Abstract
BACKGROUND:
Gastric cancer (GC) is one of the most common tumors. There were several classifications of GC recently. The value of Lauren classification in evaluating the prognosis after radical gastrectomy was still unclear and the prognosis of gastric cancer remained relatively poor in the absence of prognostic biomarkers. This study aimed to explore microRNA (miRNA) in the prognosis of GC with different Lauren classification.
METHODS:
A retrospective study of 1144 patients was performed in this study. Quantificational reverse transcription-PCR (qRT-PCR) was used to examine the expression of miRNAs. Univariate and multivariate analysis were performed to evaluate prognosis value of Lauren classification.
RESULTS:
Total 1144 GC patients were recruited in this cohort, including 302 diffuse type (26.4%), 436 intestinal type (38.1%) and 406 mixed type (35.5%) GC. Multivariate analysis showed that Lauren classification, patients’ age, tumor size, tumor infiltrating depth, vascular nerve infiltrating and metastatic lymph nodes ration were significantly correlated with GC patients’ OS and DFS. The miR-141-3p, miR-200b-3p and miR-133a-5p were significantly down-regulated in diffuse type compared to intestinal type GC tissues, the miR-105-5p had significant lower expression in diffuse type compared with intestinal type and mixed type GC tissues. As a consequence of univariate analysis, low miR-141-3p in diffuse type GC showed significant worse OS and DFS than high miR-141-3p.
CONCLUSIONS:
Lauren classification was an independent prognostic factor in GC. MiR-141-3p was an independent prognostic factor and a promising prognostic biomarker in Lauren classification GC.
Keywords: Gastric cancer, Lauren classification, microRNA, prognosis
1. Introduction
Gastric cancer (GC) is the third leading contributor of cancer mortality worldwide [1]. To date, the anatomical American Joint Committee on Cancer (AJCC) and Union for International Cancer Control (UICC) tumor-node-metastasis (TNM) staging system is the most widely used for describing GC states [2]. However, GC is a multifactorial and multistage disease [3]. It is hard to have better understanding on prognostic value just using TNM classification without reference to its pathology [4]. Since 1965, the Lauren classification has been proposed and become one of effective methods based on the histological structure of GC cells. GC is divided into diffuse type, intestinal type and mixed type according to the Lauren classification [5]. The histopathology of intestinal type is gland like structures and is considered to be associated with chronic inflammation induced by Helicobacter Pylori infection, smoking, obesity and other dietary factors [6, 7], diffuse type is isolated-cell carcinoma and induced by active inflammation which leads to poor prognosis [8]. Thus, the prognostic relevance of GC using Lauren classification still remains unilluminated.
Primary studies reported that different moleculars played important roles in GC prognosis prediction, such as KRAS, APC, TP53 and so on [9, 10]. MicroRNAs (MiRNAs), endogenous non-coding RNAs with 20–22 nucleotides in size, are known as oncogenes or tumor suppressors in various human cancers through regulating target mRNAs [11, 12]. MiR-205/miR-338-3p regulated BCL-2 expression to suppress prostate cancer cells apoptosis [13]. Besides, miRNAs become popular biomarkers for cancer diagnosis and prognosis [14]. MiR-16 was confirmed the diagnosis value in common cancer like lung cancer, gastric cancer and endometrioid endometrial cancer [15]. MiR-487a worked as prognostic biomarker in hepatocelluar carcinoma [16]. MiR-194 was verified as favourable prognosis biomarker in GC [17]. It was reported that the down-modulation of miR-375 is specifically linked to Lauren’s classification [18]. There is also research that suggests miR-18a-5p plays diagnostic and therapeutic potencies in mixed-type gastric cancer [19]. However, the application of miRNA on predicting prognosis in Lauren classification GC is still underway and needs further investigation.
To the point of controversy, we performed a retrospective study of 1144 patients who received radical gastrectomy and analyzed the clinical characteristics and significance in Lauren classification. In addition, we examined the expression levels and evaluated the prognostic value of related miRNAs from different Lauren classification GC tissues. This study provided the theoretical basis for the potential use of miRNAs in diagnosis and prognosis of gastric cancer with different Lauren classification.
2. Materials and methods
2.1. Study cohort
We collected data on 1144 patients who received radical gastrectomy at First Affiliated Hospital of Nanjing Medical University from January, 2005 to December, 2011. After surgery, GC patients were followed up every 3 months at first 2 years then every 6 months within 5 years, annually after 5 years, the last follow-up was in June, 2017. All patients from the cohort underwent radical gastrectomy and histologically confirmed. Clinical stage and histological classifications of GC were used the WHO classification criteria and the eighth edition of the AJCC TNM classification for GC.
2.2. Sample collection
We collected 145 GC tissues from the study cohort. Inclusion criteria include (1) complete clinical data, (2) Standard D2 lymph node dissection, (3) TNM stage I, to III, (4) no preoperative radiotherapy or chemotherapy, (5) postoperative chemotherapy regimen based on 5-FU and completed at least 4 cycles.
2.3. RNA extraction
Sections (8 m slices) were prepared from paraffin-embedded specimen. Paraffin was removed by dewaxing reagent to obtain GC tissues after centrifugation. Total RNA was extracted from samples using Trizol (Invitrogen, America) method [20]. The concentration and quality of total RNA were evaluated by the ultraviolet spectrophotometer.
2.4. Quantitative RT-PCR
The cDNA was conducted using the specific primers of reverse transcription (RT) (Exiqon, Denmark). The amplification of miRNA was used Bulge-Loop miRNA qRT-PCR Primer Set (RiboBio, China) and evaluated the product fluorescence by SYBR Green (TaKaRa, China). RT reaction was carried out at 42∘C for 60 min followed by 70∘C for 10 min. The quantitative RT-PCR (qRT-PCR) was carried on LightCycler® 480 Real-Time PCR System (Roche Diagnostics, Germany) in 384-well plates at 95∘C for 20 s, followed by 40 cycles of 95∘C for 10 s, 60∘C for 20 s and then 70∘C for 10 s, the melting curve program was run immediately. All reactions were performed in triplicate. The RNU6B () was used as the standard for miRNA expression normalization [21]. The miRNA relative expression levels were calculated by the 2 - ΔΔCt method, Ct Ct (miRNA) – Ct (U6).
