 2004).The gold standard management of symptomatic gallstones is removal of the gallbladder, a procedure known as a cholecystectomy.
In the United States, 1.5 million cholecystectomies are performed annually. In the United Kingdom, approximately 50,000 cholecystectomies are performed annually and the majority of these are performed laparoscopically. Currently, approximately 16,500 of cholecystectomies in the United Kingdom are performed as day‐surgery procedures (HES 2012), with people being discharged the same day as the operation. While it is obvious that performing laparoscopic cholecystectomy can result in cost savings, concerns exist regarding the safety of the patient. The main serious adverse events associated with laparoscopic cholecystectomy are bleeding and bile duct injury (Keulemans 1998; Shamiyeh 2004). The main concern is whether performing day‐surgery can have a negative impact on people because of these serious adverse events because of late recognition of complications. There are also concerns about the adequacy of post‐operative pain control at home.
Description of the intervention
Day‐surgery is defined as patient admission and discharge within the same day, with day‐surgery as the intended management (AAGBI 2011)
How the intervention might work
Reducing post‐operative hospital stay may improve recovery in part by reducing the risk of venous thromboembolism and hospital‐acquired infections (AAGBI 2011).
Why it is important to do this review
Laproscopic cholecystectomy is a major surgical procedure and increasingly this is being conducted in the form of day‐surgery. It is necessary to have up‐to‐date information regarding the safety of laparoscopic cholecystectomy performed as day‐surgery.
This is an update of a previous Cochrane review, last published in 2008 (Gurusamy 2008b).
Objectives
To compare the benefits and harms of day‐surgery versus overnight stay laparoscopic cholecystectomy.
Methods
Criteria for considering studies for this review
Types of studies
We considered only randomised clinical trials for this review, irrespective of language, blinding, or publication status. Quasi‐randomised studies (where the method of allocating participants to a treatment is not strictly random (eg, date of birth, hospital record number, alternation)), cohort studies, and case‐control studies were excluded for benefits because of the bias in their study designs. We considered including quasi‐randomised studies for harms related to day‐surgery.
Types of participants
People undergoing laparoscopic cholecystectomy.
Types of interventions
We included trials comparing day‐surgery laparoscopic cholecystectomy versus overnight stay laparoscopic cholecystectomy.
Types of outcome measures
Primary outcomes
-
Mortality
Short‐term mortality (30‐day mortality or in‐hospital mortality).
Long‐term mortality (at longest follow‐up).
Serious adverse events. Adverse events are defined as any untoward medical occurrence not necessarily having a causal relationship with the treatment, but resulting in a dose reduction or discontinuation of treatment (ICH‐GCP 1997). Serious adverse events are defined as any event that would increase mortality, is life‐threatening, requires inpatient hospitalisation, results in a persistent or significant disability, or any important medical event, which might have jeopardised the participant or requires intervention to prevent it.
Quality of life.
Secondary outcomes
Pain (visual analogue score (VAS) between four and eight hours).
Time to return to normal activity.
Time to return to work.
-
Hospital readmissions:
Total number of hospital readmissions.
Number of people requiring hospital admissions.
Failed discharge.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2012) and the Cochrane Central Register of Controlled Trials (CENTRAL) inThe Cochrane Library, MEDLINE, EMBASE, Science Citation Index Expanded (Royle 2003), and the metaRegister of Controlled Trials (http://www.controlled‐trials.com/mrct/) until September 2012. We have given the search strategies in Appendix 1 with the time spans for the searches.
Searching other resources
We searched the references of the identified trials to identify further relevant trials.
Data collection and analysis
We performed the systematic review following the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (CHBG module).
Selection of studies
Two authors (JV and KG) identified the trials for inclusion independently of each other. We have listed the excluded studies with the reasons for the exclusion. We resolved any differences through discussion.
Data extraction and management
Two of the review authors (JV and KG) independently extracted the following data:
Year and language of publication.
Country.
Date and duration of the trial.
Inclusion and exclusion criteria.
Sample size.
Post‐randomisation drop‐outs and reasons for these.
Participant characteristics such as age and gender.
Risk of bias (described below).
Outcomes (described above).
We sought any unclear or missing information by contacting the authors of the individual trials. If there was any doubt whether the trials shared the same participants, completely or partially (by identifying common authors and centres), we planned to contact the authors of the trials to clarify whether the trial report had been duplicated. However, we did not find any such trials. We resolved any differences in opinion through discussion until we reached a consensus.
Assessment of risk of bias in included studies
We followed the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (CHBG module). According to empirical evidence (Schulz 1995; Moher 1998; Kjaergard 2001; Wood 2008; Lundh 2012; Savović 2012a; Savović 2012b), the risk of bias of the trials was assessed based on the following domains: sequence generation, allocation concealment, blinding of participants, personnel, and outcome assessors, incomplete outcome data, selective outcome reporting, and vested interest bias. Risk of bias domains were classified as follows:
Allocation sequence generation
Low risk of bias: sequence generation was achieved using computer random number generation or a random number table. Drawing lots, tossing a coin, shuffling cards, and throwing dice are adequate if performed by an independent research assistant not otherwise involved in the trial.
Uncertain risk of bias: the method of sequence generation was not specified.
High risk of bias: the sequence generation method was not random.
Allocation concealment
Low risk of bias: the participant allocations could not have been foreseen in advance of, or during, enrolment. Allocation was controlled by a central and independent randomisation unit. The allocation sequence was unknown to the investigators (eg, if the allocation sequence was hidden in sequentially numbered, opaque, and sealed envelopes).
Uncertain risk of bias: the method used to conceal the allocation was not described so that intervention allocations may have been foreseen in advance of, or during, enrolment.
High risk of bias: the allocation sequence was likely to be known to the investigators who assigned the participants.
Blinding of participants and personnel
Low risk of bias: blinding was performed adequately, or the assessment of outcomes was not likely to be influenced by lack of blinding.
Uncertain risk of bias: there was insufficient information to assess whether blinding was likely to introduce bias into the results.
High risk of bias: no blinding or incomplete blinding, and the assessment of outcomes was likely to be influenced by lack of blinding.
It is impossible to blind the participants as to whether they received day‐surgery or overnight stay surgery. We rated all the subjective outcomes as being at high risk of bias.
Blinding of outcome assessors
Low risk of bias: blinding was performed adequately, or the outcome measurement is not likely to be influenced by lack of blinding.
Uncertain risk of bias: there is insufficient information to assess whether the type of blinding used is likely to introduce bias into the estimate of effect.
High risk of bias: no blinding or incomplete blinding, and the outcome or the outcome measurement is likely to be influenced by lack of blinding.
Incomplete outcome data
Low risk of bias: missing data were unlikely to make treatment effects depart from plausible values. Sufficient methods, such as multiple imputation, have been employed to handle missing data.
Uncertain risk of bias: there was insufficient information to assess whether missing data in combination with the method used to handle missing data were likely to introduce bias into the results.
High risk of bias: the results were likely to be biased due to missing data.
Selective outcome reporting
Low risk of bias: all outcomes were pre‐defined (for example, in a published protocol) and reported, or all clinically relevant and reasonably expected outcomes (mortality and serious adverse events) were reported.
Uncertain risk of bias: it is unclear whether all pre‐defined and clinically relevant and reasonably expected outcomes were reported.
High risk of bias: one or more clinically relevant and reasonably expected outcomes were not reported, and data on these outcomes were likely to have been recorded.
Vested Interest
Low risk of bias: the trial appears to be free of other components that could put it at risk of bias.
Uncertain risk of bias: the trial may or may not be free of other components that could put it at risk of bias.
High risk of bias: there are other factors in the trial that could put it at risk of bias, eg, for‐profit involvement, authors have conducted trials on the same topic etc.
We considered trials which were classified as being at low risk of bias in all the above domains as trials with a low risk of bias. The remaining trials were classified as trials with a high risk of bias.
Measures of treatment effect
For dichotomous variables, we calculated the risk ratio (RR) with a 95% confidence interval (CI). We reported the risk difference if this was different from the risk ratio (ie, if the risk difference was statistically significant and the risk ratio was not statistically significant, and vice versa). Risk difference takes the 'zero event trials' into account while the risk ratio does not. For continuous variables, we calculated the mean difference (MD) or standardised mean difference (SMD) with a 95% CI. For count data such as serious adverse events, we reported the rate ratio with a 95% CI.
Unit of analysis issues
The unit of analysis was the participant undergoing laparoscopic cholecystectomy.
Dealing with missing data
We performed an intention‐to‐treat analysis whenever possible (Newell 1992). We imputed data for binary outcomes using various scenarios such as 'best‐case' scenario, 'worst‐case' scenario, 'best‐worst case' scenario, and 'worst‐best case' scenario analyses (Gurusamy 2009; Gluud 2012).
For continuous outcomes, we planned to use available‐case analysis. For continuous outcomes, we imputed the standard deviation from P values according to the instructions given in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and used the median for the meta‐analysis when the mean was not available. If it was not possible to calculate the standard deviation from a P value or a confidence interval, we imputed the standard deviation as the highest standard deviation noted for that group under that outcome.
Assessment of heterogeneity
We explored heterogeneity by Chi² test with significance set at a P value of 0.10, and measured the quantity of heterogeneity by I² (Higgins 2002).
Assessment of reporting biases
We planned to use visual asymmetry on a funnel plot to explore reporting bias (Egger 1997; Macaskill 2001). We planned to perform the linear regression approach described by Egger 1997 to determine the funnel plot asymmetry. We performed neither of these because fewer than 10 trials were included in this review.
