Abstract
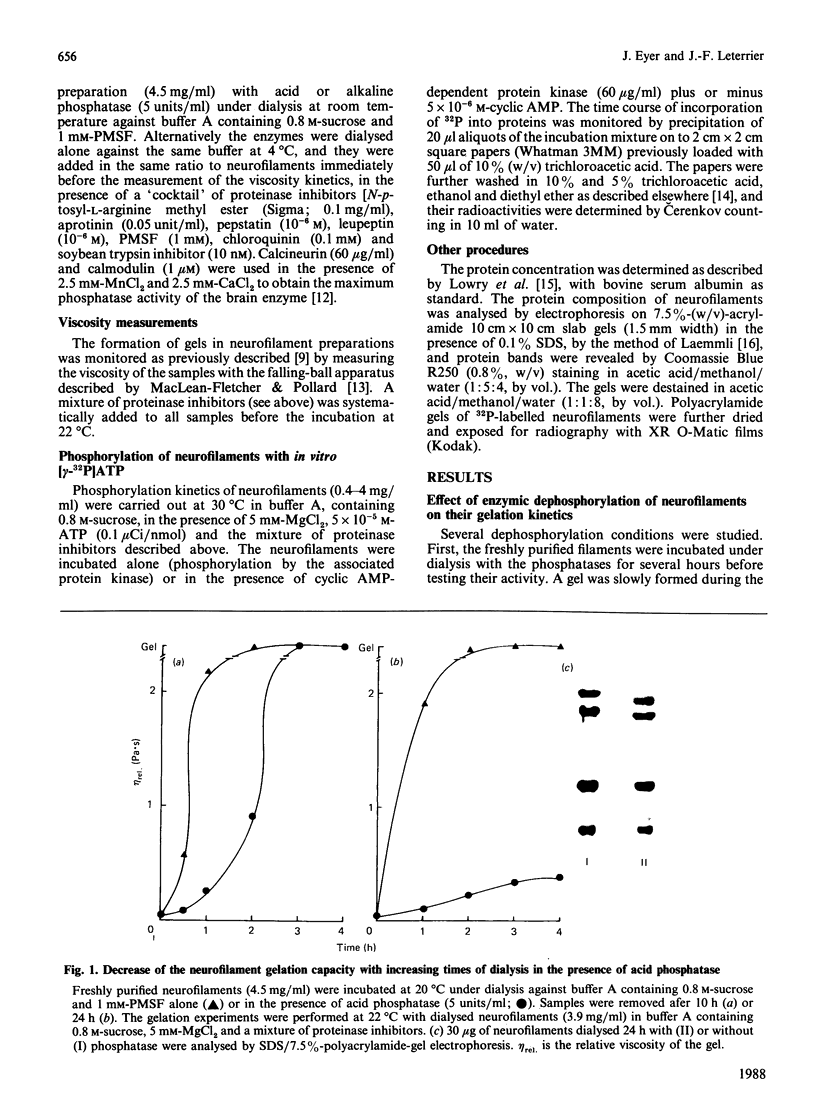
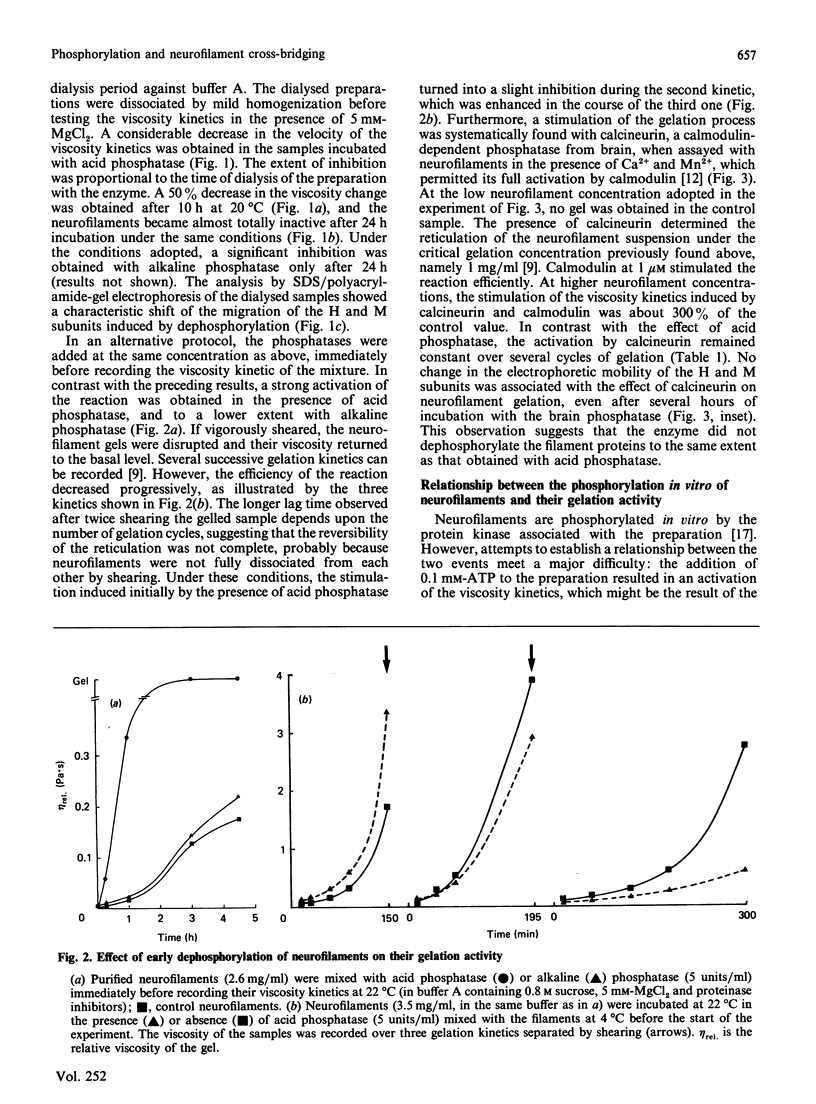
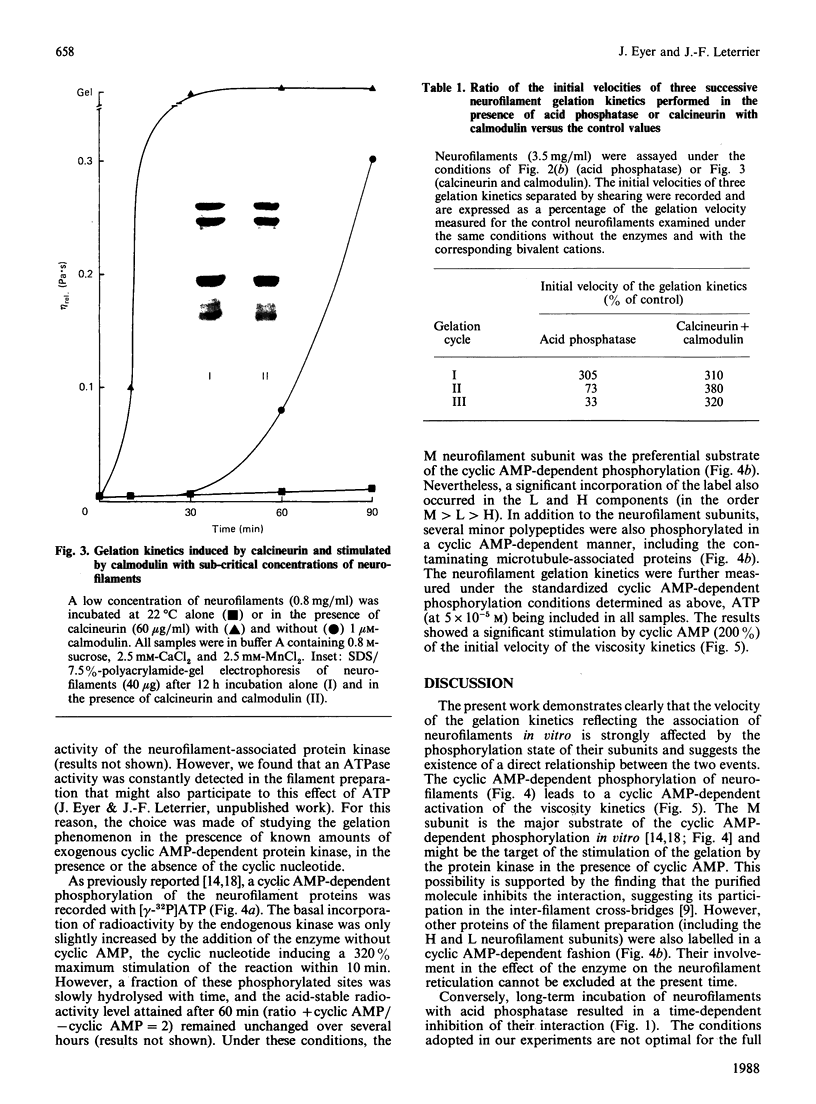
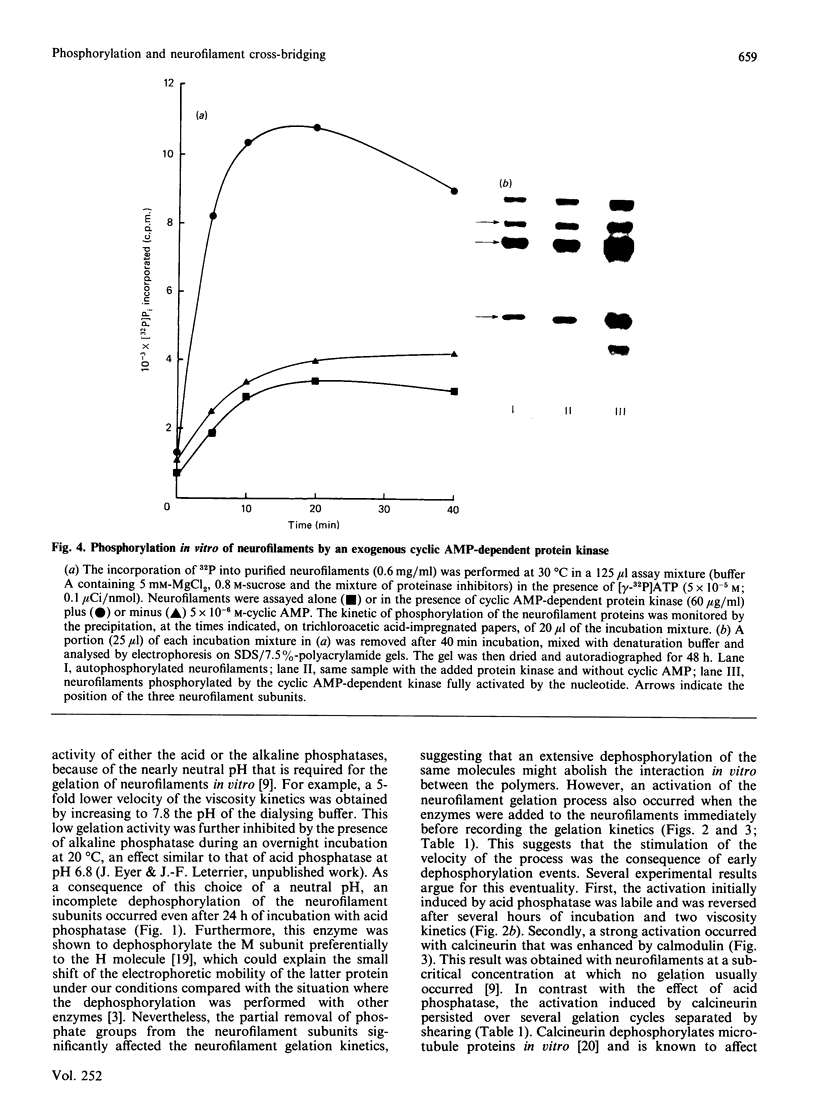
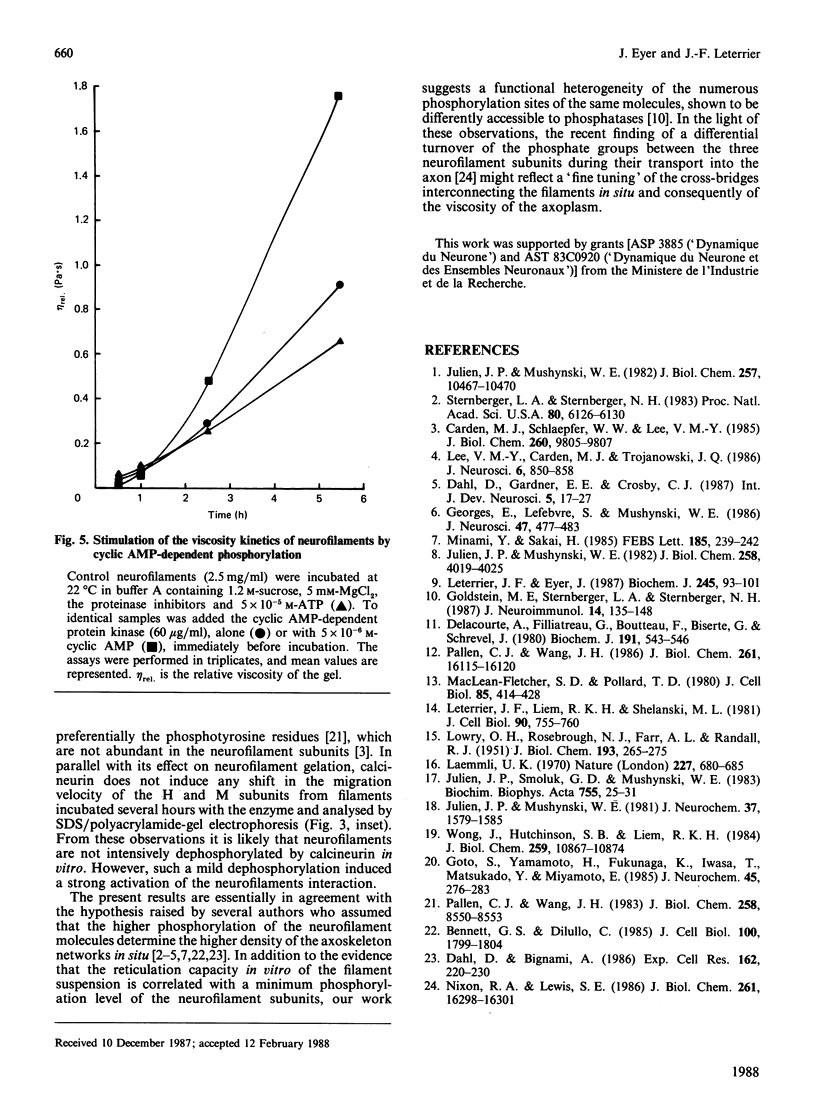
The extensive enzymic dephosphorylation of neurofilaments determined the progressive loss of their capacity to interconnect in vitro into a reticulated network, measured by the formation of highly viscous gels in purified preparations of neurofilaments [Leterrier & Eyer (1987) Biochem. J. 245, 93-101]. Conversely, a cyclic AMP-dependent activation of the gelation process was obtained by phosphorylation of the neurofilament proteins by the cyclic-nucleotide-dependent protein kinase added to the preparation. These findings argue for a direct relationship between the high phosphorylation level of the neurofilament subunits and the cross-bridging of the polymers in vitro. However, a transient stimulation of the neurofilament viscosity kinetics was also observed during the early steps of dephosphorylation with acid phosphatase, which, moreover, disappeared with longer incubation times before the net inhibition was obtained. In the same way, the calmodulin-dependent brain phosphatase, calcineurin, induced a permanent activation of the phenomenon, correlated with a low dephosphorylation capacity of the neurofilament molecules. Taken together, these results suggest a functional