Abstract
Influenza vaccines that induce greater cross-reactive or heterosubtypic immunity (Het-I) may overcome limitations in vaccine efficacy imposed by the antigenic variability of influenza A viruses. We have compared mucosal versus traditional parenteral administration of inactivated influenza vaccine for the ability to induce Het-I in BALB/c mice and evaluated a modified Escherichia coli heat-labile enterotoxin adjuvant, LT(R192G), for augmentation of Het-I. Mice that received three intranasal (i.n.) immunizations of H3N2 vaccine in the presence of LT(R192G) were completely protected against lethal challenge with a highly pathogenic human H5N1 virus and had nasal and lung viral titers that were at least 2,500-fold lower than those of control mice receiving LT(R192G) alone. In contrast, mice that received three vaccinations of H3N2 vaccine subcutaneously in the presence or absence of LT(R192G) or incomplete Freund's adjuvant were not protected against lethal challenge and had no significant reductions in tissue virus titers observed on day 5 post-H5N1 virus challenge. Mice that were i.n. administered H3N2 vaccine alone, without LT(R192G), displayed partial protection against heterosubtypic challenge. The immune mediators of Het-I were investigated. The functional role of B and CD8+ T cells in Het-I were evaluated by using gene-targeted B-cell (IgH-6−/−)- or β2-microglobulin (β2m−/−)-deficient mice, respectively. β2m−/− but not IgH-6−/− vaccinated mice were protected by Het-I and survived a lethal infection with H5N1, suggesting that B cells, but not CD8+ T cells, were vital for protection of mice against heterosubtypic challenge. Nevertheless, CD8+ T cells contributed to viral clearance in the lungs and brain tissues of heterotypically immune mice. Mucosal but not parenteral vaccination induced subtype cross-reactive lung immunoglobulin G (IgG), IgA, and serum IgG anti-hemagglutinin antibodies, suggesting the presence of a common cross-reactive epitope in the hemagglutinins of H3 and H5. These results suggest a strategy of mucosal vaccination that stimulates cross-protection against multiple influenza virus subtypes, including viruses with pandemic potential.
The introduction of an influenza A virus possessing a novel hemagglutinin (HA) into an immunologically naive human population has the potential to cause the next influenza pandemic. Avian species are the natural host of influenza A viruses of 15 different HA and nine neuraminidase (NA) subtypes. In 1997, an avian influenza A (H5N1) virus emerged in humans in Hong Kong and caused 18 cases of human respiratory disease, six of them fatal. The outbreak resulted from the direct transmission of H5N1 viruses from infected poultry to humans and was the first known occurrence of a wholly avian virus causing respiratory disease and death in humans (4, 7, 8, 27, 32, 52, 57, 58, 72). The severity of the H5N1 infections in apparently healthy individuals aged 13 to 60 years was of particular concern. This event created a new awareness of the potential of avian influenza A viruses to cause a pandemic and renewed interest in developing vaccine strategies capable of inducing more broadly cross-reactive immunity against novel influenza variants.
Protective immunity provided by current, parenterally administered influenza vaccine is largely based on the induction of strain-specific immunoglobulin G (IgG) neutralizing antibodies directed against the HA. The vaccine provides optimal protection against viruses that are antigenically closely matched with those in the vaccine, but it is less effective against antigenic variants within a subtype and provides little, if any, resistance to infection with a different subtype of virus (1). In contrast, immunity induced by influenza virus infection or live intranasal (i.n.) vaccines in mice provides not only protection against the homologous virus but also cross-protection against heterologous strains (2, 17, 28, 34, 46, 51, 60). In humans, natural infection or i.n. vaccination with live-attenuated viruses can also provide protection against heterologous viruses (3, 20).
Infection with an influenza A virus of one subtype can provide partial protection against challenge with an influenza A virus of a different subtype, and this effect is termed heterosubtypic immunity (Het-I) (17, 28, 39, 51, 63). Heterotypically immune animals show decreased viral titers and duration of viral shedding in the respiratory tract 3 to 7 days following virus challenge. Most efforts to induce Het-I in mice have used either live virus infections (17, 28, 41, 51), influenza recombinant viruses (16, 48, 61), or DNA-expressed influenza proteins (15, 67, 68), but the specific immune effector(s) responsible for mediating this cross-protection has not been fully elucidated. The role of T cells in Het-I has been given the most consideration (15–17, 28, 39, 67, 69). While a consistent role for CD4+ T cells has not been identified (15, 17, 28), many studies have provided evidence that CD8+ cytotoxic T lymphocytes (CTL) directed against viral epitopes conserved among influenza A viruses, such as those within the nucleoprotein (NP), contribute to Het-I (18, 70, 71). Influenza virus NP-specific CTL generated through vaccination or introduced by adoptive transfer lead to a more rapid viral clearance and recovery of the host and protection from death (1, 31, 50, 64, 70). However, mice depleted of CD8+ T cells or made devoid of the T-cell subset through the targeted disruption of β2-microglobulin (β2m) were protected against heterosubtypic lethal challenge (16, 17). These results suggest that immune components other than T cells may also mediate effector function(s) in Het-I.
While a role for anti-HA, anti-NA, and anti-matrix protein 2 (M2) antibodies in virus clearance and recovery from infection has been established (1, 22, 38, 73), their role as antiviral antibodies in Het-I is still uncertain. Although several studies have identified antibody responses to infection with broader cross-reactive properties (6, 29, 33, 62), the transfer of serum or mucosal antibody generated by infection with a live virus has generally failed to protect naive recipients from heterosubtypic challenge (17, 62).
Here we test the ability of an inactivated H3N2 vaccine to induce heterosubtypic protection against a highly pathogenic avian H5N1 virus isolated from a fatal human case. This non-mouse-adapted virus replicates most efficiently in the respiratory tract, disseminates to nonrespiratory organs including the brain, induces lymphocytic depletion, and is highly lethal for mice (21, 24, 30, 66). Thus, this vaccine model provides a most stringent test for protective Het-I. In an attempt to induce strong cross-protective immunity, we administered the vaccine i.n. together with a mutant derivative of heat-labile enterotoxin (LT) from Escherichia coli LT(R192G). The genetically altered LT(R192G) protein possesses negligible toxicity, retains adjuvant properties similar to those of the native LT molecule (11, 25, 26), and as a result has been given consideration as a useful mucosal adjuvant in humans (M. L. Oplinger, S. Baqar, A. Trofa, J. D. Clements, P. Gibbs, G. Pazzaglia, A. L. Bourgeois, and D. A. Scott, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. G-10, 1997). We demonstrate that mice immunized i.n. with H3N2/LT(R192G) vaccine were protected against heterosubtypic challenge, whereas mice immunized subcutaneously (s.c.) were not. Subtype cross-reactive anti-HA antibody responses were associated with heterosubtypic protection against lethal infection, whereas CD8+ T cells reduced the level of virus replication in the respiratory tract and brain.
MATERIALS AND METHODS
Mice.
Six- to eight-week-old female BALB/c mice were purchased from Jackson Laboratories (Bar Harbor, Maine). Mouse strains with a targeted disruption of the locus carrying the β2-microglobulin (β2m−/−) gene (BALB/cJ-β2mtm1Unc) or a targeted mutation in the gene (C57BL/6-IgH-6tmlcgn) for immunoglobulin heavy chain 6 (IgH-6−/−) were also obtained from Jackson Laboratories. Female mutant (−/−) mice and their wild-type (wt) (+/+) counterparts were used at 6 to 10 weeks of age. For the IgH-6−/− mice, the lack of CD45R/B220+ B cells and serum antibody was confirmed by fluorescence-activated cell sorter analysis and enzyme-linked immunosorbent assay (ELISA) (see procedures below), respectively.
Virus.
The influenza viruses used in this study were A/Hong Kong/483/97 (H5N1) (HK/483); the reassortant human influenza A virus, X-31 (which possesses the surface glycoprotein genes of A/Aichi/2/68 [H3N2] and the internal protein genes of A/Puerto Rico/8/34); B/Harbin/7/94 (B/Har); and mouse-adapted A/Taiwan/1/86 (H1N1) (A/TW), originally derived by P. Wyde (Baylor College of Medicine, Houston, Texas) and kindly provided by J. Matthews (Aventis Pasteur, Swiftwater, Pa.). Additional influenza A viruses used as antigens for serologic and CTL assays were A/Duck/Singapore/Q/F119-3/97 (H5N3) (dk/Sing) and A/Hong Kong/156/97 (H5N1) (HK/156). Virus stocks were propagated in the allantoic cavity of 10-day-old embryonated hens' eggs at 34°C (X-31, A/TW, B/Har, and dk/Sing) or 37°C (HK/483 and HK156). The allantoic fluids were harvested 24 (HK/483 and HK/156), 48 (A/TW, dk/Sing, and X-31), or 72 h (B/Har) postinoculation. Infectious allantoic fluid was divided into aliquots and stored at −70°C until use. Fifty percent egg infectious dose (EID50) titers were determined by serial titration of viruses in eggs calculated by the method of Reed and Muench (45). HK/483 and X-31 had infectivity titers in eggs of 9.0 and 8.5 log10 EID50/ml, respectively. Fifty percent mouse infectious dose (MID50) titers were determined as previously described (30). MID50 titers were calculated by the method of Reed and Muench and are expressed as mean log10 EID50/ml ± standard error. All experiments using infectious pathogenic avian H5N1 viruses, including work with animals, were conducted using biosafety level 3+ containment procedures (47).