Data synthesis
We performed the meta‐analyses according to the recommendations of The Cochrane Collaboration (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (CHBG module). We used the software package Review Manager 5 (RevMan 2012). We used a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987). In case of discrepancy between the two models we have reported both results; otherwise we have reported the results of the fixed‐effect model. We summarised the evidence in the 'Summary of findings' table using GRADEpro (GradePro 3.6).
Trial sequential analysis
We used the trial sequential analysis to control for random errors due to sparse data and repetitive testing of the accumulating data for the primary outcomes (CTU 2011; Thorlund 2011). We added the trials according to the year of publication, and if more than one trial was published in a year, we added the trials in alphabetical order according to the last name of the first author. We planned to construct the trial sequential monitoring boundaries on the basis of the diversity‐adjusted required information size (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010).
We applied trial sequential analysis (CTU 2011; Thorlund 2011) using a diversity‐adjusted required information size calculated from an alpha error of 0.05, a beta error of 0.20, a proportion with the outcome in the control group obtained from the results of the meta‐analysis, and a relative risk reduction of 20% for binary outcomes with two or more trials to determine whether more trials are necessary on this topic. If the trial sequential monitoring boundary or the required information size is reached or the futility zone is crossed, then more trials may not be necessary (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009, Wetterslev 2009; Thorlund 2010). For continuous outcomes, we calculated the required sample size from an alpha error of 0.05, a beta error of 0.20, the variance estimated from the meta‐analysis results of trials with low risk of bias, and a minimal clinically relevant difference of one cm for pain on a visual analogue scale and one day for time to return to activity and planned to calculate the required sample size for time to return to work on the same basis as time to return to activity.
It is not possible to perform trial sequential analysis for count data and for continuous outcomes such as quality of life where standardised mean difference was used as the effect measure.
Subgroup analysis and investigation of heterogeneity
We planned to perform the following subgroup analysis:
trials with low risk of bias compared to trials with high risk of bias.
We planned to use the 'test for subgroup differences' to identify the differences between subgroups.
Sensitivity analysis
We planned to perform sensitivity analyses of different methods of imputation for the binary outcomes. We performed a sensitivity analysis excluding the trials in which mean and standard deviation were imputed for the continuous outcomes.
Results
Description of studies
Results of the search
We identified 722 references through the electronic searches of the Cochrane Hepato‐Biliary Group Controlled Trials Register and Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (n = 84), MEDLINE (n = 371), EMBASE (n = 116), Science Citation Index Expanded (n = 151), and mRCT (n = 0). We have shown the flow of references in Figure 1. We excluded 242 duplicates and 462 clearly irrelevant references through reading abstracts. We retrieved 18 references for further assessment. We did not find any additional references through scanning reference lists of the identified randomised trials. Of the 18 references, we excluded seven for the reasons listed under the table 'Characteristics of excluded studies'. Eleven publications describing six randomised trials fulfilled the inclusion criteria.
1.
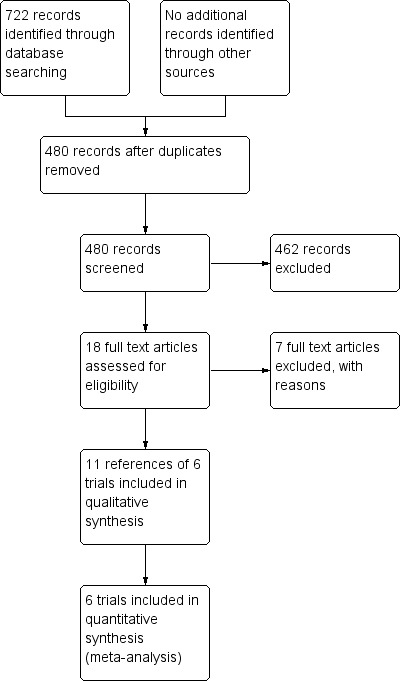
Prisma Flow Chart
Included studies
All six trials were completed and could provide data for the analyses (Keulemans 1998; Hollington 1999; Young 2001; Dirksen 2001; Johansson 2006; Barthelsson 2008). Details of the trials are shown in the table 'Characteristics of included studies'. All the trials assessed day‐surgery versus overnight stay for elective laparoscopic cholecystectomy. Four trials clearly stated that the laparoscopic cholecystectomy was performed for symptomatic gallstones (Keulemans 1998; Dirksen 2001; Johansson 2006; Barthelsson 2008). Two trials did not state the indication for laparoscopic cholecystectomy (Hollington 1999; Young 2001).
A total of 492 participants undergoing elective laparoscopic cholecystectomy were randomised to day‐surgery (n = 239) versus overnight stay surgery (n = 253). The number of participants in each trial ranged from 28 to 150. The proportion of women in the trials varied between 74% and 84%. The mean or median age in the trials varied between 40 and 47 years. Other details have been described in Characteristics of included studies.
Excluded studies
The reasons for exclusion of the studies are listed under the table 'Characteristics of excluded studies'.
Risk of bias in included studies
The risk of bias is summarised in the risk of bias graph (Figure 2) and risk of bias summary (Figure 3). All the trials were at high risk of bias as seen in Figure 3.
2.
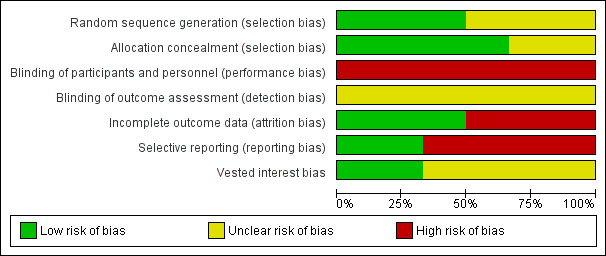
Methodological quality graph: review authors' judgments about each methodological quality item presented as percentages across all included studies.
3.
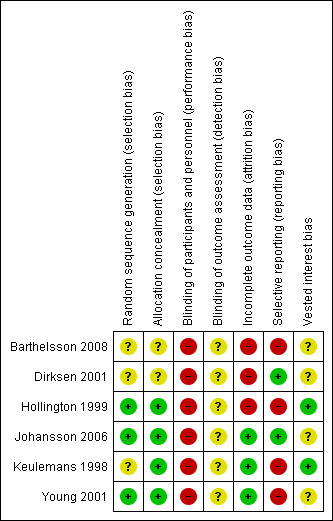
Methodological quality summary: review authors' judgments about each methodological quality item for each included study.
Allocation (selection bias)
Three trials (50%) were considered to have low risk of bias due to the description of random sequence generation (Hollington 1999; Young 2001; Johansson 2006). Four trials (67%) were considered to have low risk of bias due to the description of allocation concealment (Keulemans 1998; Hollington 1999; Young 2001; Johansson 2006).
Blinding (performance bias and detection bias)
None of the trials reported blinding.
Incomplete outcome data (attrition bias)
Two trials (33%) had featured exclusion of participants with acute cholecystitis, acute cholangitis or who postponed surgery because of lack of symptoms. This reflects a real‐life situation and therefore these post‐randomisation drop‐outs would not introduce bias due to missing outcome data (Keulemans 1998; Johansson 2006). One trial (17%) reported no post‐randomisation drop‐outs (Young 2001).
Selective reporting (reporting bias)
Three trials (50%) reported all the primary outcomes and were considered free from bias due to selective reporting (Hollington 1999; Dirksen 2001; Johansson 2006).
Other potential sources of bias
Two trials (33%) demonstrated that there was no vested interest bias (Keulemans 1998; Hollington 1999), while the other four provided no information on this.
Effects of interventions
See: Table 1
The main results are summarised in the Table 1.
Mortality
One trial reported short‐term mortality (Dirksen 2001). However, there were no deaths in this trial. Even in the other trials, although short‐term mortality was not reported explicitly, from the type of adverse events that participants suffered, and based on the number of participants included in the other outcomes, we could infer that there was no short‐term mortality in either group. Since there was no mortality in either group, we were unable to use the control group proportion for the calculation of the required information size of the trial sequential analysis. Instead, we used a proportion of 0.2% in the control group based on data from approximately 30,000 patients included in a database in Switzerland (Giger 2011). The proportion of information accrued was only 0.14% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn by the program (Figure 4).
4.
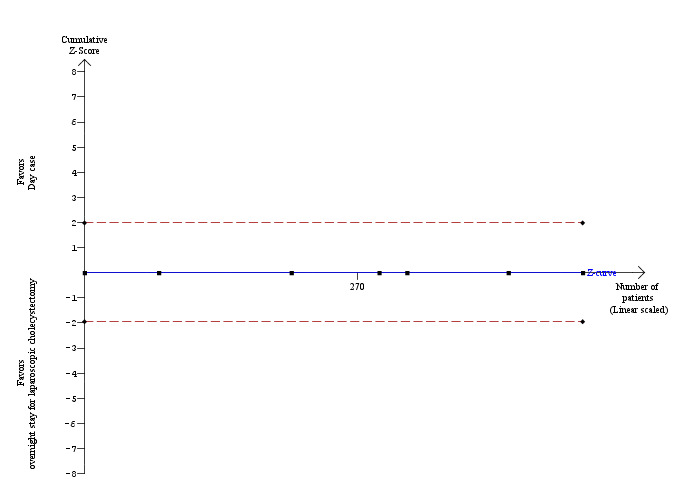
Trial sequential analysis of mortality The diversity‐adjusted required information size (DARIS) was calculated to 352,564 patients, based on the proportion of patients in the control group with the outcome of 0.2%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing a total of 492 patients in six trials, only 0.14% of the DARIS has been reached. Accordingly, the trial sequential analysis program does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries have also not been crossed by the cumulative Z‐curve.
