Abstract
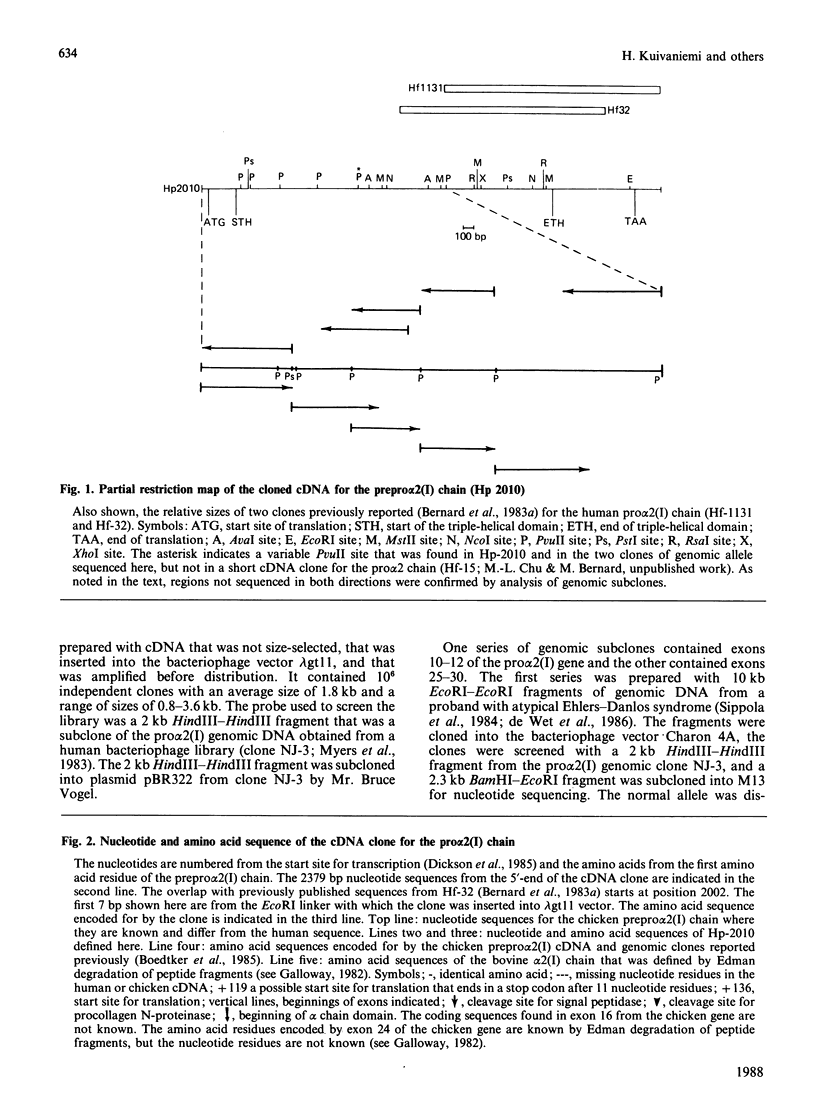
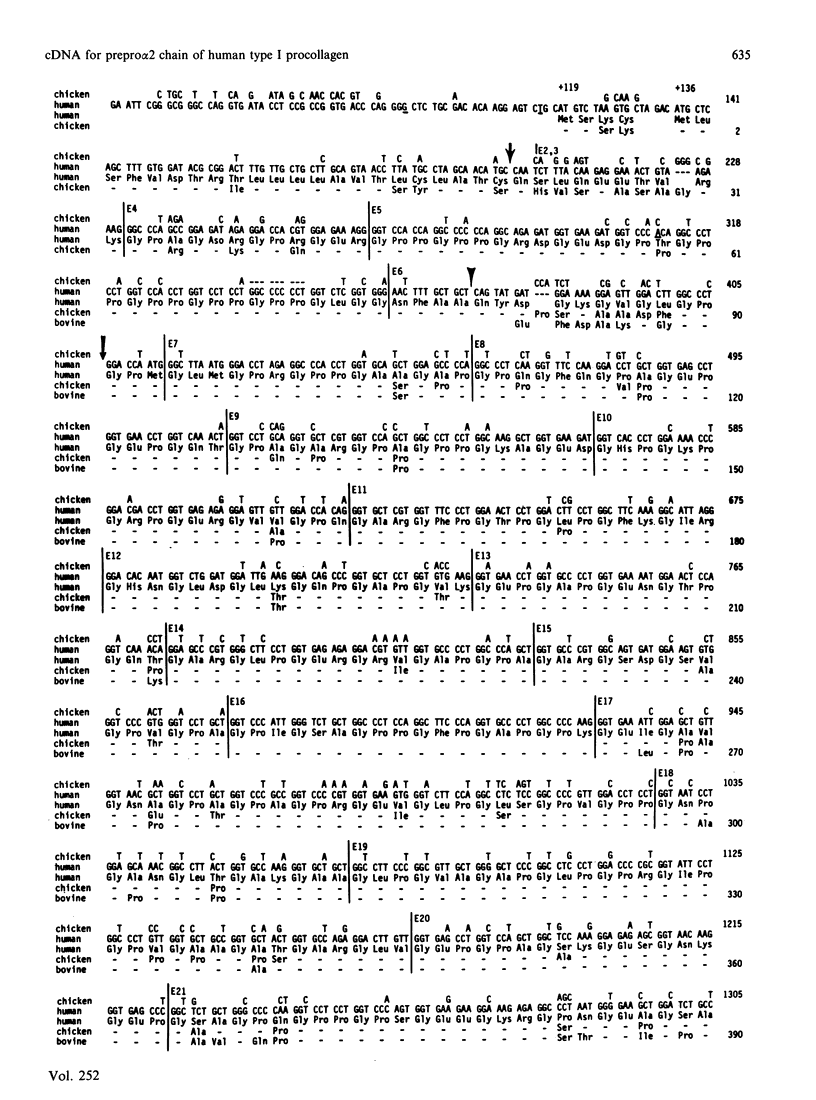
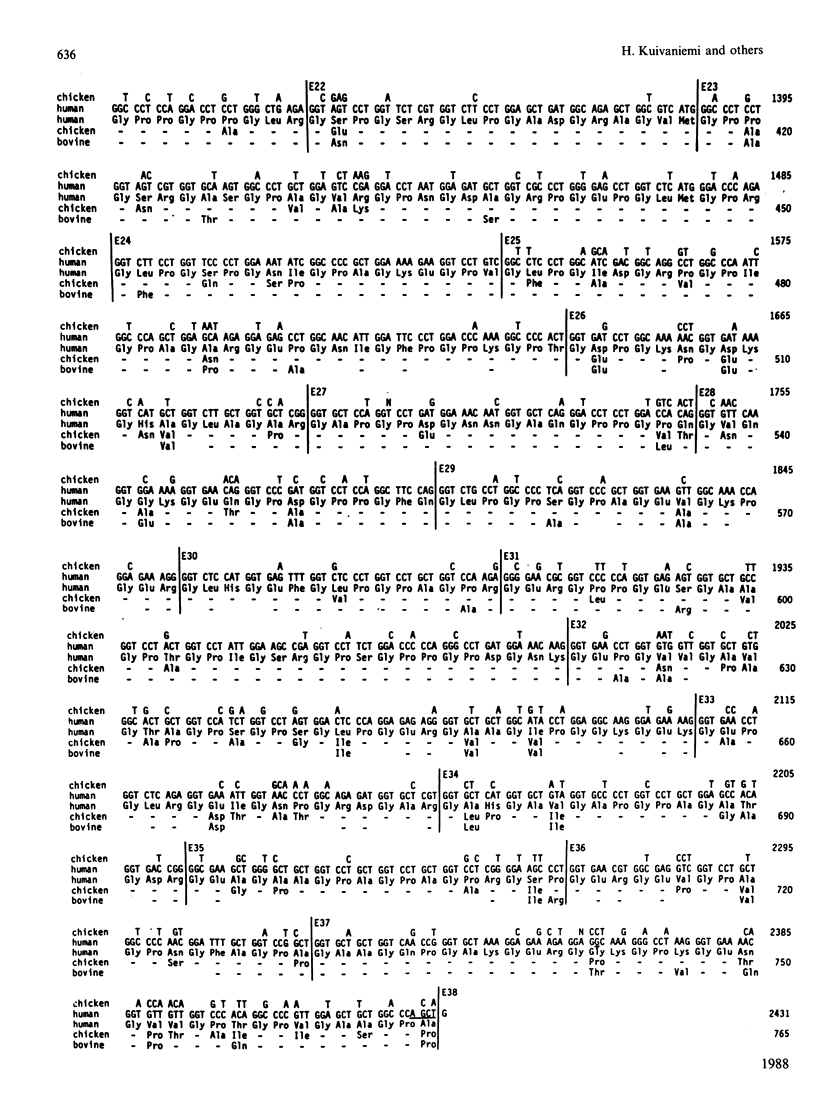
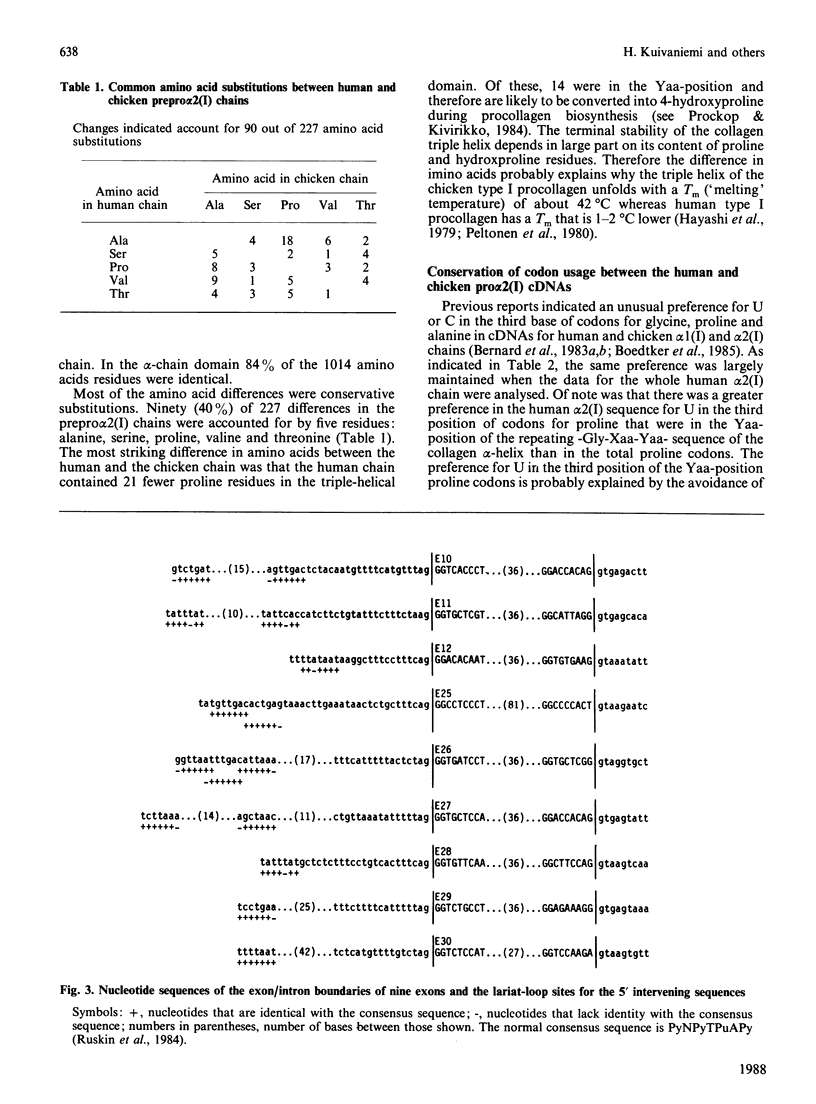
A cDNA clone from a human placental library was found to consist of an essentially full-length cDNA of 4.6 kb for the prepro alpha 2(I) chain of type I procollagen. Nucleotide sequencing of the 5'-end of the cDNA provided a sequence of 1617 nucleotide residues and codons for 539 amino acid residues not previously defined. Comparison of the complete structure of the prepro alpha 2(I) cDNA with previously reported sequences for the chicken pro alpha 2(I) gene indicated that 83% of 1366 total amino acid residues were conserved. In the alpha-chain domain 84% of 1014 amino acid residues were conserved. Also, there was conservation of the previously noted preference for U and C in the third position of codons for glycine, proline and alanine. One major difference between the human and the chicken prepro alpha 2(I) chain was that the human chain contained 21 fewer proline residues, an observation that probably explains why the triple helix of human type I procollagen unfolds at temperatures that are 1-2 degrees C lower. In parallel experiments, sequencing of intron-exon boundaries for nine exons of genomic subclones confirmed and extended previous observations that the pro alpha 2(I) gene, like other genes from fibrillar collagens, has an unusual 54-base pattern of exon sizes that is highly conserved through evolution.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernard M. P., Chu M. L., Myers J. C., Ramirez F., Eikenberry E. F., Prockop D. J. Nucleotide sequences of complementary deoxyribonucleic acids for the pro alpha 1 chain of human type I procollagen. Statistical evaluation of structures that are conserved during evolution. Biochemistry. 1983 Oct 25;22(22):5213–5223. doi: 10.1021/bi00291a023. [DOI] [PubMed] [Google Scholar]