heterogeneity of the numerous phosphate groups of the neurofilament subunits and raise the hypothesis of a highly controlled regulation of the neurofilament cross-bridging by selective phosphorylation-dephosphorylation mechanisms.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett G. S., DiLullo C. Slow posttranslational modification of a neurofilament protein. J Cell Biol. 1985 May;100(5):1799–1804. doi: 10.1083/jcb.100.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carden M. J., Schlaepfer W. W., Lee V. M. The structure, biochemical properties, and immunogenicity of neurofilament peripheral regions are determined by phosphorylation state. J Biol Chem. 1985 Aug 15;260(17):9805–9817. [PubMed] [Google Scholar]
- Dahl D., Bignami A. Neurofilament phosphorylation in development. A sign of axonal maturation? Exp Cell Res. 1986 Jan;162(1):220–230. doi: 10.1016/0014-4827(86)90440-4. [DOI] [PubMed] [Google Scholar]
- Dahl D., Gardner E. E., Crosby C. J. Axonal maturation in development--I. Characterization of monoclonal antibodies reacting with axon-specific neurofilament epitopes. Int J Dev Neurosci. 1987;5(1):17–27. doi: 10.1016/0736-5748(87)90044-x. [DOI] [PubMed] [Google Scholar]
- Delacourte A., Filliatreau G., Boutteau F., Biserte G., Schrevel J. Study of the 10-nm-filament fraction isolated during the standard microtubule preparation. Biochem J. 1980 Nov 1;191(2):543–546. doi: 10.1042/bj1910543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georges E., Lefebvre S., Mushynski W. E. Dephosphorylation of neurofilaments by exogenous phosphatases has no effect on reassembly of subunits. J Neurochem. 1986 Aug;47(2):477–483. doi: 10.1111/j.1471-4159.1986.tb04526.x. [DOI] [PubMed] [Google Scholar]
- Goldstein M. E., Sternberger L. A., Sternberger N. H. Varying degrees of phosphorylation determine microheterogeneity of the heavy neurofilament polypeptide (Nf-H). J Neuroimmunol. 1987 Mar;14(2):135–148. doi: 10.1016/0165-5728(87)90048-8. [DOI] [PubMed] [Google Scholar]
- Goto S., Yamamoto H., Fukunaga K., Iwasa T., Matsukado Y., Miyamoto E. Dephosphorylation of microtubule-associated protein 2, tau factor, and tubulin by calcineurin. J Neurochem. 1985 Jul;45(1):276–283. doi: 10.1111/j.1471-4159.1985.tb05504.x. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. A comparison of in vitro- and in vivo-phosphorylated neurofilament polypeptides. J Neurochem. 1981 Dec;37(6):1579–1585. doi: 10.1111/j.1471-4159.1981.tb06330.x. [DOI] [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. Multiple phosphorylation sites in mammalian neurofilament polypeptides. J Biol Chem. 1982 Sep 10;257(17):10467–10470. [PubMed] [Google Scholar]
- Julien J. P., Mushynski W. E. The distribution of phosphorylation sites among identified proteolytic fragments of mammalian neurofilaments. J Biol Chem. 1983 Mar 25;258(6):4019–4025. [PubMed] [Google Scholar]