Vaccine preparation.
Viruses used as vaccines or purified proteins on ELISA plates were concentrated from allantoic fluid and purified by equilibrium density centrifugation through a 30 to 60% linear sucrose gradient as previously described (10). The X-31 (H3N2), B/Har, and A/TW (H1N1) inactivated whole-virus vaccines were prepared by treating purified virus at a concentration of 1 mg/ml with 0.025% formalin at 4°C for 3 days. The treatment resulted in the complete loss of infectivity of virus, as determined by titration of vaccine preparations in eggs. The vaccine doses given throughout are expressed as amounts of total protein measured by Bradford assay (Bio-Rad Laboratories, Hercules, Calif.). Evaluation of the HA protein content of purified X-31 and B/Har used in vaccine studies was determined using a high-resolution sodium dodecyl sulfate polyacrylamide gel system as previously described (43). The HA protein was estimated to make up 29.3 and 28.5% of the total protein of purified X-31 and B/Har, respectively.
Immunization of mice.
For immunization with formalin-fixed viruses, groups of mice were lightly anesthetized with CO2 and vaccinated i.n. three times at weekly intervals with 50 μl containing 20 μg of purified X-31 (H3N2), A/TW (H1N1), or B/Har suspended in phosphate-buffered saline (PBS) in the presence or absence of a previously optimized dose (2 μg) of E. coli mutant LT(R192G). The LT mutant R192G used in these studies was genetically engineered and purified as previously described (11). For the parenteral vaccinations, mice received a volume of 0.1 ml containing 20 μg of protein in the presence or absence of 2 μg of LT(R192G) administered s.c. In one experiment, H3N2 antigen was diluted in PBS, emulsified with equal volumes of incomplete Freund's adjuvant (IFA) (Sigma, St. Louis, Mo.), and administered s.c. Control mice received LT(R192G) or IFA only. For the live X-31 infections, CO2-anesthetized mice were inoculated i.n. with 50 μl containing 100 MID50 of virus diluted in PBS.
Viral challenge.
Two weeks after final vaccination, mice were challenged i.n. with 100 MID50 of HK/483 (H5N1) or A/TW in a volume of 50 μl. Following infection, mice were monitored daily for disease signs for 14 days postinfection (p.i.). Individual body weights were recorded for each group on various days p.i. For determination of infectious virus, nose, lung, and brain tissue samples of 4 or 5 mice per group were removed on day 5 p.i. Clarified homogenates were titrated for virus infectivity in eggs from initial dilutions of 1:10 (lung and nose) or 1:2 (brain). The limit of virus detection was 101.2 EID50/ml for lung and nose and 100.8 EID50/ml for brain tissue.
Antibody sample collection.
Two weeks after the final vaccine boost, 4 or 5 mice from each group were anesthetized by intraperitoneal (i.p.) administration of avertin (2,2,2-tribromethanol; Sigma) at 0.15 ml/10 g of body weight; blood samples from the orbital plexus were used to prepare immune sera. Bronchoaveolar (lung) wash samples were obtained from euthanatized animals as previously described (25).
Antibody assays.
Serum and lung washes were tested by ELISA for the presence of antiviral IgG and IgA. All sera were initially diluted 1:10 in receptor-destroying enzyme from Vibrio cholerae (Denka Seiken, Tokyo, Japan) and incubated at 37°C overnight to destroy nonspecific serum inhibitor activity. Immunolon II plates (Dynatech Laboratories, Chantilly, Va.) were coated with 50 hemagglutinating units of purified homologous H3N2 (X-31), heterologous H5N1 (HK/483), or control B/Har virus in PBS and incubated at room temperature overnight. Some ELISA plates were coated with 2 μg of bromelain-cleaved (13), purified H3HA (from X-31) or 2 μg of purified H5HA recombinant (rHA) protein (derived from HK/483; Protein Sciences Corporation, Meriden, Conn.). The bound antibody was detected by the addition of goat anti-mouse IgG or IgA conjugated to horseradish peroxidase (Kirkegaard & Perry, Gaithersburg, Md.). The absorbance was measured at 405 nm 30 min following the addition of 2,2′-azinobis(3-ethylbenzthiazoline sulfonic acid) (Kirkegaard & Perry). Titers are expressed as the highest dilution that yielded an optical density greater than the mean plus two standard deviations of similarly diluted LT(R192G) control sera. Hemagglutination inhibition (HAI) assays were performed in V-bottom 96-well microtiter plates (Corning Costar Co., Cambridge, Mass.) with 0.5% turkey erythocytes by standard methods. Titers of neutralizing antibody were determined essentially as previously described (49) and were determined as the reciprocal of the highest dilution of serum that gave 50% neutralization of 100 50% tissue culture infectious doses. Serum and lung wash collection were performed 2 weeks after the final vaccine boost. Five mice from each vaccine and LT(R192G) control group were analyzed from two independent experiments. A positive control of goat antiserum to A/Term/South Africa/61 (H5N2) gave a titer of 3,200 on H5N1 virus and <100 on H3N2 virus. The H3-immune sera were also analyzed for cross-reactive anti-HA antibodies by Western immunoblotting with the purified rH5HA (Protein Sciences) as previously described (49).
In vivo depletion of functional subpopulations of T cells.
Groups of mucosally vaccinated mice were depleted of their CD4+ and/or CD8+ T-cell population by in vivo treatment of rat monoclonal antibodies (MAb) specific for L3T4 (clone GK1.5; 1 mg per injection) and Lyt 2.2 (clone 2.43; 1 mg per injection; American Type Culture Collection, Manassas, Va.), respectively. Control mice received 1 mg of rat IgG (Sigma) or rat MAb specific for human HLA DR5 (clone SFR3-DR5; ATCC). The mice received i.p. injections of MAb 2 days before live H5N1 virus challenge and 2, 6, and 10 days after challenge to maintain depletion. Two or three individual mice were included in each study to monitor the efficacy of in vivo lymphocyte depletion. Flow cytometry analysis was performed on cells from spleen, lungs, and mediastinal lymph nodes of mice collected on day 2 postchallenge (p.c.) as previously described (66). Briefly, 1 ml of cell suspensions containing 106 cells were incubated on ice for 40 min with combinations of fluorescein isothiocyanate (FITC)- and phycoerythrin (PE)-labeled antibodies (PharMingen, San Diego, Calif.). Accordingly, lymphocyte populations were dually stained with either FITC–anti-mouse CD4 (clone RM4-5) and PE–anti-mouse CD8a (53-6.7) or FITC–anti-mouse CD3 (17A2) and PE–anti-mouse CD45R/B220 (RA3-6B2). The cells were then washed, resuspended in 1 ml of 2% paraformaldehyde, and analyzed on a FACScan with CellQUEST software (Becton Dickinson, Mountain View, Calif.). A total of 10,000 events, gated for lymphocytes, were performed in three independent experiments. In vivo treatment with anti-L3T4 MAb resulted in a 94 to 96% reduction of CD4 cells, with no reduction of the CD8 population. Similarly, in vivo treatment with anti-Lyt 2.2 resulted in a 95 to 98% depletion of CD8 cells, with no reduction in the CD4 population.
RESULTS
Mucosal but not parenteral influenza virus vaccination induces Het-I which is augmented by LT(R192G).