- Bernard M. P., Myers J. C., Chu M. L., Ramirez F., Eikenberry E. F., Prockop D. J. Structure of a cDNA for the pro alpha 2 chain of human type I procollagen. Comparison with chick cDNA for pro alpha 2(I) identifies structurally conserved features of the protein and the gene. Biochemistry. 1983 Mar 1;22(5):1139–1145. doi: 10.1021/bi00274a023. [DOI] [PubMed] [Google Scholar]
- Bird A. P. CpG-rich islands and the function of DNA methylation. Nature. 1986 May 15;321(6067):209–213. doi: 10.1038/321209a0. [DOI] [PubMed] [Google Scholar]
- Boedtker H., Finer M., Aho S. The structure of the chicken alpha 2 collagen gene. Ann N Y Acad Sci. 1985;460:85–116. doi: 10.1111/j.1749-6632.1985.tb51159.x. [DOI] [PubMed] [Google Scholar]
- Brown W. R., Bird A. P. Long-range restriction site mapping of mammalian genomic DNA. 1986 Jul 31-Aug 6Nature. 322(6078):477–481. doi: 10.1038/322477a0. [DOI] [PubMed] [Google Scholar]
- Cheah K. S. Collagen genes and inherited connective tissue disease. Biochem J. 1985 Jul 15;229(2):287–303. doi: 10.1042/bj2290287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson L. A., de Wet W., Di Liberto M., Weil D., Ramirez F. Analysis of the promoter region and the N-propeptide domain of the human pro alpha 2(I) collagen gene. Nucleic Acids Res. 1985 May 24;13(10):3427–3438. doi: 10.1093/nar/13.10.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixit S. N., Seyer J. M., Kang A. H., Gross J. Covalent structure of collagen: amino acid sequence of chick skin collagen alpha1(1)-CB6B. Biochemistry. 1978 Dec 26;17(26):5719–5722. doi: 10.1021/bi00619a018. [DOI] [PubMed] [Google Scholar]