None of the trials reported long‐term mortality.
Serious adverse events
Four trials reported serious adverse events such as subphrenic collection requiring computerised tomographic scan‐guided drainage, pancreatitis, bleeding requiring intervention, pleuritic chest pain, bile duct injury, and haematoma at port site requiring treatment (Keulemans 1998; Hollington 1999; Dirksen 2001; Johansson 2006). There was no significant difference in the number of serious adverse events between the two groups (rate ratio 3.24; 95% confidence interval (CI) 0.74 to 14.09) (Analysis 1.1). It is not possible to perform trial sequential analysis for rate ratios.
1.1. Analysis.
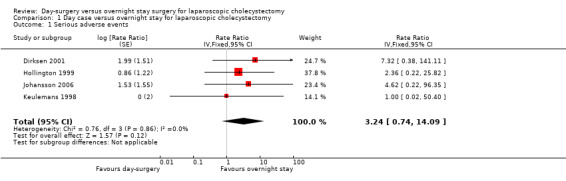
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 1 Serious adverse events.
Quality of life
Four trials reported quality of life at one week (Keulemans 1998; Dirksen 2001; Johansson 2006; Barthelsson 2008).There was no significant difference in the quality of life between the two groups (standardised mean difference (SMD) ‐0.11; 95% CI ‐0.33 to 0.10) (Analysis 1.2). It is not possible to perform trial sequential analysis for standardised mean difference data.
1.2. Analysis.
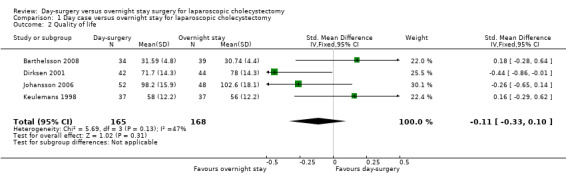
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 2 Quality of life.
Pain
Three trials reported pain (Keulemans 1998; Young 2001; Barthelsson 2008). There was no significant difference in the pain reported between the two groups (mean difference (MD) 0.02 cm visual analogue scale scores; 95% CI ‐0.69 to 0.73) (Analysis 1.3). Trial sequential analysis revealed that the futility zone area was reached suggesting that future trials are unlikely to show clinically significant difference in pain between the two groups (Figure 5).
1.3. Analysis.
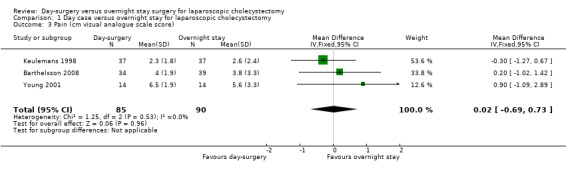
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 3 Pain (cm visual analogue scale score).
5.
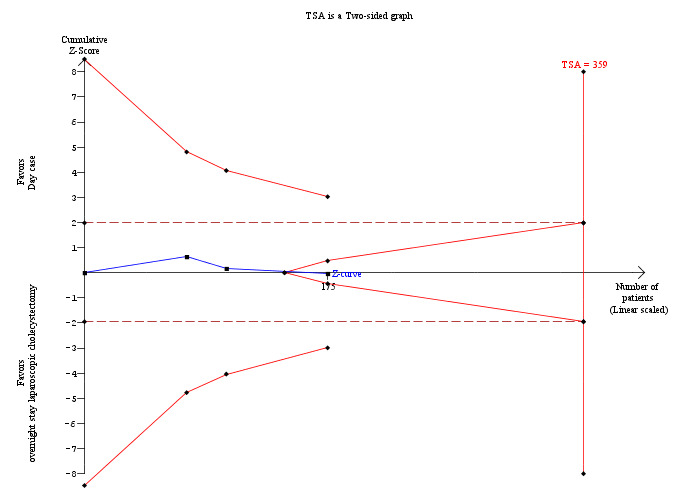
Trial sequential analysis of pain Trial sequential analysis of pain showing that the cumulative Z‐curve (blue line) enters the futility area after the third trial. The diversity‐adjusted required information size (DARIS) was 359 participants based on a minimal relevant difference (MIRD) of 1 cm on the visual analogue scale, a variance (VAR) of 11.41, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. The results are compatible with lack of difference in pain scores between day‐surgery and overnight stay surgery for laparoscopic cholecystectomy.
Time to return to activity
Two trials reported time taken to return to activity (Hollington 1999; Dirksen 2001). There was no significant difference in the time taken to return to activity between the two groups (MD ‐0.55 days; 95% CI ‐2.18 to 1.08) (Analysis 1.4). Trial sequential analysis revealed that the information was too small (9.21%) to draw the futility area, and the trial sequential boundaries for benefits or harms of day‐surgery were not crossed (Figure 6).
1.4. Analysis.

Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 4 Time to return to activity (days).
6.
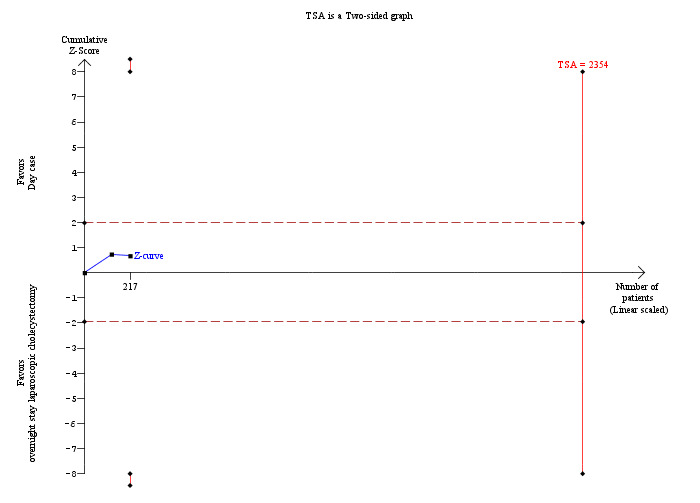
Trial sequential analysis of time to return to activity The diversity‐adjusted required information size (DARIS) was 2,354 participants based on a minimal relevant difference (MIRD) of 1 day, a variance (VAR) of 74.96, an alpha (a) of 5%, a beta (b) of 20%, and a diversity (D2) of 0%. After accruing 217 participants in two trials, only 9.2% of the DARIS has been reached. Accordingly, the trial sequential analysis program does not show the futility area. Neither the trial sequential boundaries nor the conventional statistical boundaries for benefits or harms of day‐surgery versus overnight stay were crossed by the cumulative Z‐curve (blue line).
Time to return to work
One trial reported time taken to return to work (Keulemans 1998). There was no significant difference in the time taken to return to work between the two groups (MD ‐2.00 days; 95% CI ‐10.34 to 6.34) (Analysis 1.5). We did not perform trial sequential analysis because of the presence of only one trial.
1.5. Analysis.
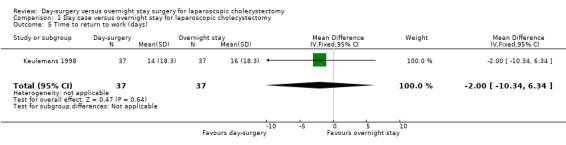
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 5 Time to return to work (days).
Number of hospital readmissions
Five trials reported the number of hospital readmissions (Keulemans 1998; Hollington 1999; Dirksen 2001; Johansson 2006; Barthelsson 2008). There was no significant difference in the rate of hospital readmissions between the two groups (rate ratio 1.25; 95% CI 0.43 to 3.63) (Analysis 1.6). It is not possible to perform trial sequential analysis for rate ratios.
1.6. Analysis.
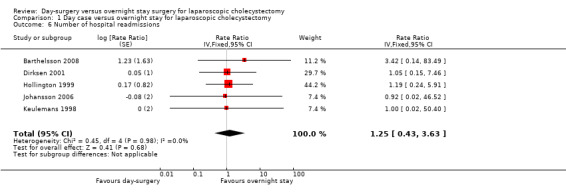
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 6 Number of hospital readmissions.
Number of people requiring readmission
Three trials reported the proportion of people requiring hospital readmissions (Hollington 1999; Dirksen 2001; Barthelsson 2008). There was no significant difference in the proportion of people requiring readmission between the two groups (risk ratio (RR) 1.09; 95% CI 0.33 to 3.60) (Analysis 1.7). The trial sequential analysis revealed that the proportion of information accrued was only 1.38% of the diversity‐adjusted required information size and so the trial sequential monitoring boundaries were not drawn (Figure 7).
1.7. Analysis.
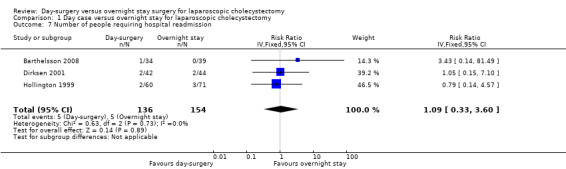
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 7 Number of people requiring hospital readmission.
7.