2.5. Statistical analysis
The differential miRNAs expression levels between GC tissues and control tissues were analyzed by Mann-Whitney test. Clinical characteristics among Lauren classification groups and the relationship with miRNAs were analyzed by one-way ANOVA or 2 test. Survival curves and univariate analysis were used the log-rank test. Five-year survival rates were estimated by life-table. Multivariate analysis was performed using Cox’s proportional hazards regression model. All the statistical analyses were performed using SPSS software (version 20.0, IBM, USA). value 0.05 was defined statistically significant.
3. Results
3.1. Clinicopathological characteristics of study subjects
Total 1144 GC patients (846 males and 298 females, mean age, 61 years) were recruited in this cohort, including 302 diffuse type (26.4%), 436 intestinal type (38.1%) and 406 mixed type (35.5%) GC, the demographic information of GC patients were summarized in Table 1. We conducted the correlation analysis between Lauren classification and the differentiated. The results indicated that GC patients with diffuse type were younger ( 45 years) ( 0.001), female predominant ( 0.001), distal stomach predominant ( 0.001), more infiltrating type and vascular nerve ( 0.001), higher incidence in signet ring carcinoma and mucinous carcinoma ( 0.001), TNM III stage predominant ( 0.001). The intestinal type GC patients showed that were older ( 60 years) ( 0.001), less distal stomach predominant ( 0.001), well differentiated ( 0.001), Borrmann type predominant ( 0.001), relative smaller tumor size (diameter 3 cm) ( 0.001), less vascular nerve infiltrating ( 0.001), less lymphovascular invasion ( 0.001), TNM I stage predominant ( 0.001).
Table 1.
The correlation analysis between Lauren classification and clinicopathological characteristics
| Variables | Lauren classification | 2 | value | ||
|---|---|---|---|---|---|
| Diffuse type | Intestinal type | Mixed type | |||
| Gender | 24.48 | 0.001 | |||
| Male | 191 (22.6%) | 352 (41.6%) | 303 (35.8%) | ||
| Female | 111 (37.2%) | 84 (28.2%) | 103 (34.6%) | ||
| Age | 71.982 | 0.001 | |||
| 45 years | 55 (59.1%) | 9 (9.7%) | 29 (31.2%) | ||
| 45–60 years | 169 (26.8%) | 248 (39.3%) | 214 (33.9%) | ||
| 60 years | 78 (18.6%) | 179 (42.6%) | 163 (38.8%) | ||
| Tumor site | 85.254 | 0.001 | |||
| Proximal | 63 (13.5%) | 236 (50.6%) | 167 (35.8%) | ||
| Middle | 122 (32.4%) | 116 (30.9%) | 138 (36.7%) | ||
| Distal | 117 (38.7%) | 84 (27.8%) | 101 (33.4%) | ||
| Tumor subtype | 58.011 | 0.001 | |||
| Non-infiltrating type | 50 (25.5%) | 101 (51.5%) | 45 (23.0%) | ||
| Borrmann type | 207 (24.0%) | 323 (37.4%) | 333 (38.6%) | ||
| Infiltrating type | 45 (52.9%) | 12 (14.1%) | 28 (32.9%) | ||
| Pathological classifications | 149.681 | 0.001 | |||
| Adenocarcinoma | 217 (22.1%) | 413 (42.0%) | 354 (36.0%) | ||
| Mucinous carcinoma | 35 (32.7%) | 23 (21.5%) | 49 (45.8%) | ||
| Signet ring carcinoma | 50 (94.3%) | 0 (0%) | 3 (5.7%) | ||
| Tumor size | 28.884 | 0.001 | |||
| 3 cm | 120 (25.5%) | 209 (44.4%) | 142 (30.1%) | ||
| 3–6 cm | 120 (23.8%) | 187 (37.1%) | 197 (39.1%) | ||
| 6 cm | 62 (36.7%) | 40 (23.7%) | 67 (39.6%) | ||
| Histopathology classification | 487.347 | 0.001 | |||
| Well differentiated | 1 (3.7%) | 25 (92.6%) | 1 (3.7%) | ||
| Moderately differentiated | 4 (1.5%) | 247 (91.5%) | 19 (7.0%) | ||
| Poor differentiated | 297 (35.1%) | 164 (19.4%) | 386 (45.6%) | ||
| Tumor infiltrating depth | 51.995 | 0.001 | |||
| T1 | 59 (29.5%) | 95 (47.5%) | 46 (23.0%) | ||
| T2 | 25 (14.7%) | 84 (55.1%) | 46 (30.1%) | ||
| T3 | 14 (19.4%) | 35 (47.8%) | 23 (32.8%) | ||
| T4 | 204 (28.5%) | 222 (31.0%) | 291 (40.6%) | ||
| Vascular nerve infiltrating | 82.582 | 0.001 | |||
| Negative | 154 (24.5%) | 310 (49.4%) | 164 (26.1%) | ||
| Positive | 148 (28.7%) | 126 (24.4%) | 242 (46.9%) | ||
| Number of metastatic lymph nodes | 110.381 | 0.001 | |||
| N0 | 92 (21.9%) | 222 (52.7%) | 107 (25.4%) | ||
| N1 | 37 (18.0%) | 90 (43.7%) | 79 (38.3%) | ||
| N2 | 63 (27.4%) | 79 (34.3%) | 88 (38.3%) | ||
| N3 | 110 (38.3%) | 45 (15.7%) | 132 (46.0%) | ||
| Metastatic lymph nodes ration | 108.674 | 0.001 | |||
| 0 | 92 (21.9%) | 222 (52.7%) | 107 (25.4%) | ||
| 0.25 | 47 (19.5%) | 112 (46.5%) | 82 (34.0%) | ||
| 0.5 | 66 (32.8%) | 50 (24.9%) | 85 (42.3%) | ||
| 0.5 | 97 (34.5%) | 52 (18.5%) | 132 (47.0%) | ||
| AJCC 8th TNM stage | 71.894 | 0.001 | |||
| I | 64 (23.9%) | 144 (53.7%) | 60 (22.4%) | ||
| II | 54 (20.7%) | 124 (47.5%) | 83 (31.8%) | ||
| III | 184 (29.9%) | 168 (27.3%) | 263 (42.8%) | ||
0.05, statistical significance.