- Fietzek P. P., Wendt P., Kell I., Kühn K. The covalent structure of collagen: amino acid sequence of 1-CB3 from calf skin collagen. FEBS Lett. 1972 Oct 1;26(1):74–76. doi: 10.1016/0014-5793(72)80545-3. [DOI] [PubMed] [Google Scholar]
- Grobler-Rabie A. F., Wallis G., Brebner D. K., Beighton P., Bester A. J., Mathew C. G. Detection of a high frequency RsaI polymorphism in the human pro alpha 2(I) collagen gene which is linked to an autosomal dominant form of osteogenesis imperfecta. EMBO J. 1985 Jul;4(7):1745–1748. doi: 10.1002/j.1460-2075.1985.tb03845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann H., Fietzek P. P., Kühn K. The role of polar and hydrophobic interactions for the molecular packing of type I collagen: a three-dimensional evaluation of the amino acid sequence. J Mol Biol. 1978 Oct 25;125(2):137–165. doi: 10.1016/0022-2836(78)90342-x. [DOI] [PubMed] [Google Scholar]
- Hyashi T., Curran-Patel S., Prockop D. J. Thermal stability of the triple helix of type I procollagen and collagen. Precautions for minimizing ultraviolet damage to proteins during circular dichroism studies. Biochemistry. 1979 Sep 18;18(19):4182–4187. doi: 10.1021/bi00586a022. [DOI] [PubMed] [Google Scholar]
- Kang A. H., Bornstein P., Piez K. A. The amino acid sequence of peptides from the cross-linking region of rat skin collagen. Biochemistry. 1967 Mar;6(3):788–795. doi: 10.1021/bi00855a019. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers J. C., Dickson L. A., de Wet W. J., Bernard M. P., Chu M. L., Di Liberto M., Pepe G., Sangiorgi F. O., Ramirez F. Analysis of the 3' end of the human pro-alpha 2(I) collagen gene. Utilization of multiple polyadenylation sites in cultured fibroblasts. J Biol Chem. 1983 Aug 25;258(16):10128–10135. [PubMed] [Google Scholar]