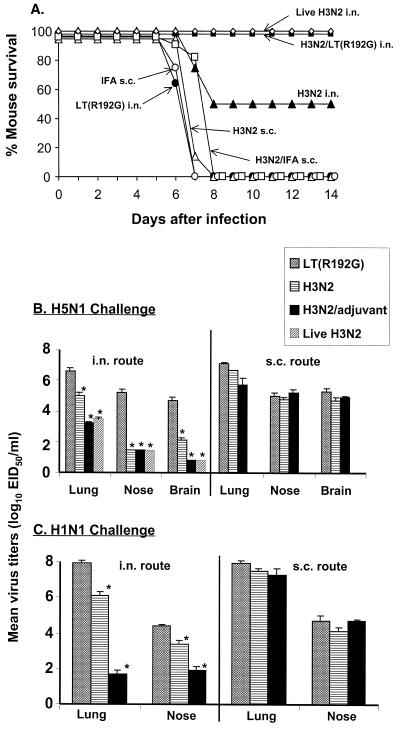
We first compared mucosal (i.n.) vaccination with parenteral (s.c.) vaccination for the ability to induce Het-I. Formalin-inactivated purified whole X-31 (H3N2) vaccine (20 μg) was coadministered i.n. with LT(R192G) (2 μg) three times at weekly intervals. Parenterally vaccinated mice received 20 μg of H3N2 vaccine with or without IFA in an attempt to generate optimal immunity. Two weeks after the final vaccine boost, mice received a lethal heterosubtypic challenge with 100 MID50 of HK/483 (H5N1) virus. For comparison, an additional group of mice were infected i.n. with live H3N2 virus 4 weeks previously and were challenged at the same time as the other groups. The extent of Het-I was measured as (i) survival over a 14-day p.c. period and (ii) virus titers in the upper respiratory tract (nose), lower respiratory tract (lung), and brain tissue of individual mice 5 days p.c. Mucosally vaccinated mice that received live or fixed H3N2/LT(R192G) virus vaccine were completely protected from death, whereas 50% of mice that were i.n. administered vaccine alone, without LT(R192G), survived the lethal H5N1 heterosubtypic challenge (Fig. 1A). In contrast, all animals vaccinated by the s.c. route, whether or not they received adjuvant, succumbed to the lethal H5N1 infection. Mean lung virus titers in mice administered H3N2/LT(R192G) vaccine by the i.n. route were at least 2,500-fold lower than those of control mice receiving LT(R192G) alone and were 63-fold lower than those of mice administered H3N2 vaccine alone (Fig. 1B). Virus was recovered from brain and nose tissue of LT(R192G) control mice on day 5 p.c., but virus was not detected in the tissues of mice vaccinated i.n. with H3N2/LT(R192G). Infectious virus was still detectable in the brain tissue of mice that received H3N2 vaccine alone. Live H3N2 virus immunization resulted in 1,500-fold lower mean lung virus titers than those of control mice receiving LT(R192G) alone. As with H3N2/LT(R192G) i.n. immunization, the live-virus-immunized group had undetectable virus in brain and nose tissue on day 5 p.c. Heterosubtypic protection of H3N2-immune mice was also observed when a non-lethal H1N1 (TW/86) virus was used as the challenge virus. These mice displayed significant reductions (106-fold) in lung virus titers compared with LT(R192G) control mice, and these titers were 25,000-fold lower than those of mice administered H3N2 vaccine alone. In contrast to mice that received mucosal vaccination, mice that received H3N2 vaccine by the s.c. route, either with or without IFA, showed only minimal reduction in lung virus titers and no reduction of nose and brain titers (Fig. 1B and C). These results suggest that mucosal influenza immunization can provide greater Het-I than parenteral influenza vaccination and that the adjuvant LT(R192G) augments Het-I induced by mucosal vaccine.
FIG. 1.
Mucosal but not parenteral influenza virus vaccination induces Het-I that is augmented by adjuvant. Groups of BALB/c mice received three i.n. or s.c. inoculations at weekly intervals of 20 μg of formalin-fixed H3N2 virus in the presence or absence of the indicated adjuvant. One additional group was vaccinated i.n. with live H3N2 virus. Control mice received adjuvant only. Four weeks after live virus vaccine boost and 2 weeks after the final fixed vaccine boost, mice were challenged i.n. with lethal H5N1 (A/Hong Kong/483/97) (A and B) or nonlethal H1N1 virus (A/Taiwan/1/86) (C) and monitored for survival (A) or euthanatized 5 days later for collection of lung, nose, and brain tissue. Individual tissues were homogenized in 1 ml of PBS and titrated for virus infectivity in eggs. Virus endpoint titers are expressed as mean log10 EID50/ml (B and C). An asterisk indicates the H3N2/LT(R192G)-vaccinated group was significantly (P < 0.05) different from the adjuvant-only control group by analysis of variance.
Mucosal vaccination with influenza B virus vaccine fails to protect against lethal influenza A virus challenge.
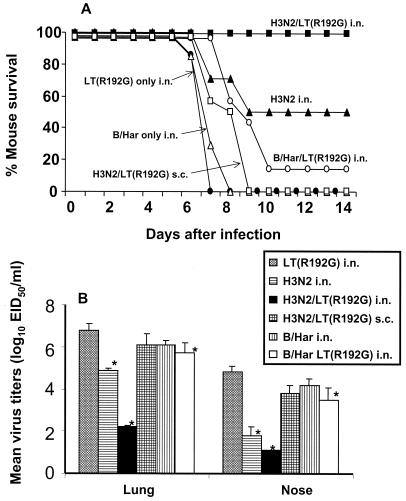
To examine whether Het-I induced by mucosal vaccination was influenza A virus specific, we next determined whether mice immunized i.n. with an influenza B virus (B/Har) vaccine in the presence or absence of LT(R192G) were protected from lethal influenza H5N1 virus challenge. Two weeks after the third weekly vaccination, mice were challenged with H5N1 virus and monitored daily for weight loss and survival. Mucosal administration of H3N2/LT(R192G) vaccine again resulted in 100% survival (Fig. 2A) compared with that of mice that received vaccine s.c. or adjuvant alone. Mice administered B/Har vaccine in the presence or absence of LT(R192G) succumbed to infection, indicating that protection from death was specific for influenza A virus. Interestingly, the onset of death of B/Har/LT(R192G)-vaccinated animals was delayed by 2 days, and a modest but significant (P = 0.04) reduction of virus titers in the lung and nose tissue was detected on day 5 p.c. compared with that of LT(R192G)-vaccinated mice (Fig. 2B). In addition, no weight loss was observed in the B/Har/LT(R192G)-vaccinated group the first 6 days p.c., whereas the LT(R192G) control group had significant weight loss from day 3 until the death of these mice on days 6 through 8 (data not shown). These results suggest that an influenza A virus-specific immune effector(s) is needed for complete heterosubtypic protection against the lethal H5N1 virus challenge but that nonspecific mechanisms may contribute to minimal reduction of virus titers and morbidity.
FIG. 2.
Mucosal vaccination with influenza B virus vaccine fails to protect against lethal influenza A virus challenge. Groups of BALB/c mice received three i.n. inoculations of 20 μg of formalin-fixed H3N2 or B/Harbin/7/94 virus in the presence or absence of 2 μg of LT(R192G). An additional group of mice received three s.c. inoculations of 20 μg of fixed H3N2 virus together with 2 μg of LT(R192G). Two weeks after the final vaccine boost, mice were challenged i.n. with a lethal dose of H5N1 virus and monitored for survival (A) or euthanatized 5 days later for collection of lung and nose tissue (B). An asterisk indicates the vaccinated group was significantly (P < 0.05) different from the adjuvant-only control group by analysis of variance.
CD8+ and/or CD4+ T cells are not required for survival, but CD8+ T cells do contribute to virus clearance in Het-I.
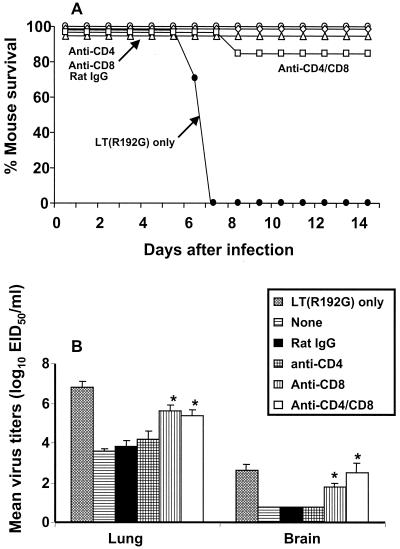
Other studies have demonstrated that CD8+ CTL recognizing determinants that are conserved among influenza A virus subtypes may contribute to Het-I (18, 31, 69). To assess the role of CD8+ and/or CD4+ T cells in Het-I induced by mucosal vaccination, T-cell subsets were depleted from H3N2/LT(R192G)-immune mice by administration of MAb 2 days before H5N1 virus challenge. Depletion of CD8+ T cells had no effect on survival (Fig. 3A) but resulted in 100-fold higher lung virus titers and 10-fold higher brain virus titers on day 5 p.c. than those of the rat IgG-treated control mice (Fig. 3B) (P < 0.01). Depletion of CD4+ T cells had no significant effect on survival or level of virus titers in the lung and brain tissue. The difference between the CD8+-depleted and dually (CD8+ and CD4+) depleted mice was not significant, and the majority of mice depleted of both T-cell subsets survived heterosubtypic challenge. As a second approach, β2m−/− mice deficient in CD8+ T cells and their wt counterparts received the H3N2/LT(R192G) vaccine i.n. Two weeks after the third vaccination, mice received a lethal challenge of H5N1 virus and were monitored for survival and weight loss. The β2m−/− mice vaccinated i.n. with H3N2/LT(R192G) survived H5N1 virus challenge and displayed transient weight loss similar to that of vaccinated wt controls. In contrast, unvaccinated wt and β2m−/− mice failed to survive virus challenge (data not shown). Taken together, these results indicate that an immune effector(s) other than T cells is required for survival following heterosubtypic challenge.
FIG. 3.