- Julien J. P., Smoluk G. D., Mushynski W. E. Characteristics of the protein kinase activity associated with rat neurofilament preparations. Biochim Biophys Acta. 1983 Jan 4;755(1):25–31. doi: 10.1016/0304-4165(83)90268-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee V. M., Carden M. J., Trojanowski J. Q. Novel monoclonal antibodies provide evidence for the in situ existence of a nonphosphorylated form of the largest neurofilament subunit. J Neurosci. 1986 Mar;6(3):850–858. doi: 10.1523/JNEUROSCI.06-03-00850.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Eyer J. Properties of highly viscous gels formed by neurofilaments in vitro. A possible consequence of a specific inter-filament cross-bridging. Biochem J. 1987 Jul 1;245(1):93–101. doi: 10.1042/bj2450093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leterrier J. F., Liem R. K., Shelanski M. L. Preferential phosphorylation of the 150,000 molecular weight component of neurofilaments by a cyclic AMP-dependent, microtubule-associated protein kinase. J Cell Biol. 1981 Sep;90(3):755–760. doi: 10.1083/jcb.90.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean-Fletcher S. D., Pollard T. D. Viscometric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J Cell Biol. 1980 May;85(2):414–428. doi: 10.1083/jcb.85.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami Y., Sakai H. Dephosphorylation suppresses the activity of neurofilament to promote tubulin polymerization. FEBS Lett. 1985 Jun 17;185(2):239–242. doi: 10.1016/0014-5793(85)80914-5. [DOI] [PubMed] [Google Scholar]
- Nixon R. A., Lewis S. E. Differential turnover of phosphate groups on neurofilament subunits in mammalian neurons in vivo. J Biol Chem. 1986 Dec 15;261(35):16298–16301. [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Calmodulin-stimulated dephosphorylation of p-nitrophenyl phosphate and free phosphotyrosine by calcineurin. J Biol Chem. 1983 Jul 25;258(14):8550–8553. [PubMed] [Google Scholar]
- Pallen C. J., Wang J. H. Stoichiometry and dynamic interaction of metal ion activators with calcineurin phosphatase. J Biol Chem. 1986 Dec 5;261(34):16115–16120. [PubMed] [Google Scholar]
- Sternberger L. A., Sternberger N. H. Monoclonal antibodies distinguish phosphorylated and nonphosphorylated forms of neurofilaments in situ. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6126–6130. doi: 10.1073/pnas.80.19.6126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong J., Hutchison S. B., Liem R. K. An isoelectric variant of the 150,000-dalton neurofilament polypeptide. Evidence that phosphorylation state affects its association with the filament. J Biol Chem. 1984 Sep 10;259(17):10867–10874. [PubMed] [Google Scholar]