- Ohkubo H., Vogeli G., Mudryj M., Avvedimento V. E., Sullivan M., Pastan I., de Crombrugghe B. Isolation and characterization of overlapping genomic clones covering the chicken alpha 2 (type I) collagen gene. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7059–7063. doi: 10.1073/pnas.77.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Grabowski P. J., Konarska M. M., Seiler S., Sharp P. A. Splicing of messenger RNA precursors. Annu Rev Biochem. 1986;55:1119–1150. doi: 10.1146/annurev.bi.55.070186.005351. [DOI] [PubMed] [Google Scholar]
- Peltonen L., Palotie A., Hayashi T., Prockop D. J. Thermal stability of type I and type III procollagens from normal human fibroblasts and from a patient with osteogenesis imperfecta. Proc Natl Acad Sci U S A. 1980 Jan;77(1):162–166. doi: 10.1073/pnas.77.1.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prockop D. J., Kivirikko K. I. Heritable diseases of collagen. N Engl J Med. 1984 Aug 9;311(6):376–386. doi: 10.1056/NEJM198408093110606. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sippola M., Kaffe S., Prockop D. J. A heterozygous defect for structurally altered pro-alpha 2 chain of type I procollagen in a mild variant of osteogenesis imperfecta. The altered structure decreases the thermal stability of procollagen and makes it resistant to procollagen N-proteinase. J Biol Chem. 1984 Nov 25;259(22):14094–14100. [PubMed] [Google Scholar]