3.2. Univariate and multivariate analysis for prognosis of gastric cancer
Among 1144 GC patients, the overall survival (OS) of 1-year, 3-year and 5-year were 90%, 75% and 66%, while the disease free survival (DFS) of 1-year, 3-year and 5-year were 78%, 66% and 59% (Fig. 1A). The univariate analysis showed that Lauren classification was strongly related to the OS and DFS ( 0.05), the OS and DFS of diffuse type, intestinal type, mixed type GC patients were 51%, 69%, 48% and 49%,67%, 47%, respectively ( Fig. 1B). In addition, the univariate analysis also showed that the prognosis of GC patients were strongly related to the patients’ age, tumor site and size, tumor subtype, pathological classifications, tumor infiltrating depth, vascular nerve infiltrating, number of metastatic lymph nodes and metastatic lymph nodes ration, AJCC 8th edition TNM classification ( 0.05) (Tables 2–3).
Figure 1.
Overall survival (OS) and disease free survival (DFS) curves plotted by the Kaplan-Meier method for (A) 1144 GC patients (B) GC patients with Lauren classification, diffuse type, intestinal type, mixed type.
Table 2.
The univariate analysis between disease free survival (DFS) and clinicopathological characteristics
| Variables | Numbers | DFS (months) | 5-year survival (%) | 2 | value |
|---|---|---|---|---|---|
| Lauren classification | 41.731 | 0.001 | |||
| Diffuse type | 302 | 44.7 | 49 | ||
| Intestinal type | 436 | * | 67 | ||
| mixed type | 406 | 36.933 | 47 | ||
| Gender | 0.924 | 0.336 | |||
| Male | 846 | * | 54 | ||
| Female | 298 | * | 58 | ||
| Age | 19.242 | 0.001 | |||
| 45 years | 93 | * | 58 | ||
| 45–60 years | 631 | * | 60 | ||
| 60 years | 420 | 37.267 | 47 | ||
| Tumor site | 8.355 | 0.015 | |||
| Proximal | 466 | 49.267 | 49 | ||
| Middle | 376 | * | 61 | ||
| Distal | 302 | * | 56 | ||
| Tumor subtype | 84.799 | 0.001 | |||
| Non-infiltrating type | 196 | * | 83 | ||
| Borrmann type | 863 | 70.2 | 51 | ||
| Infiltrating type | 85 | 18.9 | 32 | ||
| Pathological classifications | 2.228 | 0.328 | |||
| Adenocarcinoma | 984 | * | 55 | ||
| Mucinous carcinoma | 107 | 68.633 | 51 | ||
| Signet ring carcinoma | 53 | 43.733 | 49 | ||
| Tumor size | 194.55 | 0.001 | |||
| 3 cm | 471 | * | 77 | ||
| 3–6 cm | 504 | 31.433 | 43 | ||
| 6 cm | 169 | 16.967 | 28 | ||
| Histopathology classification | 53.592 | 0.001 | |||
| Well differentiated | 27 | * | 96 | ||
| Moderately differentiated | 270 | * | 70 | ||
| Poor differentiated | 847 | 46.5 | 49 | ||
| Tumor infiltrating depth | 258.912 | 0.001 | |||
| T1 | 200 | * | 97 | ||
| T2 | 155 | * | 79 | ||
| T3 | 72 | * | 68 | ||
| T4 | 717 | 26.167 | 37 | ||
| Number of metastatic lymph nodes | 404.815 | 0.001 | |||
| N0 | 421 | * | 87 | ||
| N1 | 206 | 52 | |||
| N2 | 230 | 31.533 | 42 | ||
| N3 | 287 | 13.233 | 20 | ||
| Metastatic lymph nodes ration | 458.228 | 0.001 | |||
| 0 | 421 | * | 87 | ||
| 0.25 | 241 | * | 57 | ||
| 0.5 | 201 | 27.9 | 36 | ||
| 0.5 | 281 | 12.267 | 19 | ||
| AJCC 8th TNM stage | 382.954 | 0.001 | |||
| I | 268 | * | 96 | ||
| II | 261 | * | 72 | ||
| III | 615 | 19.7 | 31 | ||
| Vascular nerve infiltrating | 144.147 | 0.001 | |||
| Negative | 628 | * | 70 | ||
| Positive | 516 | 26.167 | 36 |
*The median survival time was not reached by follow-up, 0.05, statistical significance.
Table 3.