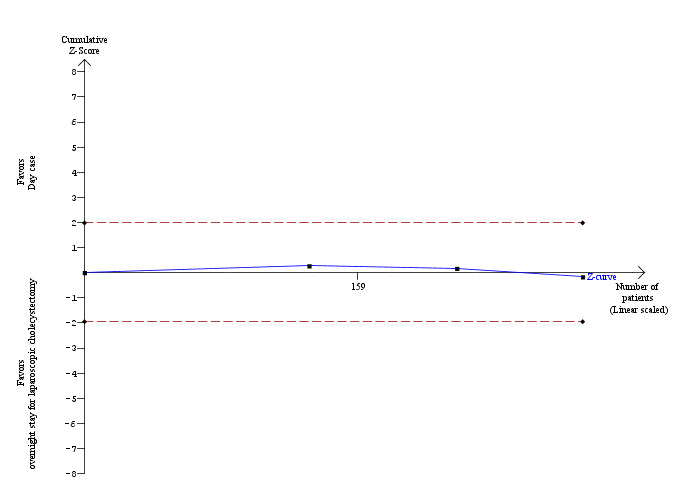
Trial sequential analysis of proportion of participants requiring hospital readmission The diversity‐adjusted required information size (DARIS) was calculated to 21,030 participants, based on the proportion of participants in the control group with the outcome of 3.24%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 0%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing 290 participants in three trials, only 1.38% of the DARIS has been reached. Accordingly, the trial sequential analysis program does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional boundaries have also not been crossed.
Failed discharge
Five trials reported failed discharge (Keulemans 1998; Hollington 1999; Young 2001; Dirksen 2001; Johansson 2006). There was no significant difference in the proportion of participants who had failed discharge between the two groups (RR 0.96; 95% CI 0.65 to 1.41) (Analysis 1.8). The trial sequential analysis revealed that the proportion of information accrued was only 2.62% of the diversity‐adjusted required information size, and so the trial sequential monitoring boundaries were not drawn (Figure 8).
1.8. Analysis.
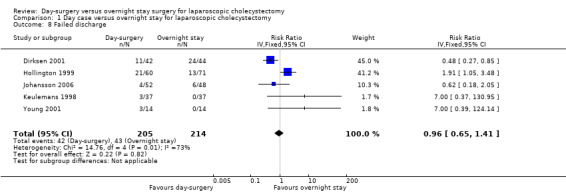
Comparison 1 Day case versus overnight stay for laparoscopic cholecystectomy, Outcome 8 Failed discharge.
8.
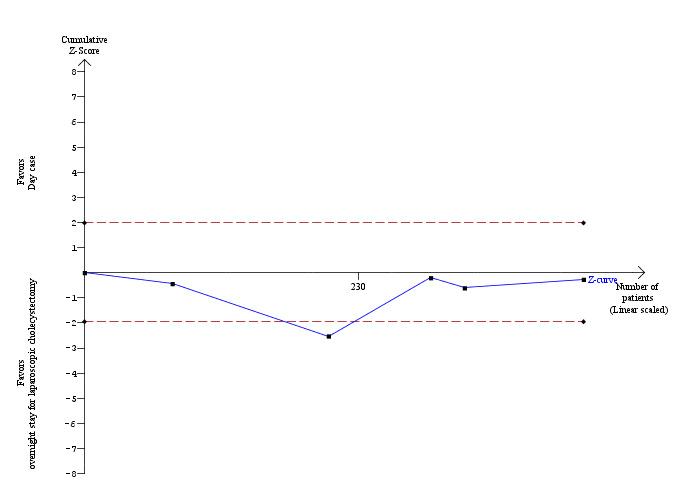
Trial sequential analysis of proportion of participants with failed discharge The diversity‐adjusted required information size (DARIS) was calculated to 15,968 participants, based on the proportion of participants in the control group with the outcome of 20.09%, a relative risk reduction of 20%, an alpha of 5%, a beta of 20%, and a diversity of 81.98%. To account for zero event groups, a continuity correction of 0.01 was used in the calculation of the cumulative Z‐curve (blue line). After accruing 419 participants in the five trials, only 2.62% of the DARIS has been reached. Accordingly, the trial sequential analysis program does not show the required information size and the trial sequential monitoring boundaries. As shown, the conventional statistical boundaries have also not been crossed at the end of five trials although the cumulative Z‐curve crossed temporarily the conventional statistical boundary favouring overnight stay after two trials. Such a finding is likely to be a random error.
Sensitivity analysis
In two trials (Keulemans 1998; Hollington 1999), the standard deviation was calculated from the standard error. In the trial by Barthelsson 2008, the standard deviation, the standard error, and the P value were not given, and we used the highest standard deviation in the outcome. Removing the imputed data from each outcome did not cause a change in the results (Analysis 2.1; Analysis 2.2; Analysis 2.3; Analysis 2.4).
2.1. Analysis.
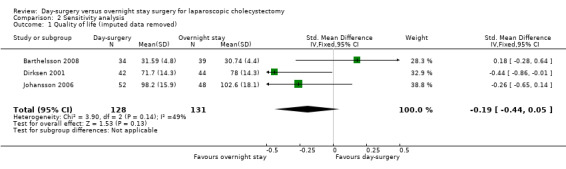
Comparison 2 Sensitivity analysis, Outcome 1 Quality of life (imputed data removed).
2.2. Analysis.
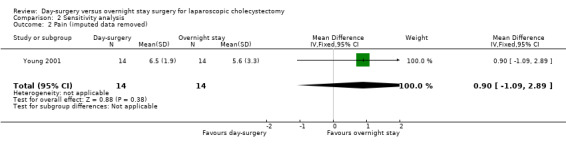
Comparison 2 Sensitivity analysis, Outcome 2 Pain (imputed data removed).
2.3. Analysis.
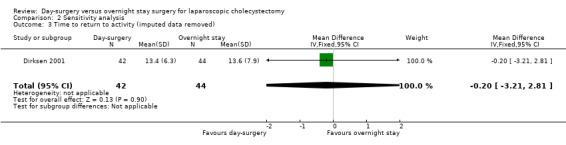
Comparison 2 Sensitivity analysis, Outcome 3 Time to return to activity (imputed data removed).
2.4. Analysis.
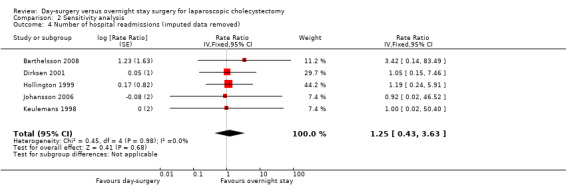
Comparison 2 Sensitivity analysis, Outcome 4 Number of hospital readmissions (imputed data removed).
Five of the six trials included in this systematic review had post‐randomisation drop‐outs (Keulemans 1998; Hollington 1999; Dirksen 2001; Johansson 2006; Barthelsson 2008). In these groups, we performed a sensitivity analysis for the three binary outcomes (short‐term mortality, number of people requiring hospital readmission, and failed discharge), to investigate best and worst outcomes. It was not possible to impute best and worst scenarios for one trial (Dirksen 2001) as the trial failed to detail the groups to which post‐randomisation drop‐outs belonged. For this reason we did not perform a sensitivity analysis on short‐term mortality, as Dirksen 2001 was the only trial that reported short‐term mortality explicitly. With regards to the number of people requiring hospital readmission, the 'best‐best' scenario results showed no change compared with the original analysis (Analysis 2.5). However, when the outcomes were imputed by the 'worst‐worst' and 'worst‐best' scenarios, the results significantly favoured overnight stay laparoscopic cholecystectomy. In contrast, imputation of outcome according to the 'best‐worst' scenario significantly favoured day‐surgery laparoscopic cholecystectomy (Analysis 2.6; Analysis 2.7; Analysis 2.8). With regards to the number of failed discharge attempts, the imputation of outcomes by the 'best‐best', 'worst‐worst', 'best‐worst', and 'worst‐best' scenarios showed no significant change in the results obtained without imputation (Analysis 2.9; Analysis 2.10; Analysis 2.11; Analysis 2.12).
2.5. Analysis.
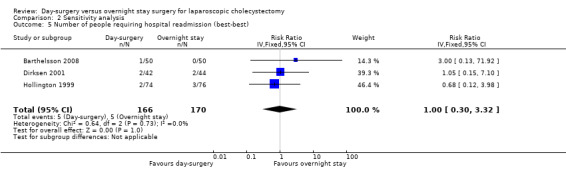
Comparison 2 Sensitivity analysis, Outcome 5 Number of people requiring hospital readmission (best‐best).
2.6. Analysis.
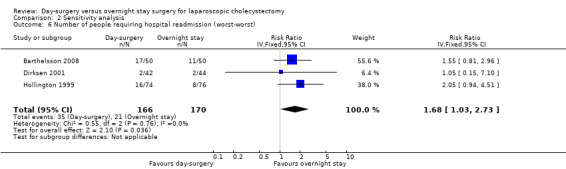
Comparison 2 Sensitivity analysis, Outcome 6 Number of people requiring hospital readmission (worst‐worst).
2.7. Analysis.
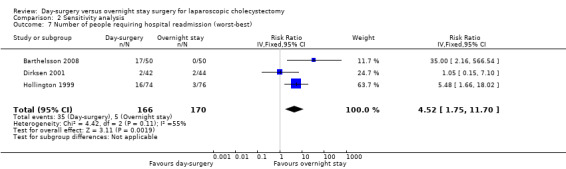
Comparison 2 Sensitivity analysis, Outcome 7 Number of people requiring hospital readmission (worst‐best).
2.8. Analysis.
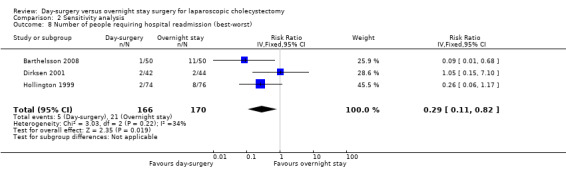
Comparison 2 Sensitivity analysis, Outcome 8 Number of people requiring hospital readmission (best‐worst).
