Abstract
Purpose
To report the course of atypical choroidal neovascularization (CNV) in a middle-aged woman experiencing sudden vision loss.
Observations
A middle-aged female presented with sudden onset vision loss. Following in depth investigations an initial diagnosis of presumed idiopathic CNV was made in her right eye. Multimodal imaging confirmed the presence of CNV, while the left eye remained unaffected. The CNV initially responded well to intravitreal Aflibercept injections, resulting in significant visual improvement. However, the patient later developed punctate inner choroidopathy (PIC), which eventually responded to systemic but not local corticosteroids. Recurrent CNV became incompletely controlled by Aflibercept. Switching to Faricimab resulted in sustained visual acuity improvement and CNV resolution.
Conclusions and Importance
The therapeutic efficacy of anti-VEGF treatments has long been underscored in the management of CNV. However, CNV in younger patients often points to a different causation and sometimes requires different management. Our case underscores the significance of inflammatory causes of CNV and including anti-inflammatories in the treatment of CNV management and outlines differences in anti-VEGF efficacy in control of CNV.
Keywords: Anti-VEGF, Choroidal neovascularization, Steroids, Spectral-domain optical coherence tomography
1. Introduction
Choroidal neovascularization (CNV) is the one of the most frequent causes of legal blindness in older individuals in the United States and is characterized by the abnormal development of new blood vessels within the choroid, penetrating Bruch's membrane.1 This pathological process is commonly linked to a range of retinal disorders and diseases including but not limited to age-related macular degeneration, pathological or degenerative myopia, punctate inner choroidopathy, and presumed ocular histoplasmosis syndrome.1,2 The etiology of CNV can be diverse, with age-related macular degeneration being the most common cause.3 However, in younger patients, CNV can arise secondary to other causes including pathological myopia, angioid streaks, trauma, or inflammation.4 Idiopathic choroidal neovascularization (ICNV) represents cases where CNV occurs without a clear identifiable underlying cause. ICNV typically affects young individuals, with patients ranging from 10 to 55 years old, and is more prevalent in females than males.4, 5, 6 While choroidal abnormalities, particularly choroidal inflammation, have been implicated in the pathophysiology of CNV,4,7 systemic inflammation has emerged as a possible factor in the development and progression of ICNV.7
This case report explores the presentation of an atypical course of CNV in a middle-aged female patient, who presented with sudden onset vision loss and mild systemic inflammation, that showed improvement with systemic steroid treatment. We aim to better characterize the phenotype and management of CNV in younger patients, offering insights into potential diagnostic and therapeutic strategies for similar atypical cases in clinical practice.
2. Case report
A 45-year-old female patient was evaluated for sudden onset of decreased vision in her right eye which was noticed three weeks prior to her initial visit. The patient's medical history was unremarkable, with no history of trauma or drugs known to have toxic effects on the retina. The patient had a long history of a corrected refractive state of high myopia (−7.50 D), in both eyes, measured by subjective refraction by the patient's optometrist. A complete ophthalmologic examination was then performed which included an assessment of best-corrected visual acuity (BCVA) using a logMAR chart, slit lamp biomicroscopy and ophthalmoscopy. Multimodal imaging was also performed, which included Spectral Domain Optical Coherence Tomography (SD-OCT) (Heidelberg Engineering, Heidelberg, Germany), ultrawide-field (UWF) pseudocolor fundus photos, fundus autofluorescence (FAF), fluorescein angiogram (FA), and indocyanine green angiogram (ICG) (Optos, Nikon, UK). Additionally, microperimetry (S-MAIA, Centervue, Italy) was performed to assess the patient's retinal sensitivity and assess central visual function.
On examination, at her initial visit, her best corrected visual acuity (BCVA) was 20/80 right eye, 20/25-1 left eye. Anterior segments of both eyes were unremarkable. However, fundus examination revealed a grayish macular lesion in the right eye (Fig. 1A) with no evidence of focal macular atrophy, posterior staphyloma or lacquer cracks. FAF demonstrated central hypo-autofluorescence (Fig. 1A) and SD-OCT imaging showed intraretinal and subretinal fluid in the right eye (Fig. 1A), together with hyper-reflective lesion in the subretinal space. FA showed a characteristic pattern of CNV with macular leakage. No evidence of retinal vasculitis was present (Fig. 2A). Ophthalmologic examination together with multimodal imaging of the left eye did not reveal any abnormalities (Fig. S1). Upon further examination, the patient was found to have an increased axial length of 26.68mm in the right eye and 26.67mm in the left eye, indicating myopia. However, there were no signs of pathological myopia such as lacquer cracks, Fuchs' spot, or chorioretinal atrophy.8,9 Fluorescein angiography (FA) revealed a type 1 membrane and occult choroidal neovascular membrane with macular leakage only evident in the late phase, without visible new vessels or vasculitis present at any stages (Fig. 2A). These FA findings, unusual for myopic CNV, led us to consider alternative diagnoses although myopic CNV remained a differential.