The univariate analysis between overall survival (OS) and clinicopathological characteristics
| Variables | Numbers | OS (months) | 5-year survival (%) | 2 | value |
|---|---|---|---|---|---|
| Lauren classification | 46.781 | 0.001 | |||
| Diffuse type | 302 | 62.633 | 51 | ||
| Intestinal type | 436 | * | 69 | ||
| Mixed type | 406 | 55.367 | 48 | ||
| Gender | 1.021 | 0.312 | |||
| Male | 846 | * | 55 | ||
| Female | 298 | * | 60 | ||
| Age | 19.161 | 0.001 | |||
| 45 years | 93 | * | 63 | ||
| 45–60 years | 631 | * | 61 | ||
| 60 years | 420 | 57.6 | 49 | ||
| Tumor site | 8.51 | 0.014 | |||
| Proximal | 466 | 65.3 | 51 | ||
| Middle | 376 | * | 62 | ||
| Distal | 302 | * | 59 | ||
| Tumor subtype | 82.088 | 0.001 | |||
| Non-infiltrating type | 196 | * | 86 | ||
| Borrmann type | 863 | 114.567 | 52 | ||
| Infiltrating type | 85 | 29.367 | 35 | ||
| Pathological classifications | 3.236 | 0.198 | |||
| Adenocarcinoma | 984 | * | 58 | ||
| Mucinous carcinoma | 107 | 70.133 | 53 | ||
| Signet ring carcinoma | 53 | 59.933 | 49 | ||
| Tumor size | 193.159 | 0.001 | |||
| 3 cm | 471 | * | 79 | ||
| 3–6 cm | 504 | 45.467 | 45 | ||
| 6 cm | 169 | 25.933 | 30 | ||
| Histopathology classification | 56.797 | 0.001 | |||
| Well differentiated | 27 | * | 96 | ||
| Moderately differentiated | 270 | * | 72 | ||
| Poor differentiated | 847 | 64.967 | 50 | ||
| Tumor infiltrating depth | 264.464 | 0.001 | |||
| T1 | 200 | * | 98 | ||
| T2 | 155 | * | 82 | ||
| T3 | 72 | * | 69 | ||
| T4 | 717 | 36.733 | 39 | ||
| Vascular nerve infiltrating | 145.628 | 0.001 | |||
| Negative | 628 | * | 72 | ||
| Positive | 516 | 36.367 | 38 | ||
| Number of metastatic lymph nodes | 412.207 | 0.001 | |||
| N0 | 421 | * | 88 | ||
| N1 | 206 | * | 56 | ||
| N2 | 230 | 43.7 | 44 | ||
| N3 | 287 | 20.233 | 22 | ||
| Metastatic lymph nodes ration | 462.637 | 0.001 | |||
| 0 | 421 | * | 88 | ||
| 0.25 | 241 | * | 60 | ||
| 0.5 | 201 | 37.267 | 40 | ||
| 0.5 | 281 | 20.433 | 19 | ||
| AJCC 8th TNM stage | 386.133 | 0.001 | |||
| I | 268 | * | 96 | ||
| II | 261 | * | 75 | ||
| III | 615 | 31.133 | 32 |
*The median survival time was not reached by follow-up, 0.05, statistical significance.
Multivariate analysis were introduced using Cox proportional hazards regression (forward LR stepwise procedure) to analyze independent prognostic predicting factors based on the statistically significant variants in univariate analysis. Multivariate analysis showed that Lauren classification, patients’ age, tumor size, tumor infiltrating depth, vascular nerve infiltrating and metastatic lymph nodes ration were significantly correlated with GC patients’ OS and DFS ( 0.001) (Table 4). The Lauren classification was an independent prognostic predicting factor in GC and the diffuse type was an independent risk factors for poor prognosis of GC.
Table 4.
The multivariate COX risk model analysis of overall survival (OS) and between disease free survival (DFS)
| Endpoint | Variables | (regression coefficient) | SE | Wald value | HR (risk ratio) 95% CI | value |
|---|---|---|---|---|---|---|
| DFS | ||||||
| Lauren classification | 0.27 | 0.074 | 13.13 | 1.175 (1.041–1.328) | 0.001 | |
| Tumor size | 0.283 | 0.069 | 16.777 | 1.336 (1.156-1.544) | 0.001 | |
| Tumor infiltrating depth | 0.397 | 0.104 | 14.621 | 1.476 (1.187–1.836) | 0.001 | |
| Vascular nerve | 0.359 | 0.096 | 13.833 | 1.433 (1.171–1.754) | 0.001 | |
| Metastatic lymph nodes ration | 0.457 | 0.092 | 24.686 | 1.558 (1.293–1.879) | 0.001 | |
| OS | ||||||
| Lauren classification | 0.309 | 0.075 | 16.723 | 1.183 (1.047–1.337) | 0.001 | |
| Tumor size | 0.295 | 0.07 | 17.836 | 1.338 (1.156–1.548) | 0.001 | |
| Tumor infiltrating depth | 0.464 | 0.098 | 13.579 | 1.612 (1.284–2.023) | 0.001 | |
| Vascular nerve | 0.36 | 0.104 | 10.941 | 1.412 (1.151–1.732) | 0.001 | |
| Metastatic lymph nodes ration | 0.472 | 0.092 | 26.185 | 1.568 (1.300–1.891) | 0.001 |
0.05, statistical significance.
3.3. Identification of candidate differentially expressed miRNAs in GC
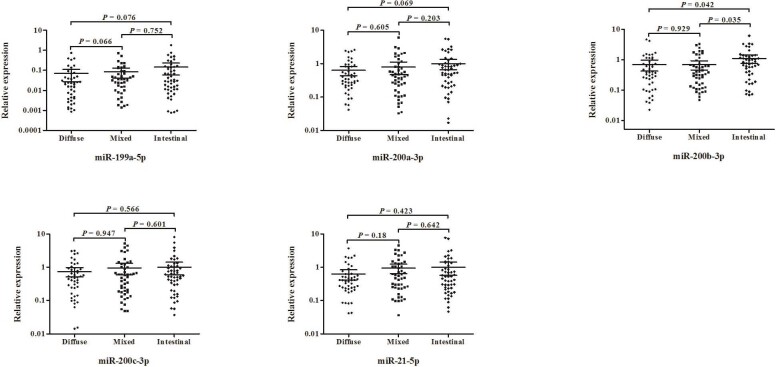
We searched keywords, gastric cancer, stomach cancer, miRNA and Lauren classification in PubMed website. The latest publication time of reference was January, 2017. Finally, a total of 8 references were in line with our topic idea after intensive reading. There were 22 candidate miRNAs, miR-105-5p, miR-100-5p, miR-199a-5p, miR-99a-5p, miR-133a-5p, miR-373-5p, miR-498, miR-202-5p, miR-32-5p, miR-141-3p, miR-182-5p, miR-125b-5p, miR-143-3p, miR-145-5p, miR-494-3p, miR-21-5p, miR-299-5p, miR-365b, miR-499a-5p, miR-200a-3p, miR-200b-3p and miR-200c-3p [22, 23, 24, 25, 26, 27, 28, 29].