2.9. Analysis.
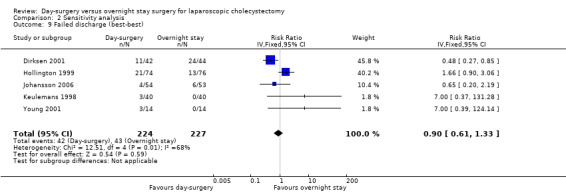
Comparison 2 Sensitivity analysis, Outcome 9 Failed discharge (best‐best).
2.10. Analysis.
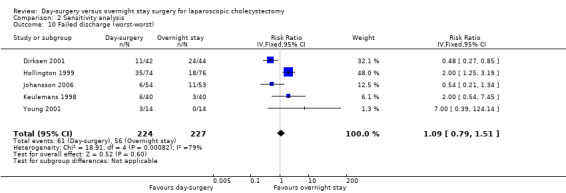
Comparison 2 Sensitivity analysis, Outcome 10 Failed discharge (worst‐worst).
2.11. Analysis.
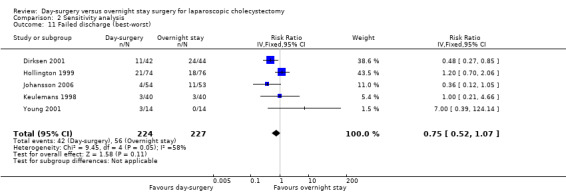
Comparison 2 Sensitivity analysis, Outcome 11 Failed discharge (best‐worst).
2.12. Analysis.
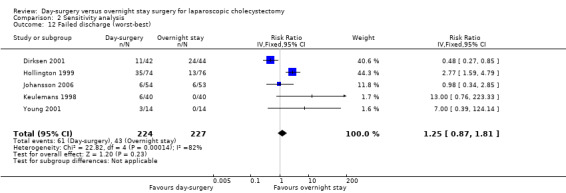
Comparison 2 Sensitivity analysis, Outcome 12 Failed discharge (worst‐best).
Variations in meta‐analysis
There was no change in the interpretation of the results by adopting the random‐effects model or by calculating the risk difference rather than the risk ratio.
Subgroup analyses
All the trials were of high risk of bias, so we did not perform any subgroup analysis.
Funnel plot
We did not produce a funnel plot due to the small number of trials (fewer than 10 trials) included in this review.
Discussion
Summary of main results
There were no statistically significant differences in any of the outcomes between day‐surgery and overnight stay laparoscopic cholecystectomy. There was no significant difference in short‐term mortality between the groups of participants undergoing day‐surgery or overnight‐stay elective laparoscopic cholecystectomy. Only one trial reported short‐term mortality explicitly. However, the serious adverse events and the number of participants included in the remaining trials suggest that there was no mortality in the remaining trials. Laparoscopic cholecystectomy is generally considered a low‐morbidity procedure. Long‐term mortality was not reported. However, it is unlikely that long‐term mortality is affected by the decision to admit the patient overnight provided that there was no difference in the short‐term mortality (which was the case in this particular comparison). There was no significant difference in serious adverse events or quality of life between the two groups. There was a suggestion of heterogeneity between the trials with regards to quality of life by the I². Two trials favoured day‐surgery (Dirksen 2001; Johansson 2006) and two trials favoured overnight‐stay surgery (Keulemans 1998; Barthelsson 2008). However, there was significant overlap of confidence intervals and the Chi² test for heterogeneity was not significant. Quality of life is difficult to quantify, and trials report it in markedly different ways. This could be the reason for the heterogeneity noted between the trials.
There was no statistically significant difference in pain, time to return to activity, time to return to work, number of hospital readmissions, number of people requiring hospital readmission, and failed discharge. Inadequate pain control at home or excessive complications because of early discharge would have resulted in an increase in one or more of the above. Considering that none of the above have been affected, it appears that there are no significant differences between day‐surgery and overnight stay laparoscopic cholecystectomy. However, there is still a possibility that these findings are because of lack of evidence of effect and not because of lack of effect. Furthermore, all trials were considered at risk of bias, that is overestimation of benefits and underestimation of harms of day‐surgery or overnight stay. That is why more randomised clinical trials are necessary.
Elective laparoscopic cholecystectomy is a low‐morbidity procedure. Major complications are likely to be noted at the time of laparoscopic cholecystectomy and those with such major complications during operation will not be discharged as day‐surgery patients even if they were admitted to the hospital with an intention to perform the surgery as day‐surgery. The only concern about discharging the people on the same day is that post‐operative complications can be missed. The issue is whether these post‐operative complications would have been identified if the patient had stayed overnight. Many post‐operative complications following laparoscopic cholecystectomy manifest with abdominal pain and so appropriate advice should be given to people to ensure that they return to the hospital if they have abdominal pain uncontrolled by routine analgesia. They should also be advised to return in the presence of dizziness or light headedness (particularly if postural), or exercise intolerance, as these may be early signs of significant bleeding. However, it must be noted that the impact of discharging people on the same day on these rare complications which are not evident within the first four to eight hours of observation in hospital cannot be assessed in randomised clinical trials because of the rare nature of the complications. On the other hand, inadequate pain control can affect the patients' quality of life, return to normal activity, and return to work. Future trials should be powered to measure differences in these outcomes.
We identified four studies that were non‐randomised (Rosen 2001; Burney 2002; Curet 2002; Sharma 2004). These do not report any specific adverse events related to day‐surgery laparoscopic cholecystectomy.
Because of the shorter hospital stay, day‐surgery laparoscopic cholecystectomy is likely to result in lower costs, which will benefit those paying for their surgery and the government in a state‐funded healthcare system.
Overall completeness and applicability of evidence
This review included participants undergoing elective laparoscopic cholecystectomy, which is the predominant management choice for symptomatic gallstones. Four trials clearly stated that the patients underwent elective laparoscopic cholecystectomy for symptomatic gallstones (Keulemans 1998; Dirksen 2001; Johansson 2006; Barthelsson 2008). The indication for elective laparoscopic cholecystectomy was not stated in the remaining two trials (Hollington 1999; Young 2001). However, the findings of this review can be applied to patients undergoing elective laparoscopic cholecystectomy for benign gallbladder polyps and gallbladder dyskinesia since there is no scientific rationale for an interaction between the timing of discharge and the indication for elective laparoscopic cholecystectomy. Hence this review is applicable to most people undergoing elective laparoscopic cholecystectomy. However, the safety of day‐surgery laparoscopic cholecystectomy in acute cholecystitis on a non‐elective basis has not been established and should be investigated further. This review also includes mostly patients at low anaesthetic risk and is applicable only for such people.
Quality of the evidence
All the trials were at a high risk of bias. Blinding of participants, healthcare providers, assessors, and investigators was not reported in any trial. Some trials had a significant proportion of post‐randomisation drop‐outs, introducing missing outcome data bias. This was evident from the sensitivity analysis where imputation of missing data by different scenarios resulted in different results.
While it may be impossible to blind the participants and healthcare providers to the intervention groups, it is possible to blind the outcome assessors and investigators to the intervention groups. Considering that the risk of conversion to open cholecystectomy in elective laparoscopic cholecystectomy is low and it is reasonable to exclude such participants from the analyses as such patients who require conversion to open cholecystectomy will be admitted in real‐life, it is possible to conduct trials with no missing outcome data bias.
Potential biases in the review process
We followed the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) for this review. There were no language, publication status, or sample size restrictions. Thus, we minimised the bias due to selection of trials. However, we have used median for the meta‐analyses when the mean was not available. We have also imputed the standard deviation from P values according to the formulae stated in the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011). If the trials stated a P value less than 0.05, we calculated the standard deviation using a P value of 0.05. If the standard deviation could not be calculated because the trial reports just state that there was no statistical significance without mentioning the exact P value, we used the highest standard deviation among the other trials included in the outcome. This imputation of standard deviation may have introduced bias. However, sensitivity analyses performed on imputed values demonstrated no difference in results. The alternative to this imputation is to exclude such trials, but this would make the results even more difficult to interpret.
Agreements and disagreements with other studies or reviews
There are no major changes in the results by following the updated methodology and searches as compared to the previous review version (Gurusamy 2008b).
Authors' conclusions
Implications for practice.
Day‐surgery for laparoscopic cholecystectomy appears to be safe, but due to risks of systematic errors (bias) and risks of random errors (play of chance) more research is needed.
Implications for research.
Further trials at low risk of bias are necessary, which are powered to measure differences in quality of life using validated quality of life measures such as EQ‐5D, return to normal activity, and return to work. Trials need to be designed according to the SPIRIT Statement (SPIRIT 2013) and need to be conducted and reported according to the CONSORT Statement (www.consort‐statement.org).
What's new
| Date | Event | Description |
|---|---|---|
| 29 July 2013 | Amended | Author list: Jessica Vaughan, Kurinchi Gurusamy, Brian R Davidson |
| 13 October 2012 | Amended | Outcomes revised according to current Cochrane guidelines. |
| 13 October 2012 | New citation required but conclusions have not changed | Conclusions not changed. |
| 13 October 2012 | New search has been performed | One new trial fulfilled the inclusion criteria of the review (Barthelsson 2008). Data from the trial were added to the meta‐analyses. |
Acknowledgements
We thank S Junnarkar for previous data extraction and Marwan Farouk for critically commenting on the first version of the review.
The Cochrane Hepato‐Biliary Group for the provided support.