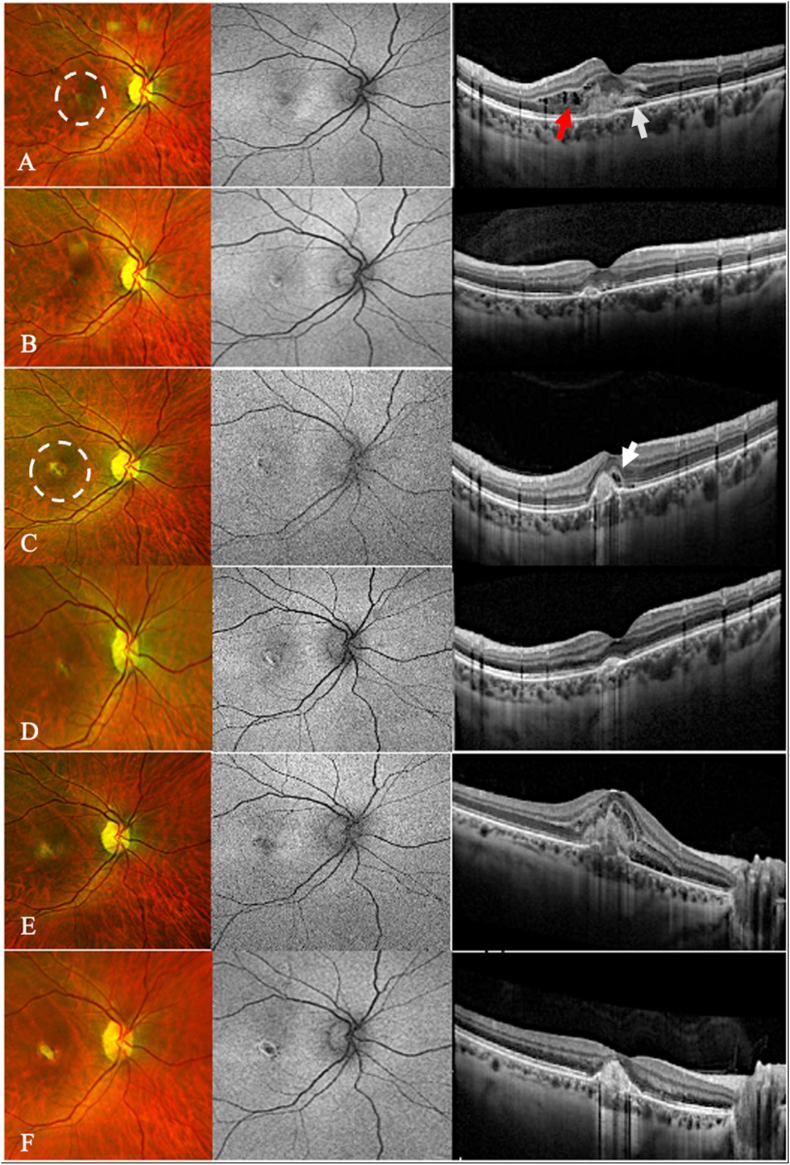
Fig. 1.