Figure 2.
continued.
The expression levels of 22 miRNAs were verified in 145 GC tissues, 47 diffuse type (32.4%), 50 intestinal type (33.1%), 48 mixed type (34.5%), the clinical features of patients were listed in Table 5. The expressed of miR-202-5p, miR-299-5p, miR-32-5p, miR-365b, miR-373-5p, miR-494-3p, miR-498 and miR-499a-5p were weakly expressed in GC tissues. As shown in Fig. 2, there were no significant difference in miR-100-5p, miR-199a-5p, miR-99a-5p, miR-182-5p, miR-125b-5p, miR-143-3p, miR-145-5p, miR-21-5p, miR-200a-3p, and miR-200c-3p among diffuse type, intestinal type and mixed type GC tissues. Compared to intestinal type GC tissues, the miR-141-3p, miR-200b-3p and miR-133a-5p were significantly down-regulated in diffuse type. Meanwhile, the miR-105-5p had significant lower expression in diffuse type compared with intestinal type and mixed type GC tissues.
Table 5.
Clinicopathological characteristics used in miRNA expression detection
| Age | |
|---|---|
| 45 years | 13 (9.0%) |
| 45–60 years | 80 (55.1%) |
| 60 years | 52 (35.9%) |
| Gender | |
| Male | 101 (69.7%) |
| Female | 44 (30.3%) |
| Tumor site | |
| Proximal | 62 (42.8%) |
| Middle | 44 (30.3%) |
| Distal | 39 (26.9%) |
| Tumor subtype | |
| Non-infiltrating type | 29 (20.0%) |
| Borrmann type | 101 (69.7%) |
| Infiltrating type | 15 (10.3%) |
| Pathological classifications | |
| Adenocarcinoma | 118 (81.4%) |
| Mucinous carcinoma | 10 (6.9%) |
| Signet ring carcinoma | 17 (11.7%) |
| Tumor size | |
| 3 cm | 62 (42.8%) |
| 3–6 cm | 57 (39.3%) |
| 6 cm | 26 (17.9%) |
| Histopathology classification | |
| Well differentiated | 3 (2.1%) |
| Moderately differentiated | 30 (20.7%) |
| Poor differentiated | 112 (77.2%) |
| Tumor infiltrating depth | |
| T1 | 40 (27.6%) |
| T2 | 15 (10.3%) |
| T3 | 30 (20.7%) |
| T4 | 60 (41.4%) |
| Number of metastatic lymph nodes | |
| N0 | 65 (44.8%) |
| N1 | 18 (12.4%) |
| N2 | 31 (21.4%) |
| N3 | 31 (21.4%) |
| Metastatic lymph nodes ration | |
| 0 | 65 (44.8%) |
| 0.25 | 30 (20.7%) |
| 0.5 | 20 (13.8%) |
| 0.5 | 30 (20.7%) |
| AJCC 8th TNM stage | |
| I | 47 (32.4%) |
| II | 28 (19.3%) |
| III | 70 (48.3%) |
| Vascular nerve infiltrating | |
| Negative | 94 (64.8%) |
| Positive | 51 (35.2%) |
| Lauren classification | |
| Diffuse type | 47 (32.4%) |
| Intestinal type | 50 (33.1%) |
| Mixed type | 48 (34.5%) |
Figure 2.
Expression levels of 14 candidate miRNAs in the diffuse type, intestinal type and mixed type GC tissues. Horizontal line: mean with 95% CI. Each value was calculated by the Mann–Whitney test. 0.05 was defined statistically significant.
3.4. Diagnostic and prognostic value of miRNAs in different Lauren classification GC
During the followed up, the median DFS and OS of 145 GC patients was 47.9 17.9 and 50.1 14.8 months, 114 (78.6%) were still alive. To further explore the prognostic value of miRNAs, log-rank test was introduced to analyze the OS and DFS between high miRNA expression and low expression of 14 miRNAs (miR-100-5p, miR-199a-5p, miR-99a-5p, miR-182-5p, miR-125b-5p, miR-143-3p, miR-145-5p, miR-21-5p, miR-200a-3p, miR-200c-3p, miR-141-3p, miR-200b-3p, miR-133a-5p and miR-105-5p) (Table 6). The results showed that only low expression of miR-141-3p lead to worse OS and DFS in contrast to high miR-141-3p ( 0.05). There was no significant difference of OS and DFS between low miRNA expression and high miRNA expression of remaining miRNAs.
Table 6.