Peer Reviewers: Mikael Victorzon, Finland; Cosmin Puia, France. Contact Editor: Saboor A'Khan, UK.
Appendices
Appendix 1. Search strategy
| Database | Period of search | Search strategy |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register and the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (Wiley) | September 2012. | #1 "day case" OR day‐case OR "day surgery" or day‐surgery OR "day care" OR day‐care OR "day stay" OR day‐stay OR ambulatory OR outpatient OR out‐patient OR (partial and (hospitalization or hospitalizations or hospitalisation or hospitalizations)) #2 MeSH descriptor Day Care explode all trees #3 MeSH descriptor Ambulatory Surgical Procedures explode all trees #4 MeSH descriptor Ambulatory Care explode all trees #5 (#1 OR #2 OR #3 OR #4) #6 (laparoscop* OR celioscop* OR coelioscop* OR abdominoscop* OR peritoneoscop*) AND (cholecystecto* OR colecystecto*) #7 MeSH descriptor Cholecystectomy, Laparoscopic explode all trees #8 (#6 OR #7) #9 (#5 AND #8) |
| MEDLINE (Pubmed) | 1987 to September 2012. | ("day case" OR day‐case OR "day surgery" or day‐surgery OR "day care" OR day‐care OR "day stay" OR day‐stay OR ambulatory OR outpatient OR out‐patient OR (partial and (hospitalization or hospitalizations or hospitalisation or hospitalizations)) OR "Day Care"[MeSH] OR "Ambulatory Surgical Procedures"[MeSH] OR "Ambulatory Care"[MeSH]) AND (((laparoscop* OR celioscop* OR coelioscop* OR abdominoscop* OR peritoneoscop*) AND (cholecystecto* OR colecystecto*)) OR "cholecystectomy, laparoscopic"[MeSH]) AND ((randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) |
| EMBASE (OvidSP) | 1987 to September 2012. | 1 (day case OR day‐case OR day surgery OR day‐surgery OR day care OR day‐care OR day stay OR day‐stay OR ambulatory OR outpatient OR out‐patient).af. 2 exp DAY‐CARE/ OR exp AMBULATORY‐SURGERY/ 3 1 OR 2 4 (LAPAROSCOP* OR CELIOSCOP* OR COELIOSCOP* OR ABDOMINOSCOP* OR PERITONEOSCOP*).af. 5 (CHOLECYSTECT* OR COLECYSTECT*).af. 6 4 AND 5 7 exp LAPAROSCOPIC‐SURGERY/ OR exp LAPAROSCOPY/ 8 exp CHOLECYSTECTOMY/ 9 7 AND 8 10 6 OR 9 11 3 AND 10 12 exp crossover‐procedure/ or exp double‐blind procedure/ or exp randomized controlled trial/ or single‐blind procedure/ 13 (random* OR factorial* OR crossover* OR placebo*).af. 14 12 OR 13 15 11 AND 14 |
| Science Citation Index Expanded (ISI Web of Knowledge) | 1987 to September 2012. | #1 TS=("day case" OR day‐case OR "day surgery" or day‐surgery OR "day care" OR day‐care OR "day stay" OR day‐stay OR ambulatory OR outpatient OR out‐patient) #2 TS=(partial) #3 TS=(hospitalization or hospitalizations or hospitalisation or hospitalizations) #4 #3 AND #2 #5 #4 OR #1 #6 TS=(laparoscop* OR celioscop* OR coelioscop* OR abdominoscop* OR peritoneoscop*) #7 TS=(cholecystecto* OR colecystecto*) #8 TS=(random* OR blind* OR placebo* OR meta‐analysis) #9 #8 AND #7 AND #6 AND #5 |
| mRCT (http://www.controlled‐trials.com/mrct/) | September 2012. | outpatient AND laparoscopic AND cholecystectomy |
Data and analyses
Comparison 1. Day case versus overnight stay for laparoscopic cholecystectomy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 4 | Rate Ratio (Fixed, 95% CI) | 3.24 [0.74, 14.09] | |
| 2 Quality of life | 4 | 333 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.11 [‐0.33, 0.10] |
| 3 Pain (cm visual analogue scale score) | 3 | 175 | Mean Difference (IV, Fixed, 95% CI) | 0.02 [‐0.69, 0.73] |
| 4 Time to return to activity (days) | 2 | 217 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [‐2.18, 1.08] |
| 5 Time to return to work (days) | 1 | 74 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐10.34, 6.34] |
| 6 Number of hospital readmissions | 5 | Rate Ratio (Fixed, 95% CI) | 1.25 [0.43, 3.63] | |
| 7 Number of people requiring hospital readmission | 3 | 290 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [0.33, 3.60] |
| 8 Failed discharge | 5 | 419 | Risk Ratio (IV, Fixed, 95% CI) | 0.96 [0.65, 1.41] |
Comparison 2. Sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life (imputed data removed) | 3 | 259 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.19 [‐0.44, 0.05] |
| 2 Pain (imputed data removed) | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐1.09, 2.89] |
| 3 Time to return to activity (imputed data removed) | 1 | 86 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐3.21, 2.81] |
| 4 Number of hospital readmissions (imputed data removed) | 5 | Rate Ratio (Fixed, 95% CI) | 1.25 [0.43, 3.63] | |
| 5 Number of people requiring hospital readmission (best‐best) | 3 | 336 | Risk Ratio (IV, Fixed, 95% CI) | 1.00 [0.30, 3.32] |
| 6 Number of people requiring hospital readmission (worst‐worst) | 3 | 336 | Risk Ratio (IV, Fixed, 95% CI) | 1.68 [1.03, 2.73] |
| 7 Number of people requiring hospital readmission (worst‐best) | 3 | 336 | Risk Ratio (IV, Fixed, 95% CI) | 4.52 [1.75, 11.70] |
| 8 Number of people requiring hospital readmission (best‐worst) | 3 | 336 | Risk Ratio (IV, Fixed, 95% CI) | 0.29 [0.11, 0.82] |
| 9 Failed discharge (best‐best) | 5 | 451 | Risk Ratio (IV, Fixed, 95% CI) | 0.90 [0.61, 1.33] |
| 10 Failed discharge (worst‐worst) | 5 | 451 | Risk Ratio (IV, Fixed, 95% CI) | 1.09 [0.79, 1.51] |
| 11 Failed discharge (best‐worst) | 5 | 451 | Risk Ratio (IV, Fixed, 95% CI) | 0.75 [0.52, 1.07] |
| 12 Failed discharge (worst‐best) | 5 | 451 | Risk Ratio (IV, Fixed, 95% CI) | 1.25 [0.87, 1.81] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barthelsson 2008.
| Methods | Randomised clinical trial. | |
| Participants | Country: Sweden. Number randomised: 100. Post‐randomisation drop‐outs: 27 (27%). Revised sample size: 73. Average age: 45 years. Women: 54 (74%). Inclusion criteria 1. Ultrasonography‐documented cholelithiasis. 2. Scheduled for planned laparoscopic cholecystectomy. 3. American Association of Anaesthesiologists (ASA) I – II. 4. 20 – 70 years old. 5. Able to understand and speak the Swedish language. Exclusion criteria 1. Immunodeficiency. 2. HIV. 3. Previous GI surgery. 4. Proven malignancy. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 34). Group 2: overnight stay laparoscopic cholecystectomy (n = 39). Participants in the day‐surgery group were admitted to the outpatient surgery department in the morning on the day of surgery and laparoscopic cholecystectomy (LC) was performed before 11:00 a.m. Participants were discharged when they were able to meet standard discharge criteria (adequate pain control: visual analogue scores (VAS) < 4), able to walk, and able to void and tolerate oral liquids. | |
| Outcomes | The outcomes reported were pain score on first post‐operative day and quality of life. | |
| Notes | We attempted to contact the authors in September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Comment: This information was not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants for this comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs which might have altered the effect estimates. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported explicitly. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Dirksen 2001.
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 94. Post‐randomisation drop‐outs: 8 (8.5%). Revised sample size: 86. Average age: 47 years. Women: 68 (79.1%). Inclusion criteria 1. Participants aged between 18 and 80 years having symptomatic gallstones. Exclusion criteria 1. Complicated gallstones. 2. Participants with an American Society of Anesthesiology [ASA] classification of III or IV. 3. Other interventions during operation. 4.No support at home until 24 hours after the operation. 5. Trip time between house and hospital longer than 30 minutes. 6. No good control of the Dutch language. 3. Participants with extensive previous abdominal surgery. 4. Clinical suspicion of common bile duct stones. 5. Acute cholecystitis. 6. Calcified gallbladder. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 42). Group 2: overnight stay laparoscopic cholecystectomy (n = 44). The discharge criteria were no or manageable nausea and/or vomitus, good pain control, spontaneous micturition and absence of a complication. | |
| Outcomes | The outcomes reported were short‐term mortality, morbidity, failed discharge, hospital readmissions, and quality of life (EUROQoL at 1 week). | |
| Notes | We attempted to contact the authors in February 2007 and September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "After informed license, patients were randomised to day‐surgery or clinical observation by the secretary with help of closed envelopes." Comment: Further details were not available. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants for this comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs which might have altered the effect estimates. |
| Selective reporting (reporting bias) | Low risk | Comment: All important outcomes (short‐term mortality and serious adverse events) were reported. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Hollington 1999.