Effect of T-cell depletion on survival of H3N2-immune mice. Groups of mice were vaccinated with H3N2 in the presence of LT(R192G) as described in Materials and Methods. The adjuvant control mice received LT(R192G) only (●). Mice received 1 mg of the anti-CD4 (○), anti-CD8 (◊), a combination of both MAbs (anti-CD4/CD8) (□), or rat IgG (▵) control antibody on days −2, +2, +6, and +10 relative to the time of challenge. Mice were challenged i.n. with a lethal dose of H5N1 and were monitored for survival (A) or euthanatized for collection of tissues on day 5 (B). Individual lung and brain tissues were titrated for virus infectivity as described in the legend to Fig. 1. An asterisk indicates the T cell-depleted group was significantly (P < 0.05) different from the rat IgG control group by analysis of variance.
Heterosubtypic protection against a lethal influenza virus infection is primarily mediated by B cells.
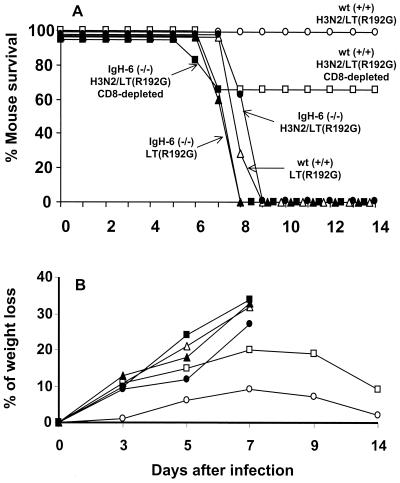
We used B-cell-deficient (IgH-6−/−) mice defective in antibody production to assess the role of B cells and antibody in Het-I. Two groups of eight IgH-6−/− mice were vaccinated three times i.n. with inactivated H3N2/LT(R192G) vaccine as described above. The contribution of CD8+ T cells in heterosubtypic protection of IgH-6−/− mice was also examined by administering anti-CD8 MAb 2 days before H5N1 challenge. A similar number of age-matched C57BL/6 wt control mice were also included. As shown in Fig. 4, lethal virus challenge of IgH-6−/− H3N2/LT(R192G)-vaccinated mice resulted in a progressive loss of body weight from day 3 p.c. and failure to survive virus challenge. The depletion of CD8+ T cells in B cell-deficient mice slightly accelerated the onset of death and resulted in increased weight loss and mortality in wt H3N2/LT(R192G)-immune mice, consistent with the ability of these cells to control virus levels. However, without B cells the CD8+ T-cell response appears incapable of providing protection against lethal heterosubtypic challenge.
FIG. 4.
Susceptibility of B-cell-deficient mice to heterosubtypic challenge. Four groups (eight per group) of mice were vaccinated i.n. three times with H3N2/LT(R192G) vaccine at weekly intervals. The immunized groups of IgH-6−/− (■) and wt (□) control mice were treated with 1 mg of anti-CD8 (clone 2.43) ascites on days −2, +2, and +6 relative to the time of virus challenge. In addition, immunized groups of IgH-6−/− (●) and wt (○) mice received control ascites (clone SFR3-DR5) in place of anti-CD8 MAb. Control wt (▵) and IgH-6−/− (▴) mice received LT(R192G) adjuvant only. Two weeks after the final vaccine boost, mice received a lethal heterosubtypic challenge with 100 MID50 of H5N1 virus. Blood samples were collected from the orbital plexus on day 3 p.c. from all mice and were tested for the presence of serum IgG and IgA antiviral titers by ELISA and HAI as described in Materials and Methods. No IgG, IgA, or HAI antibodies could be detected in IgH-6−/− H3N2-immunized mice, whereas wt-immunized mice had mean HAI titers of 2,560 and IgA or IgG serum titers of ≥64,000 to X-31 virus. In addition, two mice from each group were euthanatized on day 3 p.c. to confirm depletion of CD8+ T cells and/or CD45R/B220+ B cells by flow cytometry. Cells from the spleen were analyzed for CD3+, CD4+, CD8+, and CD45R/B220+ B cells as described in Materials and Methods. Anti-CD8 treatment resulted in more than 95% reduction of the T-cell subpopulation in IgH-6−/− (■) and wt (□) mice. Analysis of CD45R/B220+ B cells in the wt mice revealed a normal range (55 to 65%) of spleen cells in comparison to 1.9 to 2.2% detected in IgH-6−/− mice.
Induction of cross-reactive antibodies by mucosal vaccination with H3N2/LT(R192G).
To further assess the contribution of antibody in Het-I, lung wash and serum samples obtained from mucosal and parenterally vaccinated mice 2 weeks after the third vaccination were initially tested for the presence of neutralizing antibodies. The virus neutralization (v.n.) antibody response to H3N2 (X-31) and H5N1 (HK/483) viruses were measured in individual serum and lung wash samples from 4 or 5 mice per group. The H3N2/LT vaccine administered either i.n. or s.c. induced mean serum neutralizing antibody titers against H3N2 virus of ≥2,100, whereas only vaccine administered i.n. induced neutralizing antibody in the lung wash samples (titer was 160). Neutralizing antibody against H5N1 virus was not detected in any group of H3N2-vaccinated mice. We next performed ELISAs to investigate the presence of H5N1 cross-reactive antibodies in mice administered H3N2 vaccine by the mucosal or parenteral route. Antiviral IgG and IgA antibodies in the sera and lung washes of i.n.- and s.c.-vaccinated mice were detected by using ELISA plates coated with purified whole H3N2 (X-31) or heterologous H5N1 (HK/483) virus. This protocol detected antibodies directed against internal NP or M1 proteins, as well as antibodies directed against the surface glycoproteins. As shown in Table 1, i.n. immunization with H3N2 vaccine resulted in a substantial induction of IgG and IgA antibody responses to homologous virus, and LT(R192G) enhanced both isotypes by fourfold. The H3N2/LT(R192G) vaccine given i.n. induced the highest level of IgA among all groups, in striking contrast to s.c., which induced little or no detectable IgA in lung washes or sera. The mucosal route of vaccination also induced lung IgG titers that were approximately 16-fold higher than those induced by s.c. vaccination. Serum IgG titers induced by either route of administration were similar. Cross-reactive IgG and IgA antibodies to heterologous whole H5N1 virus were also examined (Table 1, bottom). Mucosal vaccination induced cross-reactive serum IgG and IgA responses that were 4- to 16-fold higher and cross-reactive lung antibodies that were 16- to 256-fold higher than those elicited by parenteral vaccination. To examine the anti-HA antibody response to vaccination, ELISAs were next performed using plates coated with either a bromelain-cleaved purified H3HA (X-31) protein or a baculovirus-expressed H5 rHA derived from HK/483. As shown in Table 2, i.n. and s.c. vaccination induced detectable levels of antibody to the homologous H3HA protein; however, cross-reactive anti-H5HA antibodies were detected only in mice that received H3N2 vaccine by the mucosal route. Similar levels of cross-reactive anti-H5HA antibodies were also detected in samples collected from mice that were mucosally vaccinated with an H1N1-inactivated vaccine in the presence of LT(R192G) (Table 2, bottom). Serum IgG represented the highest titers of cross-reactive anti-H5HA, while lung IgG and IgA antibody titers were considerably lower. Live H3N2 virus vaccine failed to induce detectable anti-H5HA antibody in the lung wash samples, and these mice had considerably lower cross-reactive serum antibody levels than mice that received fixed H3N2/LT(R192G) vaccine. Western immunoblotting was performed as a second serologic assay to detect the presence of cross-reactive serum IgG antibodies to H5HA. The Western blot test confirmed the ELISA results by detecting cross-reactive anti-H5HA antibodies only in mice that received H3N2 vaccine by the mucosal route (data not shown). Taken together, these results demonstrated that mucosal vaccination with fixed virus and adjuvant induced a population of v.n.-negative, cross-reactive anti-HA antibodies that were not detectable following parenteral vaccination.
TABLE 1.
Antiviral antibody responses to whole homologous H3N2 or heterologous H5N1 viruses after vaccination with H3N2 virus
| Coating antigen | Treatment | Vaccination routea | Antiviral antibody titerb
|
|||
|---|---|---|---|---|---|---|
| Serum IgG | Lung IgG | Serum IgA | Lung IgA | |||
| Whole H3N2 | LT(R192G) | i.n. | <250 | <25 | <250 | <25 |
| H3N2 | i.n. | 1 × 106 | 25,600 | 16,000 | 6,400 | |
| H3N2 and LT(R192G) | i.n. | 4 × 106 | 102,400 | 64,000 | 25,600 | |
| LT(R192G) | s.c. | <250 | <25 | <250 | <25 | |
| H3N2 | s.c. | 1 × 106 | 1,600 | 250 | <25 | |
| H3N2 and LT(R192G) | s.c. | 1 × 106 | 6,400 | 250 | <25 | |
| Whole H5N1 | LT(R192G) | i.n. | <250 | <25 | <250 | <25 |
| H3N2 | i.n. | 64,000 | 1,600 | 1,000 | 1,600 | |
| H3N2 and LT(R192G) | i.n. | 256,000 | 6,400 | 4,000 | 6,400 | |
| LT(R192G) | s.c. | <250 | <25 | <250 | <25 | |
| H3N2 | s.c. | 16,000 | 25 | <250 | <25 | |
| H3N2 and LT(R192G) | s.c. | 64,000 | 400 | 250 | <25 | |
Mice received three inoculations at weekly intervals of 20 μg of formalin-inactivated H3N2 (X-31) virus plus 2 μg of LT(R192G) or 2 μg of LT(R192G) alone. Two weeks after the final vaccine boost, mice from each vaccine and LT(R192G) control group were euthanatized for serum and lung wash collection. Samples pooled from five mice per group were used.