Log-rank test analysis of overall survival (OS) and disease-free survival (DFS) of candidate miRNA
| miRNA | Expression status | Overall survival (OS) | Disease-free survival (DFS) | ||
|---|---|---|---|---|---|
| Mean SD (months) | -value | Mean SD (months) | -value | ||
| miR-99a-5p | Low | 48.8 14.1 | 0.201 | 46.2 17.9 | 0.47 |
| High | 51.5 15.2 | 49.7 17.8 | |||
| miR-100-5p | Low | 50.3 13.7 | 0.956 | 48.4 16.6 | 0.602 |
| High | 49.9 15.8 | 47.4 19.3 | |||
| miR-105-5p | Low | 50.3 13.5 | 0.396 | 48.6 16.0 | 0.287 |
| High | 50.3 15.8 | 47.6 19.4 | |||
| miR-125b-5p | Low | 50.6 12.7 | 0.71 | 48.7 15.7 | 0.346 |
| High | 49.6 16.7 | 47.1 19.9 | |||
| miR-133a-5p | Low | 48.9 14.3 | 0.205 | 46.2 18.3 | 0.457 |
| High | 51.3 15.2 | 49.7 17.4 | |||
| miR-141-3p | Low | 47.7 16.0 | 0.007 | 44.8 19.8 | 0.036 |
| High | 52.8 12.8 | 51.3 15.0 | |||
| miR-143-3p | Low | 50.1 13.6 | 0.653 | 47.5 17.8 | 0.953 |
| High | 50.1 15.9 | 48.3 18.3 | |||
| miR-145-5p | Low | 50.1 13.9 | 0.654 | 48.0 17.1 | 0.896 |
| High | 50.1 15.6 | 47.8 18.7 | |||
| miR-182-5p | Low | 50.5 15.1 | 0.396 | 48.1 18.7 | 0.903 |
| High | 49.3 14.7 | 47.3 17.4 | |||
| miR-199a-5p | Low | 48.2 15.3 | 0.083 | 45.7 18.8 | 0.251 |
| High | 52.0 13.9 | 50.2 16.8 | |||
| miR-200a-3p | Low | 48.9 14.6 | 0.203 | 46.2 18.4 | 0.457 |
| High | 51.4 14.9 | 49.6 17.3 | |||
| miR-200b-3p | Low | 48.8 14.6 | 0.577 | 46.4 17.6 | 0.48 |
| High | 51.5 14.9 | 49.4 18.1 | |||
| miR-200c-3p | Low | 51.7 11.6 | 0.236 | 50.0 14.9 | 0.086 |
| High | 48.5 17.3 | 45.8 20.4 | |||
| miR-21-5p | Low | 52.1 13.1 | 0.146 | 50.6 15.7 | 0.089 |
| High | 48.1 16.0 | 45.1 19.6 | |||
0.05, statistical significance.
The univariate analysis indicated that the prognostic value of GC patients was related to tumor size, tumor infiltrating depth, vascular nerve infiltrating, number of metastatic lymph nodes and metastatic lymph nodes ration, TNM stage, Lauren classification and miR-141-3p. Multivariate analysis of OS and DFS revealed that metastatic lymph nodes ration, Lauren classification and miR-141-3p were independent prognostic factors (Table 7).
Table 7.
The COX risk model analysis of overall survival (OS) and disease-free survival (DFS) of miR-141-3p
| Variables | Overall survival (OS) | Disease free survival (DFS) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
| value | HR | 95%CI | value | value | HR | 95%CI | value | |
| Age | 0.288 | 0.689 | ||||||
| Gender | 0.106 | 0.108 | ||||||
| Tumor site | 0.858 | 0.768 | ||||||
| Tumor subtype | 0.063 | 0.057 | ||||||
| Pathological classifications | 0.054 | 0.106 | ||||||
| Tumor size | 0.002 | 1.421 | 0.665–3.036 | 0.364 | 0.002 | 1.589 | 0.770–3.276 | 0.21 |
| Histopathology classification | 0.0503 | 0.062 | ||||||
| Tumor infiltrating depth | 0.001 | 1.95 | 0.709–5.364 | 0.196 | 0.001 | 1.974 | 0.770–5.061 | 0.157 |
| Number of metastatic lymph nodes | 0.001 | 1.488 | 0.867–2.555 | 0.149 | 0.001 | 1.508 | 0.877–2.592 | 0.138 |
| Metastatic lymph nodes ration | 0.001 | 0.217 | 0.065–0.729 | 0.013 | 0.001 | 0.244 | 0.084–0.705 | 0.009 |
| AJCC 8th TNM stage | 0.001 | 0.757 | 0.131–4.384 | 0.756 | 0.001 | 0.941 | 0.194–4.571 | 0.94 |
| Vascular nerve infiltrating | 0.007 | 0.971 | 0.448–2.106 | 0.942 | 0.009 | 0.899 | 0.430–1.878 | 0.776 |
| adjuvant chemotherapy | 0.346 | 0.196 | ||||||
| Lauren classification | 0.012 | 0.562 | 0.347–0.911 | 0.019 | 0.004 | 0.502 | 0.314–0.804 | 0.004 |
| miR-141-3p | 0.007 | 0.408 | 0.169–0.988 | 0.047 | 0.036 | 0.563 | 0.258–1.228 | 0.149 |
0.05, statistical significance.
To explore the prognostic value of miR-141-3p in Lauren classification GC, we conducted univariate analysis of miR-141-3p among diffuse type, intestinal type and mixed type GC. As a consequence of univariate analysis, low miR-141-3p in diffuse type GC showed significant worse OS and DFS than high miR-141-3p ( 0.05). There was no significant difference of miR-141-3p in intestinal type and mixed type GC (Fig. 3).
Figure 3.
Overall survival (OS) and disease free survival (DFS) curves of low miR-141-3p and high miR-141-3p group plotted by the Kaplan-Meier method in (A–B) diffuse type, (C–D) intestinal type, (E–F) mixed type GC. Each value was calculated by the log-rank test. 0.05 was defined statistically significant.