| Methods | Randomised clinical trial | |
| Participants | Country: Australia. Number randomised: 150. Post‐randomisation drop‐outs: 19 (12.7%). Revised sample size: 131. Average age: 47 years. Women: 106 (80.9%). Inclusion criteria 1. ASA status < IV. 2. Adequate motivation. 3. Presence of family member or carer at home. Exclusion criteria 1. Participants who were assessed as being at significant risk of requiring conversion to open operation such as those with multiple upper abdominal surgical scars. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 60). Group 2: overnight stay laparoscopic cholecystectomy (n = 71). Participants were admitted to hospital on the morning of their operation, which was scheduled to commence prior to midday, and day‐stay‐only participants left hospital that evening after review to confirm suitability for discharge.The day‐stay‐only participants were subsequently reviewed a minimum of 4 hours post‐operatively, and discharged if their pain and nausea were controlled, they were not drowsy and were able to walk. If these criteria were not met they were transferred to the overnight‐stay ward. | |
| Outcomes | The outcomes reported were morbidity, failed discharge, and hospital readmissions. | |
| Notes | We attempted to contact the authors in February 2007 and September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed by a member of nursing staff using 200 cards (100 for each group) which had been shuffled and sealed in plain envelopes, during patient assessment in the outpatient clinic, after consent was obtained for both the procedure and the randomization." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was performed by a member of nursing staff using 200 cards (100 for each group) which had been shuffled and sealed in plain envelopes, during patient assessment in the outpatient clinic, after consent was obtained for both the procedure and the randomization". Comment: Allocation concealment was probably performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients were aware of which group they had been randomized to prior to admission." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: There were post‐randomisation drop‐outs which might have altered the effect estimates. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported explicitly. |
| Vested interest bias | Low risk | Quote: "South Australian Health Commission, in the form of funding for an ‘Elective Surgery Strategy’ to reduce waiting lists for elective surgery." |
Johansson 2006.
| Methods | Randomised clinical trial. | |
| Participants | Country: Sweden. Number randomised: 107. Post‐randomisation drop‐outs: 7 (6.5%). Revised sample size: 100. Average age: not stated Women: not stated Inclusion criteria 1. ASA status I ‐ II. 2. Participants between the ages of 18 and 70 years presenting for gallstone disease surgery. 3. Lived less than 50 km from the hospital. 4. Availability of an adult to accompany the participant home and stay there overnight. Exclusion criteria 1. Obesity. 2. Extensive previous abdominal surgery. 3. Common bile duct stones. 4. Acute cholecystitis. 5. Pancreatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 52). Group 2: overnight stay laparoscopic cholecystectomy (n = 48). The operating surgeon reviewed the participants before 18.00 hours. Discharge was allowed if the participant required oral pain medication only, tolerated oral fluids, had passed urine spontaneously and felt confident of managing at home. | |
| Outcomes | The outcomes reported were morbidity, failed discharge, hospital readmissions, and quality of life (Psychological General Well‐Being Index at 1 week). | |
| Notes | Authors replied to some questions in March 2007. We attempted to contact the authors again in September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was achieved by computer generated random numbers with stratification for sex, age and body mass index." |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was done with sealed envelopes" (author replies). Comment: Allocation concealment was probably performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants for this comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Participants with acute cholecystitis were excluded from both groups. This reflects real‐life situations. These post‐randomisation drop‐outs will not cause bias due to missing outcome data. |
| Selective reporting (reporting bias) | Low risk | Comment: Mortality was not reported explicitly. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Keulemans 1998.
| Methods | Randomised clinical trial. | |
| Participants | Country: Netherlands. Number randomised: 80. Post‐randomisation drop‐outs: 6 (7.5%). Revised sample size: 74. Average age: 44 years. Women: 62 (83.8%). Inclusion criteria 1. ASA status I ‐ II. 2. Age < 70 years. 3. Participants undergoing laparoscopic cholecystectomy for symptomatic gallstones. 4. Living within 50 km from the hospital. 5. Participants who had an adult willing to accompany them home and to stay with them for at least 24 hours. Exclusion criteria 1. Obesity. 2. Extensive previous abdominal surgery. 3. Common bile duct stones. 4. Acute cholecystitis. 5. Pancreatitis. | |
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 37). Group 2: overnight stay laparoscopic cholecystectomy (n = 37). The LC procedures were performed during the morning. Discharge was allowed if participants required oral pain medication only, tolerated oral fluids, could walk to the lavatory, had passed urine spontaneously, and felt confident that they could manage at home. The decision about discharge was made by both the surgeon and the anaesthesiologist before 7 pm. | |
| Outcomes | The outcomes reported were morbidity, failed discharge (clearly reported for day‐surgery group but not for overnight stay group), hospital readmissions, quality of life (EuroQoL at 1 week), and return to work. | |
| Notes | We attempted to contact the authors in February 2007 and September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: This information was not available. |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were then randomly allocated, by opening a sealed envelope, to either the day‐care group or the clinical observation group." Comment: Allocation concealment was probably performed adequately. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants for this comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: Participants with acute cholecystitis, acute cholangitis or who postponed surgery because of lack of symptoms were excluded from both groups. This reflects the real‐life situation. These post‐randomisation drop‐outs will not cause bias due to missing outcome data. |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported explicitly. |
| Vested interest bias | Low risk | Quote: "Supported by a grant from the Richtlijnen Commissie AMC (Committee for Development of Clinical Guidelines)." |
Young 2001.
| Methods | Randomised clinical trial. | |
| Participants | Country: Australia.
Number randomised: 28.
Post‐randomisation drop‐outs: 0 (0%).
Revised sample size: 28.
Average age: 40 years.
Women: 23 (82.1%).
Inclusion criteria
1. ASA status I ‐ II.
2. Age < 50 years.
3. Spoke conversational English. Exclusion criteria: none. |
|
| Interventions | Participants were randomly assigned to two groups. Group 1: day‐case laparoscopic cholecystectomy (n = 14). Group 2: overnight stay laparoscopic cholecystectomy (n = 14). Day‐case participants were discharged within 8 hours. Overnight stay participants were discharged within 23 hours. | |
| Outcomes | The outcomes reported were morbidity, failed discharge, and pain scores. | |
| Notes | Authors replied to some questions in February 2007. We attempted to contact the authors again in September 2012. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer generated numbers sequence" (author replies). |
| Allocation concealment (selection bias) | Low risk | Quote: "Patient allocation ID were placed in an envelope and put inside the Laparoscopic cholecystectomy preadmission patients packs. It was then opened once patients had consented to participate in the study. The doctor recruiting patients was not part of the research team" (author replies). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: It is not possible to blind the participants for this comparison. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: This information was not available. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "There were no patient drop outs or withdrawals from the study" (author replies). |
| Selective reporting (reporting bias) | High risk | Comment: Mortality was not reported explicitly. |
| Vested interest bias | Unclear risk | Comment: This information was not available. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bews‐Hair 2000 | Comment about an included trial (Hollington 1999). |
| Burney 2002 | Not a randomised clinical trial. We were unable to determine the complications specific to discharge of patient on the same day as the surgery. |
| Curet 2002 | The allocation sequence was generated by hospital number. So, this is a quasi‐randomised trial. We were unable to determine the complications specific to discharge of patient on the same day as the surgery. |
| Parvaiz 2006 | Comment about an included study (Johansson 2006). |
| Rosen 2001 | Not a randomised clinical trial. We were unable to determine the complications specific to discharge of patient on the same day as the surgery. |
| Selas 2004 | Editorial with reference to a retrospective study. |
| Sharma 2004 | Not a randomised clinical trial. We were unable to determine the complications specific to discharge of patient on the same day as the surgery. |
Differences between protocol and review
New outcomes were added based on the outcomes reported in the trials.
Difference between previous versions and this version
One new randomised trial has been added (Barthelsson 2008).
The outcomes have been revised and ordered according to their clinical significance.
The methods of assessment of risk of bias have been updated in line with the updated Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011) and the Cochrane Hepato‐Biliary Group Module (CHBG module).
Trial sequential analysis was conducted.
Contributions of authors
JV is the lead author for this version of the review. She identified the trials for inclusion, extracted the data, and wrote the review. KG independently identified trials for inclusion and extracted the data from all the trials. BRD critically commented on the review and suggested improvements.
Sources of support
Internal sources
None, Not specified.