Multimodal imaging of the right eye during the treatment course. Left panel represents zoomed-in images of Ultra-wide field (UWF) pseudo-color fundus photos, middle panel represents zoomed-in images of Ultra-wide field (UWF) fundus autofluorescence, and the right panel represents SD-OCT images. A: During the initial visit, the UWF pseudo-color fundus and the UWF autofluorescence photos demonstrated a small and grayish subretinal lesion and hyper-reflective lesions with fuzzy borders at the retinal pigment epithelium (white dashed circle). The SD-OCT imaging demonstrated central ellipsoid, and external limiting membrane with both intraretinal (red arrow) and subretinal fluid (white arrow). B: Following the initial injection, the intraretinal fluid cleared on SD-OCT, while minimal subretinal fluid persisted. C: After the sudden decline in vision, UWF pseudo-color fundus and autofluorescence photos revealed the emergence of a pigmented scar at the lesion site (white dashed circle). SD-OCT was significant for external limiting membrane (ELM) disruption (white arrow), ellipsoid zone (EZ) loss, subretinal fluid, subretinal hyperreflective material (SRHM), RPE irregularity, small serous pigment epithelial detachment (PED). D: Following the completion of a systemic steroid course, a significant resolution of CNV and resolving AF were seen on SD-OCT. E: After completing a series of six Faricimab treatment sessions, a significant improvement was noted on SD-OCT, characterized by the absence of SHRM and subretinal fluid. F: Following the latest Faricimab treatment, there appeared to be a significant improvement on SD-OCT, with no signs of SHRM and no subretinal fluid present. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
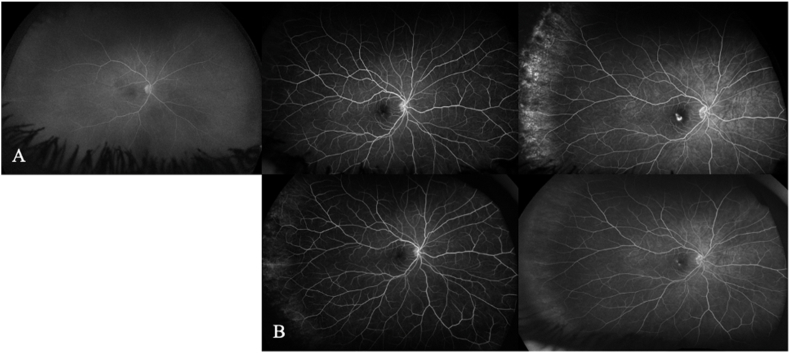
Fig. 2.
Fluorescein angiography (FA) images during the treatment. The left panel represents the early phase of fluorescein angiography at 15 seconds, middle panel represents Mid phase at 30 seconds and the right panel represents late-phase at 3 minutes. A: FA showed the characteristic pattern of an occult choroidal neovascular membrane with macular leakage only evident in late phase, however no evidence of new vessels or vasculitis was present at any stages during the initial visit. B: Sudden drop of vision with clear blind spot in the central part of her vision. FA continued to show the characteristic pattern of a choroidal neovascular membrane with slow macular leakage only evident in late phase with no evidence of new vessels or lesions.
Treatment was then initiated with a series of seven intravitreal injections of 2 mg of Aflibercept at four to six-week intervals. After the first injection, the patient endorsed improvement in her vision with a BCVA of 20/20 in the right eye, however persistent distortion remained. On SD-OCT, the intraretinal fluid resolved with persistent minimal subretinal fluid (Fig. 1B). Following the initiation of treatment with intravitreal injections of 2 mg of Aflibercept at four to six-week intervals, the patient consistently maintained stable vision in her right eye, coupled with improvements in both intraretinal and subretinal fluid.
During her fourth visit, despite good treatment of her CNV, a small, whitish lesion was noted close to the CNV. This lesion became gradually more prominent and was associated with another whitish lesion which was more punctate which developed in the nasal mid-periphery during the sixth visit (Fig. S2).