Titers are expressed as the highest dilution of serum having a mean optical density at 405 nm greater than the mean plus two standard deviations of similarly diluted LT(R192G) control serum.
TABLE 2.
Antiviral antibody responses to recombinant H3HA or H5HA antigen after vaccination with H3N2 virus
| Coating antigen | Treatment | Vaccination routea | Antiviral antibody titerb
|
|||
|---|---|---|---|---|---|---|
| Serum IgG | Lung IgG | Serum IgA | Lung IgA | |||
| H3HA | LT(R192G) | i.n. | <250 | <25 | <250 | <25 |
| H3N2 | i.n. | 1 × 106 | 6,400 | 16,000 | 1,600 | |
| H3N2 and LT(R192G) | i.n. | 1 × 106 | 6,400 | 64,000 | 25,600 | |
| LT(R192G) | s.c. | <250 | <25 | <250 | <25 | |
| H3N2 | s.c. | 256,000 | <25 | <250 | <25 | |
| H3N2 and LT(R192G) | s.c. | 1 × 106 | 1,600 | <250 | <25 | |
| H5HA | LT(R192G) | i.n. | <250 | <25 | <250 | <25 |
| H3N2 | i.n. | 16,000 | 100 | 250 | 400 | |
| H3N2 and LT(R192G) | i.n. | 64,000 | 400 | 250 | 400 | |
| H1N1 and LT(R192G) | i.n. | 64,000 | 1,600 | 1,000 | 1,600 | |
| Live H3N2 | i.n. | 1,000 | <25 | <250 | <25 | |
| LT(R192G) | s.c. | <250 | <25 | <250 | <25 | |
| H3N2 | s.c. | <250 | <25 | <250 | <25 | |
| H3N2 and LT(R192G) | s.c. | <250 | <25 | <250 | <25 | |
Mice received three inoculations at weekly intervals of 20 μg of formalin-inactivated H3N2 (X-31) or H1N1 (A/TW) virus plus 2 μg of LT(R192G) or 2 μg of LT(R192G) alone. Two weeks after the final vaccine boost, five mice from each vaccine and LT(R192G) control group were euthanatized for serum and lung wash collection. Samples pooled from five mice per group were used.
ELISA plates were coated with 2 μg of bromelain-cleaved, purified H3HA or 2 μg of purified rH5HA protein. Titers are expressed as the highest dilution of serum having a mean optical density at 405 nm greater than the mean plus two standard deviations of similarly diluted LT(R192G) control serum.
DISCUSSION
The global spread of the next pandemic virus will likely be rapid, allowing little time for the development and production of a traditional strain-specific vaccine. This limitation will be particularly true if the pandemic strain is an avian virus with high pathogenicity for its avian host as well as its human host, as was the case with the Hong Kong H5N1 viruses. Production of vaccines against such viruses may be complicated by the higher levels of biosafety containment required in the initial stages of development. The H5N1 influenza virus outbreak and the recent emergence of H9N2 viruses in Hong Kong (44) have shown us that influenza virus subtypes that were not thought to infect humans are able to cross the species barrier and cause disease. This observation further highlighted the need for development of vaccines that are protective against multiple subtypes of influenza virus. In this study we have used a formalin-fixed whole H3N2 virus vaccine coadministered with LT(R192G) adjuvant to induce cross-protection from an H5N1 virus isolated from a fatal human case in Hong Kong in 1997. This virus was among a group of H5N1 viruses previously characterized to be of high pathogenicity in mice (21, 24, 30, 66) and therefore provided a stringent evaluation of protective efficacy. We compared the traditional parenteral route and a mucosal route of vaccination. Mucosal (i.n.) vaccination was clearly superior to parenteral (s.c.) vaccination for the induction of Het-I, and LT(R192G) delivered with vaccine serves as a potent mucosal adjuvant that enhanced protection. H3N2/LT(R192G)-immune mice were protected against mortality and significant weight loss and had accelerated virus clearance from the brain, nose, and lung tissues following a lethal H5N1 virus challenge. The cross-protective effect of mucosal vaccination was associated with the induction of subtype cross-reactive anti-HA antibodies not detected in mice that received s.c. delivery of vaccine in the presence or absence of adjuvant. We found that nasal vaccination of a fixed-virus vaccine together with LT(R192G) had similar cross-protective efficacy compared with the response in mice that received live H3N2 virus for induction of Het-I. Both methods of priming resulted in complete protection against death and a comparable reduction in viral titers in lung, brain, and nose tissue 5 days after H5N1 virus challenge (Fig. 1). For the vaccine dose and regimen tested in this study, the results also demonstrate a requirement for LT(R192G) adjuvant, since mice that received H3N2 vaccine alone were not completely protected against death.
To understand the immunologic bases of Het-I, we initially tested whether CD4+ and/or CD8+ T cells accounted for the cross-protection. Acute depletion of CD4+ T cells did not significantly reduce the strength of Het-I. These results are consistent with those of previous studies on Het-I induced by infection of mice where depletion of CD4+ T cells had no effect on survival or reductions in lung virus titers (15, 17). However, in the study by Liang et al., depletion of CD4+ T cells led to partial reduction of Het-I in the upper respiratory tract but was without effect in the lower respiratory tract (28). By depleting CD8+ T cells in heterotypically immune mice, the present study demonstrated that this T-cell subset aids in controlling virus levels in the lung and brain tissue but was not vital for the host's survival following lethal virus challenge. These results were confirmed using β2m−/− mice genetically deficient in functional CD8+ T cells, which were protected against lethal heterosubtypic challenge. Using live virus for induction of Het-I, Epstein et al. also observed Het-I in β2m−/− mice or mice depleted of CD8+ T cells (15–17). However, Stevenson et al. found that CD8+ T cells induced by i.n. infection of mice with an H3N2 virus were critical for protection in the brain following intracerebral challenge with A/WSN/33 virus (56). CD8+ T cells may control virus levels through direct lysis of infected cells; however, we could detect only a secondary virus-specific CTL response following restimulation of spleen cells from mice vaccinated with live H3N2 virus and not inactivated H3N2/LT(R192G) vaccine (data not shown). These results suggest that this mucosal vaccination strategy induces CD8+ T cells that control virus replication in vivo by mechanisms other than the direct lysis of virus-infected cells. CD8+ T cells may be mediating their effect indirectly by the secretion of antiviral cytokines such as gamma interferon or tumor necrosis factor alpha (12). Recently, using a knockout mouse model, Nguyen et al. (40) demonstrated that gamma interferon was not required for Het-I induced by i.n. infection of mice with a live virus.
Because we identified only an accessory role for T cells in Het-I, we next evaluated the role of B cells and antibody in Het-I by using B-cell-deficient mice incapable of antibody production. Unlike wt control animals, H3N2-immune, B-cell-deficient mice do not survive heterosubtypic challenge with H5N1 virus. Our observations are consistent with the recent results of Nguyen et al., where Het-I was not observed in B-cell-deficient mice, although these mice could mount cross-reactive CTL responses (41). The protective role of B cells in Het-I may relate to a functional role of B cells, the production of cross-reactive antibodies, or a combination of both. The ability of B cells to secrete cytokines and act as antigen-presenting cells suggests that B cells may be needed for optimal activation of T-cell responses in some models (22), although others have shown that the development of CD4+ and CD8+ T-cell responses to influenza virus infection did not require B cells (14, 23, 36, 37, 65). Our results suggest that the induction of potent Het-I in mucosally vaccinated mice was due to the production of antibodies directed against cross-reactive viral determinants. Characterization of the postvaccination antibody responses identified a difference between antibody responses induced by the mucosal and parenteral routes of vaccination. Although s.c. and i.n. administration of vaccine together with LT(R192G) induced comparable levels of H3-specific IgG and v.n. activity in the serum, only i.n. administration of vaccine induced a substantial local H3-specific IgA antibody response. These results are consistent with those of a previous study, where i.n. administration of H3N1 vaccine induced both mucosal and systemic antibody responses and protected mice from lethal H5N1 virus challenge (59). In this study we also observed that mucosal vaccination with H3N2 vaccine induced antibodies that reacted with H5HA whereas parenteral vaccination did not, suggesting that these subtype cross-reactive antibodies may be responsible for conferring protection from lethal H5N1 virus challenge. The highest levels of cross-reactive antibody were the IgG isotype found in the serum, whereas lower levels of anti-H5HA IgG and IgA were detected in lung wash samples. Although these antibodies failed to neutralize H5N1 virus in vitro, it is possible that the cross-reactive anti-HA antibodies act by additional mechanisms in vivo to neutralize progeny virus and/or enhance clearance of virus-infected cells (22). While the overall mammalian antibody response to HA is primarily directed against the globular head region (1), antibodies to the conserved stem region are produced in small amounts (6, 42, 54), and passive immunization with a MAb directed to a conformational epitope in the middle of the HA stem region protects mice from lethal influenza virus infection (54). Studies are under way to delineate the cross-reactive epitope(s) on HA. Such studies may provide further insight for the development of vaccine strategies against multiple influenza virus subtypes. Antibodies to the conserved viral transmembrane M2 have also been associated with control of influenza virus in mice challenged with heterologous viruses (19, 22, 38, 53, 73). Since the present study used purified whole virus vaccine that would contain very minimal quantities of M2 (73), it is unlikely that induction of anti-M2 antibody contributes significantly to Het-I in this model system. Antibodies to other serologically cross-reactive proteins, such as NP and M1, are induced following influenza A virus infection; however, antibodies to NP and M1 appear not to provide protective immunity (17, 22, 68).
