Abstract
A generation ago thrombolytic therapy led to a paradigm shift in myocardial infarction (MI), from Q-wave/non-Q-wave to ST-segment elevation MI (STEMI) vs non-STEMI. Using STE on the electrocardiogram (ECG) as a surrogate marker for acute coronary occlusion (ACO) allowed for rapid diagnosis and treatment. But the vast research catalyzed by the STEMI paradigm has revealed increasing anomalies: 25% of “non-STEMI” have ACO with delayed reperfusion and higher mortality. Studying these limitations has given rise to the occlusion MI (OMI) paradigm, based on the presence or absence of ACO in the patient rather than STE on ECG. The OMI paradigm shift harnesses advanced ECG interpretation aided by artificial intelligence, complementary bedside echocardiography and advanced imaging, and clinical signs of refractory ischemia, and offers the next opportunity to transform emergency cardiology and improve patient care. This State-of-the-Art Review examines the paradigm shifts from Q wave to STEMI to OMI.
Key words: acute myocardial infarction, electrocardiogram, occlusion myocardial infarction, ST-segment elevation myocardial infarction
Highlights
-
•
The STEMI paradigm transformed emergency cardiology, but there is increasing recognition of its limitations.
-
•
STEMI criteria is a poor surrogate marker for acute coronary occlusion, leading to delayed reperfusion.
-
•
Evidence-based advances can identify OMI not meeting STEMI criteria, and false positive STEMI.
-
•
The OMI paradigm harnesses advanced ECG interpretation aided by artificial intelligence, echocardiography, and advanced imaging.
Central Illustration
“Scientific revolutions are inaugurated by a growing sense, again often restricted to a narrow subdivision of the scientific community, that an existing paradigm has ceased to function adequately in the exploration of an aspect of nature to which that paradigm itself had previously led the way.”
—Thomas Kuhn1
The concept of a “paradigm shift” was introduced by the philosopher of science Thomas Kuhn, and detailed in his landmark book “The Structure of Scientific Revolutions.”1 As he explained, scientific progress requires paradigms (or theoretical models) to guide problem-solving activities—including defining which problems to address, and which methods to address them. While a paradigm can lead to progress, these same advances reveal anomalies that cannot be explained by the paradigm itself. Some anomalies are ignored, others are absorbed into the paradigm, but eventually they provoke a crisis and search for a new paradigm. Scientific progress, therefore, does not just incrementally advance but goes through periodic paradigm shifts that question previous assumptions and replace them with new theoretical models and new methods of investigation and practice.
For a generation, the ST-segment elevation (STE) myocardial infarction (MI) paradigm has led the way in the classification and treatment of MI, but recently the occlusion MI (OMI) paradigm has emerged.2 A 2021 article in Circulation explained: “Although the STEMI/non-STEMI dichotomous classification scheme has served the cardiology community well over the last several decades, it is likely that the constituent pathophysiological event determining prognosis and natural history is acute vessel occlusion itself rather than STE.”3 As the new American College of Cardiology expert consensus on acute chest pain warns, “The application of STEMI electrocardiogram (ECG) criteria on a standard 12-lead ECG alone will miss a significant minority of patients with acute coronary occlusion (ACO).”4
Despite nearly 30 years of the STEMI paradigm, de Alencar found only 3 studies verified the diagnostic accuracy of current STEMI criteria for the underlying finding of ACO, and pooled sensitivity was only 43.6%.5 Conversely, most cardiac centers experience significantly high rates of false positive STEMI, including necessary cath lab activations for diagnostic clarification but also avoidable cath lab activations causing patient harm and wasted resources.6
In this State-of-the-Art Review paper, we review the paradigm shifts from Q-wave to STEMI to OMI through the phases described by Kuhn. This can help explain the paradox of how the STEMI paradigm, which was once a major advance in our understanding and treatment of MI, is increasingly limited. It also explains how the study of these limitations has given rise to the OMI paradigm, the opportunities it offers to transform emergency cardiology and patient care, and the challenges it faces (Figure 1).
Figure 1.
The Paradigm Shifts From Q-Wave to ST-Segment Elevation to Occlusion MI
Myocardial infarction paradigm evolution reflects Thomas Kuhn's concept of scientific progress, emphasizing the cyclical nature of normal science leading to the discovery of anomalies and then crises that lead to paradigm shifts. Thrombolytic therapy led to the STEMI paradigm, which replaced the previous Q-wave paradigm. As understanding and technology advanced, anomalies within the STEMI paradigm were recognized, inducing a crisis in diagnostic accuracy and treatment effectiveness. This crisis has given rise to the OMI paradigm, challenging the STEMI/non-STEMI dichotomy. MI = myocardial infarction; OMI = occlusion myocardial infarction; STEMI = ST-segment elevation myocardial infarction.