Approximately one week after the seventh Aflibercept injection, the patient encountered a sudden decline in vision, accompanied by the emergence of a distinct blind spot in the central aspect of her visual field; BCVA was 20/25+ right eye and the clinical exam was notable for a pigmentation ring, and a scar, and a new pale nodule supratemporal to the fovea (Fig. 1C). Repeat FA continued to show a pattern characteristic active CNV with macular leakage only evident in late phase with no evidence of other new vessels or lesions (Fig. 2B). At this time, the patient underwent extensive laboratory work up to rule out possible systemic and ocular causes of the deterioration of her condition (Table S1). Her findings were all within normal limits except for mildly elevated inflammatory markers for ESR: 32 mm/hr (normal ≤20 mm/hr) and CRP: 0.7 mg/dL (normal ≤0.3 mg/dL) suggesting mild systemic inflammation. The patient was initially treated with 40 mg of Triamcinolone Acetonide (40mg/mL) via sub-Tenon injection, aiming to address the inflammatory component. However, despite this intervention, the patient's vision continued to deteriorate (BCVA 20/125 OD) and subsequent evaluation via SD-OCT showed sub-retinal hyper-reflective material (SHRM). Two weeks later, systemic steroids were added in addition to her usual Aflibercept. Oral Prednisolone was initially started at 60mg daily for seven days and tapered over four weeks. After finishing the course of the systemic steroids, the patient again noticed an improvement in her vision in the right eye (BCVA 20/80) and the clinical exam showed significant improvement and resolution of subretinal fluid on SD-OCT images (Fig. 1D). The patient received another series of three intravitreal 2 mg Aflibercept injections with continued improvement of her vision in the right eye 20/63 and SD-OCT showed Ellipsoid zone regeneration. However, after the third monthly injection, the patient noticed another episode of a drop in vision to 20/125 and SD-OCT showed continued ELM disruption and SHRM suggesting incomplete control of CNV with monthly aflibercept (Fig. 1E). The decision was then made to switch to intravitreal injections of 6 mg Faricimab (Vabysmo). The patient received a series of six intravitreal injections of 6 mg Faricimab. These were initially administered at four-weekly intervals and then extended to six-weekly intervals. During the Faricimab treatment regimen, there was improvement in the patient's right eye visual acuity with the final BCVA 20/63 + 1 after six injections, together with improvement in SD-OCT findings and no signs of SHRM and no recurrence of subretinal fluid suggesting control of the CNV (Fig. 1F). Furthermore, sub-foveal choroidal thickness measurements (in μM) were obtained for all stages of treatment using SD-OCT images to help evaluate the effectiveness of treatments (Table 2S). A reduction in choroidal thickness after treatment may indicate a positive response, while an increase could suggest disease progression or recurrence. However, although there were fluctuations, we did not see a clear correlation between sub-foveal choroidal thickness and treatment response.
3. Discussion
We present a case of a middle-aged female who presented with CNV in her right eye. This was initially thought to be a case of ICNV due to the lack of clear etiology after extensive testing. The single creamy lesion adjacent to the CNV at week 4 was an unusual finding. However, the development of a further lesion in the nasal periphery suggested that the disease could be an atypical presentation of punctate inner choroidopathy (PIC), given the lack of other clear etiologies for the lesion (Fig. S2). This case was firstly unusual as the patient presented with CNV prior to the classical chorioretinal lesions. The patient is in the right demographic for PIC, being female and being myopic. Additionally, the late staining observed on FA (Fig. 2B) is also supportive of a diagnosis of PIC.10 While most PIC cases present as multifocal lesions located in the posterior pole, paucifocal PIC (2–4 lesions) accounts for 28 % of cases, and 21 % involve the posterior pole and mid-periphery, similar to the presentation in this particular case, emphasizing the need to consider PIC even when the clinical picture is not entirely typical.11
PIC typically presents with multiple, small, yellow-white fundus lesions in young myopic women.11 This case does not meet the classical criteria for PIC. The initial lesion in this case was solitary, while PIC is usually characterized by multiple lesions, the lesion was not multifocal initially or bilateral, which is commonly seen in PIC,11 the lesion did not exhibit the typical whitish appearance at the end-stage, as described in PIC and; the OCT findings do not show the classical features associated with PIC lesions.12 PIC has also been reported with a single lesion in some cases.11 Furthermore, the previously noted signs, combined with the success of treatment using systemic and local steroids, point towards a diagnosis of PIC.13