- Sykes B., Ogilvie D., Wordsworth P., Anderson, Jones N. Osteogenesis imperfecta is linked to both type I collagen structural genes. Lancet. 1986 Jul 12;2(8498):69–72. doi: 10.1016/s0140-6736(86)91609-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsipouras P., Myers J. C., Ramirez F., Prockop D. J. Restriction fragment length polymorphism associated with the pro alpha 2(I) gene of human type I procollagen. Application to a family with an autosomal dominant form of osteogenesis imperfecta. J Clin Invest. 1983 Oct;72(4):1262–1267. doi: 10.1172/JCI111082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogeli G., Ohkubo H., Avvedimento V. E., Sullivan M., Yamada Y., Mudryj M., Pastan I., de Crombrugghe B. A repetitive structure in the chick alpha 2-collagen gene. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):777–783. doi: 10.1101/sqb.1981.045.01.096. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Avvedimento V. E., Mudryj M., Ohkubo H., Vogeli G., Irani M., Pastan I., de Crombrugghe B. The collagen gene: evidence for its evolutinary assembly by amplification of a DNA segment containing an exon of 54 bp. Cell. 1980 Dec;22(3):887–892. doi: 10.1016/0092-8674(80)90565-6. [DOI] [PubMed] [Google Scholar]
- de Wet W. J., Pihlajaniemi T., Myers J., Kelly T. E., Prockop D. J. Synthesis of a shortened pro-alpha 2(I) chain and decreased synthesis of pro-alpha 2(I) chains in a proband with osteogenesis imperfecta. J Biol Chem. 1983 Jun 25;258(12):7721–7728. [PubMed] [Google Scholar]
- de Wett W., Sippola M., Tromp G., Prockop D., Chu M. L., Ramirez F. Use of R-loop mapping for the assessment of human collagen mutations. J Biol Chem. 1986 Mar 15;261(8):3857–3862. [PubMed] [Google Scholar]