External sources
None, Not specified.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barthelsson 2008 {published data only}
- Barthelsson C, Anderberg B, Ramel S, Bjorvell C, Giesecke K, Nordstrom G. Outpatient versus inpatient laparoscopic cholecystectomy: A prospective randomized study of symptom occurrence, symptom distress and general state of health during the first post‐operative week. Journal of Evaluation in Clinical Practice 2008;14(4):577‐84. [DOI] [PubMed] [Google Scholar]
Dirksen 2001 {published data only}
- Dirksen CD, Schmitz RF, Hans KM, Nieman FHM, Hoogenboom LJ, Go PMNYH. Laparoscopic cholecystectomy in an ambulatory treatment is just as effective as an overnight stay and from a social perspective is cheaper; a randomised study. Nederlands Tijdschrift voor Geneeskunde 2001;145(50):2434‐9. [PubMed] [Google Scholar]
Hollington 1999 {published data only}
- Hollington P, Toogood GJ, Padbury RT. A prospective randomized trial of day‐stay only versus overnight‐stay laparoscopic cholecystectomy. Australia and New Zealand Journal of Surgery 1999;69(12):841‐3. [DOI] [PubMed] [Google Scholar]
Johansson 2006 {published and unpublished data}
- Johansson M, Thune A, Nelvin L, Lundell L. Randomized clinical trial of day‐care versus overnight‐stay laparoscopic cholecystectomy. British Journal of Surgery 2006;93(1):40‐5. [DOI] [PubMed] [Google Scholar]
- Thune A, Nelvin L, Johansson MG, Lundell L. Randomized clinical trial of day‐care versus overnight stay laparoscopic cholecystectomy. Gastroenterology 2005;128(4):A785. [DOI] [PubMed] [Google Scholar]
Keulemans 1998 {published data only}
- Keulemans Y, Eshuis J, Haes H, Wit LT, Gouma DJ. Laparoscopic cholecystectomy: day‐care versus clinical observation. Annals of Surgery 1998;228(6):734‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keulemans YCA, Eshuis JH, Haes J, Leeuwenberg A, Wit TL, Gouma DJ. Day care or hospital admission after laparoscopic cholecystectomy, a prospective randomized trial. Gastroenterology 1998;114(4):A1271. [Google Scholar]
- Keulemans YCA, Eshuis JH, Haes JCJM. Laparoscopic cholecystectomy: admittance or day care? A randomised study. Nederlands Tijdschrift voor Geneeskunde 1998;142(14):822. [Google Scholar]
- Keulemans YCA, Eshuis JH, Haes JCJM, Wit L, Gouma DJ. Laparoscopic cholecystectomy in day care as effective as during hospitalization, and cheaper; a randomized trial. Nederlands Tijdschrift voor Geneeskunde 1999;143(12):621‐6. [Google Scholar]
- Keulemans YCA, Eshuis JH, Haes JCJM, Leeuwenberg A, Wit L, Gouma DJ. Day care or hospital admission after laparoscopic cholecystectomy, a prospective randomized trial [abstract]. European Journal of Gastroenterology & Hepatology 1998;10(Suppl 12):A25. [Google Scholar]
Young 2001 {published and unpublished data}
- Young J, O'Connell B. Recovery following laparoscopic cholecystectomy in either a 23 hour or an 8 hour facility. Journal of Quality in Clinical Practice 2001;21(1‐2):2‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bews‐Hair 2000 {published data only}
- Bews‐Hair M, Coulter G, Frizelle FA. A prospective randomized trial of day‐stay only versus overnight‐stay laparoscopic cholecystectomy: comment [1]. Australian and New Zealand Journal of Surgery 2000;70(10):743. [DOI] [PubMed] [Google Scholar]
Burney 2002 {published data only}
- Burney RE, Jones KR. Ambulatory and admitted laparoscopic cholecystectomy patients have comparable outcomes but different functional health status. Surgical Endoscopy 2002;16(6):921‐6. [DOI] [PubMed] [Google Scholar]
Curet 2002 {published and unpublished data}
- Curet MJ, Contreras M, Weber DM, Albrecht R. Laparoscopic cholecystectomy ‐ outpatient vs inpatient management. Surgical Endoscopy 2002;16(3):453‐7. [DOI] [PubMed] [Google Scholar]
Parvaiz 2006 {published data only}
- Parvaiz MA, Hafeez R. Randomized clinical trial of day‐care versus overnight‐stay laparoscopic cholecystectomy (Br J Surg 2006; 93: 40‐5). British Journal of Surgery 2006;93(5):639‐40. [DOI] [PubMed] [Google Scholar]
Rosen 2001 {published data only}
- Rosen MJ, Malm JA, Tarnoff M, Zuccala K, Ponsky JL. Cost‐effectiveness of ambulatory laparoscopic cholecystectomy. Surgical Laparoscopy, Endoscopy and Percutaneous Techniques 2001;11(3):182‐4. [PubMed] [Google Scholar]
Selas 2004 {published data only}
- Selas PR, Santiuste AC. Laparoscopic cholecystectomy and outpatient surgery. Revista Espanola de Enfermedades Digestivas 2004;96(7):435‐8. [DOI] [PubMed] [Google Scholar]
Sharma 2004 {published data only}
- Sharma A, Hayden JD, Reese RA, Sedman PC, Royston CMS, O’Boyle CJ. Prospective comparison of ambulatory with inpatient laparoscopic cholecystectomy: Outcome, patient preference and satisfaction. Ambulatory Surgery 2004;11(1‐2):23‐6. [Google Scholar]
Additional references
AAGBI 2011
- AAGBI. Day case and short stay surgery: 2. Anaesthesia 2011;66(5):417‐34. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
CTU 2011
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. http://ctu.dk/tsa/ 2011 (accessed 5 July 2013).
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7(3):177‐88. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey SG, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical Research Ed) 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Giger 2011
- Giger U, Ouaissi M, Schmitz SF, Krahenbuhl S, Krahenbuhl L. Bile duct injury and use of cholangiography during laparoscopic cholecystectomy. The British Journal of Surgery 2011;98(3):391‐6. [DOI] [PubMed] [Google Scholar]
Gluud 2012
- Gluud C, Nikolova D, Klingenberg SL, Alexakis N, Als‐Nielsen B, Colli A, et al. Cochrane Hepato‐Biliary Group. About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). 2013, Issue 1. Art. No.: LIVER.
GradePro 3.6 [Computer program]
- Brozek JL, Oxman A, Schünemann HJ. Grade Profiler 3.6. Version 3.6. Grade Profiler, 2004‐2007.
Gurusamy 2009
- Gurusamy KS, Gluud C, Nikolova D, Davidson BR. Assessment of risk of bias in randomized clinical trials in surgery. British Journal of Surgery 2009;96(4):342‐9. [DOI] [PubMed] [Google Scholar]
Halldestam 2004
- Halldestam I, Enell EL, Kullman E, Borch K. Development of symptoms and complications in individuals with asymptomatic gallstones. British Journal of Surgery 2004;91(6):734‐8. [DOI] [PubMed] [Google Scholar]
HES 2012
- Hospital Episode Statistics. Main operations. 4 character. http://www.hesonline.nhs.uk/Ease/servlet/ContentServer?siteID=1937&categoryID=215 (accessed 5 July 2013).
Higgins 2002
- Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice1997 CFR & ICH Guidelines. Vol. 1, PA 19063‐2043, USA: Barnett International/PAREXEL, 1997. [Google Scholar]
Jørgensen 1987
- Jørgensen T. Prevalence of gallstones in a Danish population. American Journal of Epidemiology 1987;126(5):912‐21. [DOI] [PubMed] [Google Scholar]
Kjaergard 2001
- Kjaergard LL, Villumsen J, Gluud C. Reported methodologic quality and discrepancies between large and small randomized trials in meta‐analyses. Annals of Internal Medicine 2001;135(11):982‐9. [DOI] [PubMed] [Google Scholar]
Lundh 2012
- Lundh A, Sismondo S, Lexchin J, Busuioc OA, Bero L. Industry sponsorship and research outcome. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.MR000033.pub2] [DOI] [PubMed] [Google Scholar]
Macaskill 2001
- Macaskill P, Walter SD, Irwig L. A comparison of methods to detect publication bias in meta‐analysis. Statistics in Medicine 2001;20(4):641‐54. [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [DOI] [PubMed] [Google Scholar]
Muhrbeck 1995
- Muhrbeck O, Ahlberg J. Prevalence of gallstone disease in a Swedish population. Scandinavian Journal of Gastroenterology 1995;30(11):1125‐8. [DOI] [PubMed] [Google Scholar]
Newell 1992
- Newell DJ. Intention‐to‐treat analysis: implications for quantitative and qualitative research. International Journal of Epidemiology 1992;21(5):837‐41. [DOI] [PubMed] [Google Scholar]
NIH 1992
- NIH. Gallstones and Laparoscopic Cholecystectomy, NIH Consens Statement Online 1992 Sep 14‐16. http://consensus.nih.gov/1992/1992GallstonesLaparoscopy090html.htm 1992 (accessed 28 February 2007); Vol. 10, issue 3:1‐20. [PubMed]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Savović 2012a
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Health Technology Assessment 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
Savović 2012b
- Savović J, Jones HE, Altman DG, Harris RJ, Jüni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomized, controlled trials. Annals of Internal Medicine 2012;157(6):429‐38. [DOI] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Shamiyeh 2004
- Shamiyeh A, Wayand W. Laparoscopic cholecystectomy: early and late complications and their treatment. Langenbecks Archives of Surgery 2004;389(3):164‐71. [DOI] [PubMed] [Google Scholar]
SPIRIT 2013
- Chan AW, Tetzlaff JM, Altman DG, Laupacis A, Gøtzsche PC, Krleža‐Jerić K, et al. SPIRIT 2013 Statement: defining standard protocol items for clinical trials. Annals of Internal Medicine 2013;158:200‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). http://ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 5 July 2013).
Todd 1996
- Todd KH, Funk JP. The minimum clinically important difference in physician‐assigned visual analog pain scores. Academic Emergency Medicine 1996;3(2):142‐6. [PUBMED: 8808375] [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Jüni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ (Clinical Research Ed) 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Gurusamy 2008a
- Gurusamy KS, Junnarkar S, Farouk M, Davidson BR. Day‐case versus overnight stay in laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006798.pub2] [DOI] [PubMed] [Google Scholar]
Gurusamy 2008b
- Gurusamy KS, Junnarkar S, Farouk M, Davidson BR. Day‐case versus overnight stay for laparoscopic cholecystectomy. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD006798.pub3] [DOI] [PubMed] [Google Scholar]
Gurusamy 2008c
- Gurusamy K, Junnarkar S, Farouk M, Davidson BR. Meta‐analysis of randomized controlled trials on the safety and effectiveness of day‐case laparoscopic cholecystectomy. British Journal of Surgery 2008;95(2):161‐8. [DOI] [PubMed] [Google Scholar]