We found that monthly aflibercept or locally administered steroids were not able to control the CNV. However, switching to Faricimab alone at 6 weekly intervals was able to control CNV. Even though Faricimab and Aflibercept are both anti-vascular endothelial growth factor (VEGF) agents used in the treatment of various retinal conditions, including CNV, they differ in their molecular structure and mechanism of action, which may contribute to their varying efficacy in certain cases.14 Faricimab is a bispecific antibody that targets both VEGF-A and angiopoietin-2 (Ang-2), a key regulator of vascular stability and permeability.15 By inhibiting both VEGF-A and Ang-2, Faricimab potentially provide a more comprehensive approach to managing CNV and associated complications, such as macular edema and retinal fluid accumulation.15 In contrast, Aflibercept is a smaller molecule that solely targets VEGF-A. While it has been effective in treating CNV and other retinal conditions, its single-target approach may be less effective in cases where additional pathways, such as Ang-2, play a significant role in disease progression.14,16 Moreover, the difference in molecular size between Faricimab and Aflibercept may also contribute to their varying efficacy. As a larger molecule, Faricimab may have a longer half-life and potentially require less frequent dosing compared to Aflibercept.14,15 In this case of suspected PIC associated CNV, we speculate that the dual-targeting mechanism of Faricimab provided a better control of the CNV compared to Aflibercept's single-target approach. To our knowledge, we are not aware of any previous reports of Faricimab being used to treat presumed PIC CNV. However, Faricimab has been used to treat CNV associated with inflammatory eye disease. We note one case of Farcimab's use for the adjuvant treatment of retinal neovascularization associated with tuberculous uveitis, although in that case, the efficacy of other intravitreal anti-VEGF to resolve the neovascularization was not compared.17 However, our patient should be closely monitored for progression of PIC lesions, which are not usually controlled by anti-VEGF agents. There should be a low threshold for addition of systemic steroid sparing agents if new PIC lesions were to develop. Our patient was keen to avoid long term systemic steroid sparing agents. However, systemic treatment may potentially also help long term control of CNV in our case.4,18
4. Conclusion
This atypical case of CNV, in a middle-aged female patient, was managed with the combination of anti-VEGF agents and systemic corticosteroids. Physicians should also be aware that atypical causes of CNV may develop in this age group and should be monitored closely during follow-up. This approach resolved exudation in the present case, and preserved visual function and suggests a low threshold for the use of systemic immunosuppressive treatment in atypical CNV in younger patients. Furthermore, in our case, Faricimab appeared to offer better control of CNV associated with presumed PIC than Aflibercept, however further studies in a larger cohort of patients with in younger patients with similar findings will be required to confirm whether Faricimab is indeed a better treatment for CNV in these cases.
Patient consent
A written informed consent was obtained from the patient.
Funding
This study received no funding or grant support.
Authorship
All authors attest that they meet the ICMJE criteria for authorship.
CRediT authorship contribution statement
Kimia Rezaei: Writing – review & editing, Writing – original draft. Shaden H. Yassin: Writing – review & editing. Henry Ferreyra: Supervision, Data curation. Shyamanga Borooah: Writing – review & editing, Supervision, Project administration, Investigation, Formal analysis, Data curation.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements and Disclosure
None.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajoc.2024.102191.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Laboratory values after the sudden decline in vision with abnormal values outside of the reference range in bold.
Sub-foveal choroidal thickness measurements (in μM) corresponding to the dates of the images presented in Fig. 1, obtained using SD-OCT images.
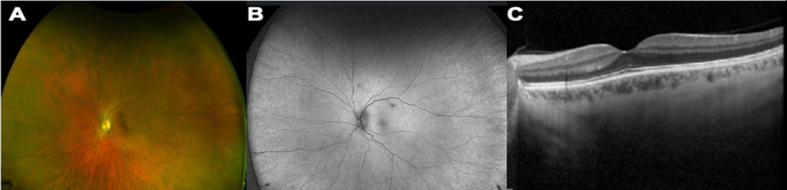
Supplementary Fig. 1.
Multimodal imaging of the left unaffected eye. A: Ultra-wide field pseudo-color fundus photo. B: Ultra-wide field fundus autofluorescence. C: Spectral domain optical coherence tomography, all three modalities did not reveal any abnormalities.
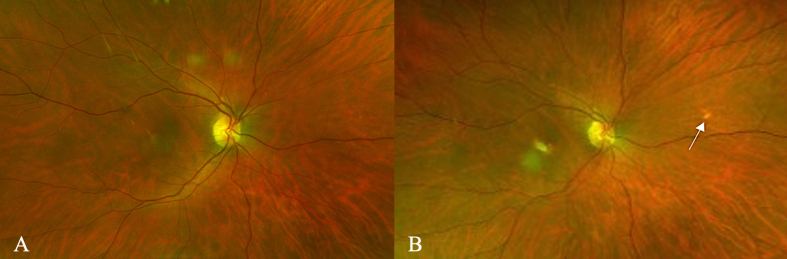
Supplementary Fig. 2.
Pseudocolor image of the right eye. A: Imaging at the initial visit showed no lesions in the nasal mid-periphery region. B: By the sixth visit, a small, whitish lesion near the treated CNV was noted in the macula and was accompanied by another punctate whitish lesion in the nasal mid-periphery (white arrow).