4. Discussion
Currently, there are several classifications of GC, including histologically and molecular [30]. It is hard to say which classification is the best one due to the morphological characteristics of GC. Choosing one classification system is insufficient to provide precise prognosis for individual treatment. Lauren classification is the most-used one, however, there is still controversial whether Lauren classification plays better roles on prognosis performance of GC. In this study, we performed a retrospective study of 1144 patients who received radical gastrectomy. The results indicated that intestinal type GC (38.1%) had higher incidence than diffuse type GC (26.4%) which was line with previous study [31]. The difference with the study was that mixed type GC had higher incidence due to regional diversity. In diffuse type, the incidence was higher in younger ones than elder ones and females were predominant which may be related with oestrogen receptor [32]. The signet ring carcinoma, mucinous carcinoma, poor differentiated, deeper infiltration and higher metastatic lymph nodes ration were found in diffuse type GC than intestinal type GC, resulting in poor prognosis in diffuse type GC. The OS, DFS and 5-year survival of intestinal type GC had obvious survival advantages than diffuse type GC. According to multivariate analysis, Lauren classification was an independent prognostic factor and diffuse type GC was an independent risk factor for poor prognosis. In addition, our study showed that diffuse type GC was predominant in younger population in our country, the late TNM stage, large tumor size, lymphatic metastasis. It may be strongly correlated with heredity and may help to further understand the tumorigenesis of diffuse type GC.
Previous researches have confirmed that miRNAs have clinical implications in pathogenesis, diagnosis and prognosis of human cancers [11, 12, 33]. To have better understanding on targeted therapies in GC, we analyzed the expression levels of miRNAs in Lauren classification GC to seek for a prognostic biomarker. In our study, the expression levels of miR-141-3p were significantly down-regulated in diffuse type compared to intestinal type GC tissues using qRT-PCR. Multivariate analysis of OS and DFS revealed that metastatic lymph nodes ration, Lauren classification and miR-141-3p were independent prognostic factors. The low expression of miR-141-3p lead to worse OS and DFS in contrast to high miR-141-3p in diffuse type GC. MiR-141 was demonstrated as tumor suppressor through regulating target gene TAZ in GC. Inhibition of miR-141 resulted in promoting GC cells proliferation, invasion and migration in vitro [34]. MiR-141-3p, a member of miR-200 family, and its target gene ZEB1 and ZEB2 (E-cadherin transcriptional repressors) were associated with epithelial to mesenchymal transition (EMT). Enhanced expression of miR-141-3p suppressed EMT, while inhibition of miR-141-3p induced EMT. Downregulation of miR-141-3p played important roles on tumor progression [35]. The results were consistent with the low expression of miR-141-3p lead to worse OS and DFS in diffuse type GC. Using DIANA-miRPath v3.0 online software to assess miR-141-3p regulatory roles and the identification of controlled pathways [36]. As shown in Table S1, KEGG molecular pathways showed that miR-141-3p was associated with P53 signaling pathway. It was confirmed that overexpression P53 indicated the poor prognosis in GC, especially in diffuse type [37]. GO pathway analysis showed that miR-141-3p was associated with epidermal growth factor receptor signaling pathway, which was confirmed to be an independent prognostic factor influenced prognosis of GC [38].
There were some limitations in our study, the number of candidate miRNAs in predicting prognosis of GC and subtype of GC were relatively small. Also, neoadjuvant chemotherapy, Helicobacter Pylori infection and HER-2 gene amplification were not included in the prognostic factors.
5. Conclusions
Taken together, the present study suggested that Lauren classification was an independent prognostic factor in GC and the diffuse type was an independent risk factors for poor prognosis of GC. Lauren classification may help clinical doctors provide a reasonable plan for individual treatment combining with other clinicopathological characteristic. MiR-141-3p was an independent prognostic factor and may become a promising prognostic biomarker in Lauren classification GC. However, the mechanism of miR-141-3p in prognosis of Lauren classification still need further investigation.
Ethics statement
The medical ethical committee of First Affiliated Hospital of Nanjing Medical University. Written informed consent was obtained from all GC patients included in the study.
Author contributions
Conception: WC, ZH, MZ and SQ.
Interpretation or analysis of data: WC, QG, ZH, and ZH. WC, QG, YD and ZH performed the experiments.
Preparation of the manuscript: WC, QG and ZH.
Revision for important intellectual content: YZ, MZ and SQ.
Supervision: MZ and SQ.
Acknowledgments
The staff authors are sincerely grateful to all volunteers who participated in this follow-up study. The work was supported by the Jiangsu Provincial Medical Key Discipline (Grant number: ZDXK202235).
Conflict of interest
The authors have declared that no potential conflict of interest exists.