The underlying mechanism responsible for generating cross-reactive anti-HA antibodies in mucossally vaccinated mice is unknown. However, the type of professional antigen-presenting cells and cytokines released during the inductive phase of the immune response may influence the antibody responses. Recently, Moran et al. demonstrated that an inactivated influenza vaccine given parenterally failed to provide Het-I unless mice received interleukin-12 (IL-12) and antibodies to IL-4 which converted Th2 immunity to a Th1 (high IL-12, low IL-4) cytokine response (35). Identification of the nature of the cytokine profile and the isotype antibody response following mucosal vaccination might suggest ways to augment such responses.
An interesting finding was the delayed protection observed in B/Har-vaccinated mice challenged with an immunologically unrelated influenza A (H5N1) virus. The delay in morbidity and mortality and moderate reductions in lung and nose virus titers on day 5 p.c. indicated that innate immunity contributes to the control of the infection. Nonspecific immune mechanisms may be local inflammatory responses accompanying vaccination that results in the recruitment and activation of cells into the respiratory tract. The restimulation of cells such as NK or γδ T cells could result in partial reduction of virus replication and morbidity observed after viral challenge (12). It has been observed that NK and γδ T cell numbers increase in the lung tissue of mice following i.n. infection with influenza virus (12, 61), and γδ T cells proliferate nonspecifically in response to virus-infected cells (5). Nonetheless, in the absence of virus type-specific immune effectors, the influenza B virus-immunized mice rapidly succumb to infection beyond the first week of virus challenge (Fig. 2).
In this study, we have shown that mucosal vaccination with an inactivated whole virus vaccine coadministered with a mucosal adjuvant is as effective as live virus vaccine for the induction of Het-I. It is noteworthy that the H3N2/LT(R192G)-immune mice remained fully protected against H5N1 lethal challenge for at least 32 weeks postvaccination (data not shown). Our studies using BALB/c mice depleted of T cells or mice deficient in B cells or CD8+ T cells revealed that B cells and antibodies are vital to Het-I, whereas CD8+ T cells control virus replication in the respiratory tract. These results are consistent with induction of cross-reactive anti-HA antibodies following a mucosal but not parenteral vaccination. Whether a formalin-fixed influenza virus vaccine coadministered with a mucosal adjuvant would induce Het-I in humans is not known. Steinhoff et al. (55) failed to demonstrate Het-I in young children previously infected with one subtype of human influenza virus and subsequently immunized i.n. with live attenuated virus vaccine of another subtype. Evaluation of the efficacy of Het-I induction in mice previously infected with influenza virus(es) and the use of fewer doses of vaccine will indicate whether this vaccine strategy would be relevant for humans in a pandemic situation or for annual vaccination. Another consideration is that although LT adjuvant itself is immunogenic, the adjuvant effect of LT is not abrogated in the presence of an anti-LT antibody response (9; J. Katz, unpublished data). Recently, a live-attenuated, cold-adapted influenza vaccine administered by nasal spray to children was shown to be highly effective against an H3N2 drift variant that was not well matched with the H3N2 vaccine virus (3). This result suggests that mucosal delivery of vaccines may indeed induce cross-reactive immunity to influenza viruses in humans, at least within a subtype. A vaccine that could induce or boost Het-I in humans could be an important first line of prevention against a novel subtype, allowing time for the development of a pandemic strain-specific vaccine.
ACKNOWLEDGMENTS
We thank Thomas Rowe for assistance with virus neutralization assays and Western blotting. We also thank John O'Connor, Nancy J. Cox, and Suryaprakash Sambhara for critical review of the manuscript.
REFERENCES
- 1.Ada G L, Jones P D. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. doi: 10.1007/978-3-642-71272-2_1. [DOI] [PubMed] [Google Scholar]
- 2.Armerding D, Rossiter H, Ghazzouli I, Liehl E. Evaluation of live and inactivated influenza A virus vaccines in a mouse model. J Infect Dis. 1982;145:320–330. doi: 10.1093/infdis/145.3.320. [DOI] [PubMed] [Google Scholar]
- 3.Belshe R B, Gruber W C, Mendelman P M, Cho I C, Reisinger K, Block S L, Wittles J, Iacuzio D, Piedra P, Treanor J, King J, Kotloff K, Bernstein D I, Hayden F G, Zangwill K, Yan L, Wolff M. Efficacy of vaccination with live attenuated, cold-adapted, trivalent, intranasal influenza virus vaccine against a variant (A/Sydney) not contained in the vaccine. J Pediatr. 2000;136:168–175. doi: 10.1016/s0022-3476(00)70097-7. [DOI] [PubMed] [Google Scholar]
- 4.Bender C, Hall H, Huang J, Klimov A, Cox N, Hay A, Gregory V, Cameron K, Lim W, Subbarao K. Characterization of the surface proteins of influenza A (H5N1) viruses isolated from humans in 1997–1998. Virology. 1999;254:115–123. doi: 10.1006/viro.1998.9529. [DOI] [PubMed] [Google Scholar]
- 5.Bukowski J F, Morita C T, Brenner M B. Recognition and destruction of virus-infected cells by human gamma delta CTL. J Immunol. 1994;153:5133–5140. [PubMed] [Google Scholar]
- 6.Burlington D B, Wright P F, van Wyke K L, Phelan M A, Mayner R E, Murphy B R. Development of subtype-specific and heterosubtypic antibodies to the influenza A virus hemagglutinin after primary infection in children. J Clin Microbiol. 1985;21:847–849. doi: 10.1128/jcm.21.5.847-849.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. Isolation of avian influenza A (H5N1) viruses from humans-Hong Kong, May-December. Morb Mortal Wkly Rep. 1997;46:1204–1207. [PubMed] [Google Scholar]
- 8.Claas E C J, Osterhaus A D M E, Van Beek R, De Jong J C, Rimmelzwaan G F, Senne D A, Krauss S, Shortridge K F, Webster R G. Human influenza A H5N1 virus related to a highly pathogenic avian influenza virus. Lancet. 1998;351:472–477. doi: 10.1016/S0140-6736(97)11212-0. [DOI] [PubMed] [Google Scholar]
- 9.Clements J D. Adjuvant activity of Escherichia coli heat-labile enterotoxin and effect on the induction of oral tolerance in mice to unrelated protein antigens. Vaccine. 1988;6:269–277. doi: 10.1016/0264-410x(88)90223-x. [DOI] [PubMed] [Google Scholar]
- 10.Cox N J, Kendal A P. Genetic stability of A/Ann Arbor/6/60 cold-mutant (temperature-sensitive) live influenza virus genes: analysis by oligonucleotide mapping of recombinant vaccine strains before and after replication in volunteers. J Infect Dis. 1984;149:194–200. doi: 10.1093/infdis/149.2.194. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson B L, Clements J D. Dissociation of Escherichia coli heat-labile enterotoxin adjuvanticity from ADP-ribosyltransferase activity. Infect Immun. 1995;63:1617–1623. doi: 10.1128/iai.63.5.1617-1623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doherty P, Allan C, Eichelberger W M. Roles of αβ and γδ T cell subsets in viral immunity. Annu Rev Immunol. 1992;10:123–151. doi: 10.1146/annurev.iy.10.040192.001011. [DOI] [PubMed] [Google Scholar]
- 13.Doms R W, Helenius A, White J. Membrane fusion activity of the influenza virus hemagglutinin. The low pH-induced conformational change. J Biol Chem. 1985;260:2973–2981. [PubMed] [Google Scholar]
- 14.Epstein M M, DiRosa F, Jankovic D, Sher A, Matzinger P. Successful T cell priming in B cell-deficient mice. J Exp Med. 1995;182:915–922. doi: 10.1084/jem.182.4.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Epstein S L, Stack A, Misplon J A, Lo C-Y, Mostowski H, Bennink J, Subbarao K. Vaccination with DNA encoding internal proteins of influenza virus does not require CD8+ cytotoxic T lymphocytes: either CD4+ or CD8+ T cells can promote survival and recovery after challenge. Int Immun. 2000;12:91–101. doi: 10.1093/intimm/12.1.91. [DOI] [PubMed] [Google Scholar]