References
- 1.Monis M., Ali S., Bhutto I., Mahar P. Idiopathic choroidal neovascularization in pregnancy: a case report. Cureus. 2023;15 doi: 10.7759/cureus.34611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waheeb S.A., Showail M.J. Idiopathic choroidal neovascular membrane in a young female. Oman J Ophthalmol. 2009;2(3):133–136. doi: 10.4103/0974-620X.57314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kesen M.R., Cousins S.W. In: Encyclopedia of the Eye. Dartt D.A., editor. Academic Press; 2010. Choroidal neovascularization; pp. 257–265. [DOI] [Google Scholar]
- 4.Yin H., Fang X., Ma J., et al. Idiopathic choroidal neovascularization: intraocular inflammatory cytokines and the effect of intravitreal ranibizumab treatment. Sci Rep. 2016;6(1) doi: 10.1038/srep31880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho A.C., Yannuzzi L.A., Pisicano K., DeRosa J. The natural history of idiopathic subfoveal choroidal neovascularization. Ophthalmology. 1995;102(5):782–789. doi: 10.1016/S0161-6420(95)30968-2. [DOI] [PubMed] [Google Scholar]
- 6.Sasahara M., Otani A., Yodoi Y., Yoshimura N. Circulating hematopoietic stem cells in patients with idiopathic choroidal neovascularization. Invest Ophthalmol Vis Sci. 2009;50(4):1575–1579. doi: 10.1167/iovs.08-1900. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham Jr, Et, Pichi F., Dolz-Marco R., Freund K.B., Zierhut M. Inflammatory choroidal neovascularization. Ocul Immunol Inflamm. 2020;28(1):2–6. doi: 10.1080/09273948.2019.1704153. [DOI] [PubMed] [Google Scholar]
- 8.Du R., Xie S., Igarashi-Yokoi T., et al. Continued increase of axial length and its risk factors in adults with high myopia. JAMA Ophthalmol. 2021;139(10):1–8. doi: 10.1001/jamaophthalmol.2021.3303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ohno-Matsui K., Yoshida T., Futagami S., et al. Patchy atrophy and lacquer cracks predispose to the development of choroidal neovascularisation in pathological myopia. Br J Ophthalmol. 2003;87(5):570–573. doi: 10.1136/bjo.87.5.570. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1771643/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meleth A.D., Sen H.N. Use of fundus autofluorescence in the diagnosis and management of uveitis. Int Ophthalmol Clin. 2012;52(4):45. doi: 10.1097/IIO.0b013e3182662ee9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Classification criteria for punctate inner choroiditis. Am J Ophthalmol. 2021;228:275–280. doi: 10.1016/j.ajo.2021.03.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Classification criteria for multifocal choroiditis with panuveitis. Am J Ophthalmol. 2021;228:152–158. doi: 10.1016/j.ajo.2021.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campos J., Campos A., Mendes S., Neves A., Beselga D., Sousa J.C. Punctate inner choroidopathy: a systematic review. Med Hypothesis, Discov Innovation (MEHDI) Ophthalmol. 2014;3(3):76–82. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348489/ [PMC free article] [PubMed] [Google Scholar]
- 14.Kodjikian L., Tadayoni R., Souied E.H., et al. Efficacy and safety of aflibercept for the treatment of idiopathic choroidal neovascularization in young patients: the intuition study. Retina. 2022;42(2):290. doi: 10.1097/IAE.0000000000003310. [DOI] [PubMed] [Google Scholar]
- 15.Yen W.T., Wu C.S., Yang C.H., Chen Y.H., Lee C.H., Hsu C.R. Efficacy and safety of intravitreal faricimab for neovascular age-related macular degeneration: a systematic review and meta-analysis. Sci Rep. 2024;14:2485. doi: 10.1038/s41598-024-52942-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Balaratnasingam C., Dhrami-Gavazi E., McCann J.T., Ghadiali Q., Freund K.B. Aflibercept: a review of its use in the treatment of choroidal neovascularization due to age-related macular degeneration. Clin Ophthalmol. 2015;9:2355–2371. doi: 10.2147/OPTH.S80040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Warter A., Galang C., Heinke A., et al. Use of faricimab (VABYSMO) for highly treatment resistant CNV in wet-age related macular degeneration. Invest Ophthalmol Vis Sci. 2023;64(8):2212. [Google Scholar]
- 18.Edwards Mayhew R.G., Li T., McCann P., Leslie L., Strong Caldwell A., Palestine A.G. Non‐biologic, steroid‐sparing therapies for non‐infectious intermediate, posterior, and panuveitis in adults. Cochrane Database Syst Rev. 2022;2022(10) doi: 10.1002/14651858.CD014831.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Laboratory values after the sudden decline in vision with abnormal values outside of the reference range in bold.
Sub-foveal choroidal thickness measurements (in μM) corresponding to the dates of the images presented in Fig. 1, obtained using SD-OCT images.