References
- [1]. Thrift A.P. and El-Serag H.B., Burden of gastric cancer, Clin Gastroenterol Hepatol 18 (2020), 534–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Zubarayev M., Min E.K. and Son T., Clinical and molecular prognostic markers of survival after surgery for gastric cancer: tumor-node-metastasis staging system and beyond, Transl Gastroenterol Hepatol 4 (2019), 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Machlowska J. et al., Gastric cancer: Epidemiology, risk factors, classification, genomic characteristics and treatment strategies, Int J Mol Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Ning F.L. et al., Prognostic value of modified Lauren classification in gastric cancer, World J Gastrointest Oncol 13 (2021), 1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Lauren P., The two histological main types of gastric carcinoma: diffuse and So-called Intestinal-type carcinoma. An attempt at a Histo-clinical classification, Acta Pathol Microbiol Scand 64 (1965), 31–49. [DOI] [PubMed] [Google Scholar]
- [6]. Quach D.T., Ha D.V. and Hiyama T., The endoscopic and clinicopathological characteristics of early-onset gastric cancer in Vietnamese patients, Asian Pac J Cancer Prev 19 (2018), 1883–1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Tang C.T. et al., Analysis of the incidence and survival of gastric cancer based on the Lauren classification: A large population-based study using SEER, Front Oncol 10 (2020), 1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Zurlo I.V. et al., Treatment of Locally advanced gastric cancer (LAGC): Back to Lauren’s classification in pan-cancer analysis era? Cancers (Basel) 12 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Oue N. et al., Molecular carcinogenesis of gastric cancer: Lauren classification, mucin phenotype expression, and cancer stem cells, Int J Clin Oncol 24 (2019), 771–778. [DOI] [PubMed] [Google Scholar]
- [10]. Venizelos A. et al., The molecular characteristics of high-grade gastroenteropancreatic neuroendocrine neoplasms, Endocr Relat Cancer 29 (2021), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Hill M. and Tran N., miRNA interplay: mechanisms and consequences in cancer, Dis Model Mech 14 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Lee Y.S. and Dutta A., MicroRNAs in cancer, Annu Rev Pathol 4 (2009), 199–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Zhang X. et al., microRNA-205 and microRNA-338-3p reduces cell apoptosis in prostate carcinoma tissue and LNCaP prostate carcinoma cells by directly targeting the B-Cell lymphoma 2 (Bcl-2) Gene, Med Sci Monit 25 (2019), 1122–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. He B. et al., miRNA-based biomarkers, therapies, and resistance in Cancer, Int J Biol Sci 16 (2020), 2628–2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Huang Z. et al., Serum miR-16 as a potential biomarker for human cancer diagnosis: results from a large-scale population, J Cancer Res Clin Oncol 145 (2019), 787–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Chang R.M. et al., miRNA-487a promotes proliferation and metastasis in hepatocellular carcinoma, Clin Cancer Res 23 (2017), 2593–2604. [DOI] [PubMed] [Google Scholar]
- [17]. Gillen S., Advancing early gastric cancer detection, FEBS Open Bio 11 (2021), 1812–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18]. Lorenzon L. et al., Down-regulated miRs specifically correlate with non-cardial gastric cancers and Lauren’s classification system, Journal of surgical oncology 116 (2017), 184–194. [DOI] [PubMed] [Google Scholar]
- [19]. Wang L. et al., Diagnostic and therapeutic potencies of miR-18a-5p in mixed-type gastric adenocarcinoma, J Cell Biochem 122 (2021), 1062–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Fu Z. et al., Construction of miRNA-mRNA-TF regulatory network for diagnosis of gastric cancer, Biomed Res Int 2021 (2021), 9121478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Das M.K. et al., Identification of endogenous controls for use in mirna quantification in human cancer cell lines, Cancer Genomics Proteomics 13 (2016), 63–68. [PubMed] [Google Scholar]
- [22]. Ahn D.H. et al., Association of the miR-146aC>G, miR-149T>C, miR-196a2T>C, and miR-499A>G polymorphisms with gastric cancer risk and survival in the Korean population, Mol Carcinog 52(Suppl 1) (2013), E39–51. [DOI] [PubMed] [Google Scholar]
- [23]. Azarbarzin S. et al., The value of miR-299-5p in diagnosis and prognosis of intestinal-type gastric adenocarcinoma, Biochem Genet 54 (2016), 413–420. [DOI] [PubMed] [Google Scholar]
- [24]. Cui L. et al., Gastric juice MicroRNAs as potential biomarkers for the screening of gastric cancer, Cancer 119 (2013), 1618–1626. [DOI] [PubMed] [Google Scholar]
- [25]. Fassan M. et al., The HER2-miR125a5p/miR125b loop in gastric and esophageal carcinogenesis, Hum Pathol 44 (2013), 1804–1810. [DOI] [PubMed] [Google Scholar]
- [26]. Li X. et al., Identification of new aberrantly expressed miRNAs in intestinal-type gastric cancer and its clinical significance, Oncol Rep 26 (2011), 1431–1439. [DOI] [PubMed] [Google Scholar]
- [27]. Ueda T. et al., Relation between microRNA expression and progression and prognosis of gastric cancer: a microRNA expression analysis, Lancet Oncol 11 (2010), 136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Wu Y.F. et al., Association of Polymorphisms in three pri-miRNAs that target pepsinogen C with the risk and prognosis of gastric cancer, Sci Rep 7 (2017), 39528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Yasui W. et al., Molecular pathology of gastric cancer: research and practice, Pathol Res Pract 207 (2011), 608–612. [DOI] [PubMed] [Google Scholar]
- [30]. Chia N.Y. and Tan P., Molecular classification of gastric cancer, Ann Oncol 27 (2016), 763–769. [DOI] [PubMed] [Google Scholar]
- [31]. Chen Y.C. et al., Clinicopathological variation of Lauren classification in gastric cancer, Pathol Oncol Res 22 (2016), 197–202. [DOI] [PubMed] [Google Scholar]
- [32]. Shimada S. et al., Identification of selective inhibitors for diffuse-type gastric cancer cells by screening of annotated compounds in preclinical models, Br J Cancer 118 (2018), 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Ali Syeda Z. et al., Regulatory Mechanism of MicroRNA Expression in Cancer, Int J Mol Sci 21 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34]. Zuo Q.F. et al., MicroRNA-141 inhibits tumor growth and metastasis in gastric cancer by directly targeting transcriptional co-activator with PDZ-binding motif, TAZ, Cell Death Dis 6 (2015), e1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Gregory P.A. et al., The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1, Nat Cell Biol 10 (2008), 593–601. [DOI] [PubMed] [Google Scholar]
- [36]. Vlachos I.S. et al., DIANA-miRPath v3.0: deciphering microRNA function with experimental support, Nucleic Acids Res 43 (2015), W460–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37]. Kim K.W. et al., Different effects of p53 protein overexpression on the survival of gastric cancer patients according to Lauren histologic classification: a retrospective study, Gastric Cancer 24 (2021), 844–857. [DOI] [PubMed] [Google Scholar]
- [38]. Xu W. et al., Human epidermal growth factor receptor 2 expressions and Janus-activated kinase/signal transducer and activator of transcription 3-suppressor of cytokine signaling 3 pathway may be associated with clinicopathological features and prognosis of gastric cancer, J Cancer Res Ther 14 (2018), S311–S318. [DOI] [PubMed] [Google Scholar]