- 16.Epstein S L, Misplon J A, Lawson C M, Subbarao K, Connors M, Murphy B R. β2-Microglobulin-deficient mice can be protected against influenza A infection by vaccination with vaccinia-influenza recombinants expressing hemagglutinin and neuraminadase. J Immunol. 1993;150:5484–5493. [PubMed] [Google Scholar]
- 17.Epstein S L, Lo C-Y, Misplon J A, Lawson C M, Hendrickson B A, Max E E, Subbarao K. Mechanisms of heterosubtypic immunity to lethal influenza A virus infection in fully immunocompetent, T cell-depleted, β2-microglobulin-deficient, and J chain-deficient mice. J Immunol. 1997;158:1222–1230. [PubMed] [Google Scholar]
- 18.Flynn K J, Belz G T, Altman J D, Ahmed R, Woodland D L, Doherty P C. Virus-specific CD8+ T cells in primary and secondary influenza pneumonia. Immunity. 1998;8:683–691. doi: 10.1016/s1074-7613(00)80573-7. [DOI] [PubMed] [Google Scholar]
- 19.Frace A M, Klimov A I, Rowe T, Black R A, Katz J M. Modified M2 proteins produce heterotypic immunity against influenza A virus. Vaccine. 1999;17:2237–2244. doi: 10.1016/s0264-410x(99)00005-5. [DOI] [PubMed] [Google Scholar]
- 20.Frank A L, Taber L H, Wells J M. Individuals infected with two subtypes of influenza A virus in the same season. J Infect Dis. 1983;147:120–124. doi: 10.1093/infdis/147.1.120. [DOI] [PubMed] [Google Scholar]
- 21.Gao P, Watanabe S, Ito T, Goto H, Wells K, McGregor M, Cooley A J, Kawaoka Y. Biological heterogeneity, including systemic replication in mice, of H5N1 influenza A virus isolates from humans in Hong Kong. J Virol. 1999;73:3184–3189. doi: 10.1128/jvi.73.4.3184-3189.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerhard W, Mozdanowska K, Furchner M, Washko G, Maiese K. Role of the B-cell response in recovery of mice from primary influenza infection. Immunol Rev. 1997;159:95–103. doi: 10.1111/j.1600-065x.1997.tb01009.x. [DOI] [PubMed] [Google Scholar]
- 23.Graham M B, Braciale T J. Resistance to and recovery from lethal influenza virus infection in B lymphocyte-deficient mice. J Exp Med. 1997;186:2063–2068. doi: 10.1084/jem.186.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz J M, Lu X, Frace M, Morken T, Zaki S R, Tumpey T M. Pathogenesis of and immunity to avian influenza A H5 viruses. Biomed Pharmacother. 2000;54:178–187. doi: 10.1016/S0753-3322(00)89024-1. [DOI] [PubMed] [Google Scholar]
- 25.Katz J M, Lu X, Young S A, Galphin J C. Adjuvant activity of the heat-labile enterotoxin from enterotoxigenic Escherichia coli for oral administration of inactivated influenza virus vaccine. J Infect Dis. 1997;175:352–363. doi: 10.1093/infdis/175.2.352. [DOI] [PubMed] [Google Scholar]
- 26.Katz J M, Lu X, Galphin J C, Clements J D. Heat labile enterotoxin from Escherichia coli as an adjuvant for oral influenza vaccination. In: Brown L E, Hampson A W, Webster R G, editors. Options for the control of influenza III. New York, N.Y: Elsevier Science Publishing Co., Inc.; 1996. pp. 292–297. [Google Scholar]
- 27.Katz J M, Lim W, Bridges C B, Rowe T, Hu-Primmer J, Lu X, Abernathy R A, Clarke M, Conn L, Kwong H, Lee M, Au G, Ho Y Y, Mak K H, Cox N J, Fukuda K. Antibody response in individuals infected with avian influenza A (H5N1) viruses and detection of anti-H5 antibody among household and social contacts. J Infect Dis. 1999;180:1763–1770. doi: 10.1086/315137. [DOI] [PubMed] [Google Scholar]
- 28.Liang S, Mozdzanowska K, Pallandino G, Gerhard W. Heterosubtypic immunity to influenza type A virus in mice. J Immunol. 1994;152:1653–1661. [PubMed] [Google Scholar]
- 29.Liew F Y, Russel S M, Appleyard G, Brand C M, Beale J. Cross-protection in mice infected with influenza A virus by the respiratory route is correlated with local IgA antibody rather than serum antibody or cytotoxic T cell reactivity. Eur J Immunol. 1984;14:350–356. doi: 10.1002/eji.1830140414. [DOI] [PubMed] [Google Scholar]
- 30.Lu X, Tumpey T M, Morken T, Zaki S R, Cox N J, Katz J. A mouse model for the evaluation of pathogenesis and immunity to influenza A (H5N1) viruses isolated from humans. J Virol. 1999;73:5903–5911. doi: 10.1128/jvi.73.7.5903-5911.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lukacher A E, Braciale V L, Braciale T J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984;160:814–819. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matrosovich M, Zhou N, Kawaoka Y, Webster R. The surface glycoproteins of H5 influenza viruses isolated from humans, chickens, and wild aquatic birds have distinguishable properties. J Virol. 1999;73:1146–1155. doi: 10.1128/jvi.73.2.1146-1155.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mazanec M B, Coudret C L, Fletcher D R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J Virol. 1995;69:1339–1343. doi: 10.1128/jvi.69.2.1339-1343.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meitin C A, Bender B S, Small P A., Jr Influenza immunization: intranasal live vaccinia recombinant contrasted with parenteral inactivated vaccine. Vaccine. 1991;9:751–756. doi: 10.1016/0264-410x(91)90292-e. [DOI] [PubMed] [Google Scholar]
- 35.Moran T M, Park H, Fernandez-Sesma A, Schulman J L. Th2 responses to inactivated influenza virus can be converted to Th1 responses and facilitate recovery from heterosubtypic virus infection. J Infect Dis. 1999;180:579–585. doi: 10.1086/314952. [DOI] [PubMed] [Google Scholar]
- 36.Mozdzanowska K, Furchner M, Maiese K, Gerhard W. CD4+ T cells are ineffective in clearing a pulmonary infection with influenza type A virus in the absence of B cells. Virology. 1997;239:217–225. doi: 10.1006/viro.1997.8882. [DOI] [PubMed] [Google Scholar]
- 37.Mozdzanowska K, Maiese K, Gerhard W. Th cell-deficient mice control influenza virus infection more effectively than Th- and B cell-deficient mice: evidence for a Th-independent contribution by B cells to virus clearance. J Immunol. 2000;164:2635–2643. doi: 10.4049/jimmunol.164.5.2635. [DOI] [PubMed] [Google Scholar]
- 38.Neirynck S, Deroo T, Saelens X, Vanlandschoot P, Jou W M, Fiers W. A universal influenza A vaccine based on the extracellular domain of the M2 protein. Nat Med. 1999;5:1157–1163. doi: 10.1038/13484. [DOI] [PubMed] [Google Scholar]
- 39.Nguyen H H, Moldoveanu Z, Novak M J, van Ginkel F W, Ban E, Kiyono H, McGhee J R, Mestecky J. Heterosubtypic immunity to lethal influenza A virus infection is associated with virus-specific CD8+ cytotoxic T lymphocyte responses induced in mucosa-associated tissues. Virology. 1999;254:50–60. doi: 10.1006/viro.1998.9521. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen H H, van Ginkel F W, Vu H L, Novak M J, McGhee J R, Mestecky J. Gamma interferon is not required for mucosal cytotoxic T lymphocyte responses or heterosubtypic immunity to influenza A virus infection in mice. J Virol. 2000;74:5495–5501. doi: 10.1128/jvi.74.12.5495-5501.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nguyen H H, van Ginkel F W, Vu H L, Novak M J, McGhee J R, Mestecky J. Heterosubtypic immunity to influenza A virus infection requires B cells but not CD8+ cytotoxic T lymphocytes. J Infect Dis. 2001;183:368–376. doi: 10.1086/318084. [DOI] [PubMed] [Google Scholar]
- 42.Okuno Y, Isegawa Y, Sasao F, Ueda S. A common neutralizing epitope conserved between the hemagglutinins of influenza A virus H1 and H2 strains. J Virol. 1993;67:2552–2258. doi: 10.1128/jvi.67.5.2552-2558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Oxford J S, Corcoran T, Hugentobler A L. Quantitative analysis of the protein composition of influenza A and B viruses using high resolution SDS polyacrylamide gels. J Biol Stand. 1981;9:483–491. doi: 10.1016/s0092-1157(81)80041-8. [DOI] [PubMed] [Google Scholar]
- 44.Peiris M, Yuen K Y, Leung C W, Chan K H, Ip P L, Lai R W, Orr W K, Shortridge K F. Human infection with influenza H9N2. Lancet. 1999;354:916–917. doi: 10.1016/s0140-6736(99)03311-5. [DOI] [PubMed] [Google Scholar]
- 45.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 46.Riberdy J M, Flynn K J, Stech J, Webster R, Altman J, Doherty P C. Protection against a lethal avian influenza A virus in a mammalian system. J Virol. 1999;73:1453–1459. doi: 10.1128/jvi.73.2.1453-1459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Richmond J Y, McKinney R W, editors. Biosafety in microbiological and biomedical laboratories. 3rd ed. Washington, D.C.: U.S. Department of Health and Human Services, CDC/NIH; 1993. [Google Scholar]
- 48.Rota P A, De B K, Shaw M W, Black R A, Gamble W C, Kendal A P. Comparison of inactivated, live and recombinant DNA vaccines against influenza virus in a mouse model. Virus Res. 1990;16:83–94. doi: 10.1016/0168-1702(90)90045-d. [DOI] [PubMed] [Google Scholar]
- 49.Rowe T, Abernathy R A, Hu-Primmer J, Thompson W W, Lu X, Lim W, Fukuda K, Cox N J, Katz J M. Detection of antibody to avian influenza A (H5N1) virus in human serum by using a combination of serologic assays. J Clin Microbiol. 1999;37:937–943. doi: 10.1128/jcm.37.4.937-943.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sastry K J, Bender B S, Bell W, Small P A, Arlinghaus R B. Effects of influenza virus-specific cytotoxic T-lymphocyte responses induced by a synthetic nucleoprotein peptide on the survival of mice challenged with a lethal dose of virus. Vaccine. 1994;12:1281–1287. doi: 10.1016/s0264-410x(94)80053-3. [DOI] [PubMed] [Google Scholar]
- 51.Schulman J L, Kilbourne E D. Induction of partial specific heterotypic immunity in mice by a single infection with influenza A virus. J Bacteriol. 1965;89:170–177. doi: 10.1128/jb.89.1.170-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shortridge K F, Zhou N N, Guan Y, Gao P, Ito T, Kawaoka Y, Kodihalli S, Krauss S, Markwell D, Murti K G, Norwood M, Senne D, Sims L, Takada A, Webster R G. Characterization of avian H5N1 influenza viruses from poultry in Hong Kong. Virology. 1998;252:331–342. doi: 10.1006/viro.1998.9488. [DOI] [PubMed] [Google Scholar]
- 53.Slepushkin V A, Katz J M, Black R A, Gamble W C, Rota P A, Cox N J. Protection of mice against influenza A virus challenge by vaccination with baculovirus-expressed M2 protein. Vaccine. 1995;13:1399–1402. doi: 10.1016/0264-410x(95)92777-y. [DOI] [PubMed] [Google Scholar]
- 54.Smirnov Y A, Lipatov A S, Gitelman A K, Okuno Y, Osterhaus A D M E, Claas E C J. An epitope shared by the hemagglutinins of H1, H2, H5 and H6 subtypes of influenza A virus. Acta Virol. 1999;43:237–244. [PubMed] [Google Scholar]
- 55.Steinhoff M C, Fries L F, Karron R A, Clements M L, Murphy B R. Effect of heterosubtypic immunity on infection with attenuated influenza A virus vaccines in young children. J Clin Microbiol. 1993;31:836–838. doi: 10.1128/jcm.31.4.836-838.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stevenson P G, Hawke S, Bangham C R M. Protection against lethal influenza virus encephalitis by intranasally primed CD8+ memory T cells. J Immunol. 1996;157:3065–3073. [PubMed] [Google Scholar]
- 57.Suarez D L, Perdue M L, Cox N J, Rowe T, Bender C, Huang J, Swayne D E. Comparisons of highly virulent H5N1 influenza A viruses isolated from humans and chickens from Hong Kong. J Virol. 1998;72:6678–6688. doi: 10.1128/jvi.72.8.6678-6688.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Subbarao K, Klimov A, Katz J, Regnery H, Lim W, Hall H, Perdue M, Swayne D, Bender C, Huang J, Hemphill M, Rowe T, Shaw M, Xu X, Fukuda K, Cox N. Characterization of an avian influenza A (H5N1) virus isolated from a child with a fatal respiratory illness. Science. 1998;279:393–396. doi: 10.1126/science.279.5349.393. [DOI] [PubMed] [Google Scholar]
- 59.Takada A, Kuboki N, Okazaki K, Ninomiya A, Tanaka H, Ozaki H, Itamura S, Nishimura H, Enami M, Tahiro M, Shortridge K F, Kida H. A virulent avian influenza virus as a vaccine strain against a potential human pandemic. J Virol. 1999;73:8303–8307. doi: 10.1128/jvi.73.10.8303-8307.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tamura S-I, Ito Y, Asanuma H, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T. Cross-protection against influenza virus infection afforded by trivalent inactivated vaccines inoculated intranasally with cholera toxin B subunit. J Immunol. 1992;149:981–988. [PubMed] [Google Scholar]
- 61.Tamura S-I, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Acceleration of influenza virus clearance by Th1 cells in the nasal site of mice immunized intranasally with adjuvant combined recombinant nucleoprotein. J Immunol. 1996;156:3892–3900. [PubMed] [Google Scholar]
- 62.Tamura S-I, Miyata K, Matsuo K, Asanuma H, Takahashi H, Nakajima K, Suzuki Y, Aizawa C, Kurata T. Cross-protection against influenza A virus infection by passively transferred respiratory tract IgA antibodies to different hemagglutinin molecules. Eur J Immunol. 1991;21:1337–1344. doi: 10.1002/eji.1830210602. [DOI] [PubMed] [Google Scholar]
- 63.Tamura S-I, Asanuma H, Ito Y, Hirabayashi Y, Suzuki Y, Nagamine T, Aizawa C, Kurata T, Oya A. Superior cross-protective effect of nasal vaccination to subcutaneous inoculation with influenza hemagglutinin vaccine. Eur J Immunol. 1992;22:477–481. doi: 10.1002/eji.1830220228. [DOI] [PubMed] [Google Scholar]
- 64.Taylor P M, Askonas B A. Influenza nucleoprotein-specific cytotoxic T-cell clones are protective in vivo. Immunology. 1986;58:417–420. [PMC free article] [PubMed] [Google Scholar]
- 65.Topham D J, Doherty P C. Clearance of an influenza A virus by CD4+ T cells is inefficient in the absence of B cells. J Virol. 1998;72:882–885. doi: 10.1128/jvi.72.1.882-885.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tumpey T M, Lu X, Morken T, Zaki S R, Katz J M. Depletion of lymphocytes and diminished cytokine production in mice infected with a highly virulent influenza A (H5N1) virus isolated from humans. J Virol. 2000;74:6105–6116. doi: 10.1128/jvi.74.13.6105-6116.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ulmer J B, Fu T-M, Deck R R, Friedman A, Guan L, DeWitt C, Liu X, Wang S, Liu M A, Donnelly J J, Caulfield M J. Protective CD4+ and CD8+ T cells against influenza virus induced by vaccination with nucleoprotein DNA. J Virol. 1998;72:5648–5653. doi: 10.1128/jvi.72.7.5648-5653.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ulmer J B, Donnelly J J, Parker S E, Rhodes G H, Filgner P L, Dwarki V J, Gromkowski S H, Deck R R, DeWitt C M, Friedman A, Hawe L A, Leander K R, Martinez D, Perry H C, Shiver J W, Montgomery D L, Liu M A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993;259:1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- 69.Webster R G, Askonas B A. Cross-protection and cross-reactive cytotoxic T cells induced by influenza virus vaccines in mice. Eur J Immunol. 1980;10:396–401. doi: 10.1002/eji.1830100515. [DOI] [PubMed] [Google Scholar]
- 70.Yap K L, Ada G L. The recovery of mice from influenza A virus infection: adoptive transfer of immunity with influenza virus-specific cytotoxic T lymphocytes recognizing a common virion antigen. Scand J Immunol. 1978;8:413–420. doi: 10.1111/j.1365-3083.1978.tb00536.x. [DOI] [PubMed] [Google Scholar]
- 71.Yewdell J W, Bennink J R, Smith G L, Moss B. Influenza A virus nucleoprotein is a major target antigen for cross-reactive anti-influenza A virus cytotoxic T lymphocytes. Proc Natl Acad Sci USA. 1985;82:1785–1790. doi: 10.1073/pnas.82.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yuen K Y, Chan P K, Peiris M, Tsang D N C, Que T L, Shortridge K F, Cheung P T, To W K, Ho E T F, Sung R, Cheng A F B Members of the H5N1 Study Group. Clinical features and rapid viral diagnosis of human disease associated with avian influenza A H5N1 virus. Lancet. 1998;351:467–471. doi: 10.1016/s0140-6736(98)01182-9. [DOI] [PubMed] [Google Scholar]
- 73.Zebede S L, Lamb R A. Influenza A virus M2 protein: monoclonal antibody restriction of virus growth and detection of M2 in virions. J Virol. 1988;62:2762–2772. doi: 10.1128/jvi.62.8.2762-2772.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]