STEMI paradigm shift
“We must recognize how very limited in both scope and precision a paradigm can be at the time of its appearance. Paradigms gain their status because they are more successful than their competitors in solving a few problems that the group of practitioners has come to recognize as acute.”1
Before thrombolytics, patients with MI were admitted to hospital and retrospectively classified by ECG as Q-wave vs non-Q-wave. The STEMI paradigm was a major advance over the Q-wave paradigm in providing emergent reperfusion, initially with thrombolytics and then with percutaneous coronary intervention (PCI). But the evidence for STEMI criteria as a surrogate marker for ACO was very limited in scope and precision.
The 1994 Fibrinolytic Therapy Trialists’ meta-analysis has been interpreted to imply that only patients whose ECGs meet STEMI criteria benefit from emergent reperfusion.7 However, these trials included patients merely with suspected MI, with limited or no ECG requirements, treated with streptokinase (almost half at more than 6 hours from symptom onset), and with MI determined by creatine kinase-MB without any angiographic outcome. ECG interpretation was crudely separated into “ST-segment elevation” or “bundle branch block,” “ST-segment depression,” or “normal,” each of which was poorly defined, and 4 trials had no ECG requirements for enrollment or had them poorly specified.7 The study authors themselves cautioned about denying reperfusion in patients without STE, stating that “numbers of death among such patients were relatively small and data-dependent emphasis on the lack of benefit in these subgroups may seriously mislead.”7
Kuhn explains that while paradigms often begin as flexible, they become rigid over time. The 1996 Acute MI guidelines advised thrombolytics for hyperacute T waves or ST-segment depression (STD) V1-V4 from posterior MI, noting that “it should be clear that certain cases require experienced interpretation of the ECG before withholding reperfusion therapy.”8 But by 1999 the Acute MI guidelines advised clinicians to “classify patients as those with STE or left bundle branch block (LBBB) (acute reperfusion indicated) and those with nondiagnostic ECGs” even if the latter included “direct posterior infarctions caused by circumflex artery occlusion.”9 Then the STEMI paradigm formally emerged, with separate guidelines for 2 supposedly distinct disease entities: non-STEMI vs STEMI. The former included an image to visualize the STEMI/non-STEMI dichotomy, with STEMI showing an occluded artery and non-STEMI a nonocclusive thrombus.10
Despite its appeal, this image unfortunately represented a clear deviation from the actual evidence. The STE millimeter criteria based on age and sex originated from studies comparing healthy individuals to those who had MI measured by creatine kinase-MB and, despite this, were now being recommended for the differentiation of MI with and without ACO, even when there was no real evidence from any angiographic data or even thrombolytic trials.11
The normal science of the STEMI paradigm
“Normal science, the puzzle-solving activity we have just examined, is a highly cumulative enterprise, eminently successful in its aim, the steady expansion of scientific knowledge…Normal science does not aim at novelties of fact or theory and, when successful, finds none.”1
Most science takes place during the long periods between paradigm shifts, in what Kuhn called “normal science.” This takes the prevailing paradigm for granted and seeks to extend knowledge and solve puzzles within this framework. The normal science of the STEMI paradigm has been geared toward solving the problem of how, in patients with ACO as identified by STEMI criteria on the ECG, to best reperfuse the arteries and how to reduce reperfusion delays.
The STEMI paradigm has been eminently successful in its aim. For health providers, it provided a surrogate marker for ACO that could be easily identified and automated by computer interpretation. This, in turn, catalyzed vast research on reperfusion strategies and techniques, resulting in myriad advances in angiography, stenting, and adjunctive medications. For the health care system, the understanding that time is myocardium led to campaigns to reduce reperfusion delays, with interventions guided by quality metrics and feedback on door-to-balloon time.12 This has helped reshape emergency cardiovascular care and interdisciplinary collaboration: emergency department nurse rapid ECG acquisition, emergency physician-initiated cath lab activation, paramedics bypassing the emergency department, and interventional cardiologists rapidly assembling their teams. For patients with ACO, the results have been an impressive improvement in reperfusion times, but only for those whose ECGs met STEMI criteria. Two quality improvement goals within the STEMI paradigm are to reduce reperfusion delays for true positive STEMI, while reducing false positive STEMI.6
But a novelty that is never found within the STEMI paradigm is false negatives, and this is due to what we call the “no false negative paradox.”2 If the ECG meets STEMI criteria and the patient has an ACO, then this is a true positive STEMI, and if there is no culprit, this is a false positive STEMI. But if the ECG does not meet STEMI criteria and the patient does have an ACO, this is not considered a false negative STEMI because it is, by definition, still a “non-STEMI.” As a result, the patient will be denied emergent reperfusion, and regardless of patient outcome (including angiography, peak troponin, or regional wall motion abnormalities), the patient will still be discharged as “non-STEMI.” As McLaren showed, discharge diagnoses change to highlight false positive STEMI but not false negative STEMI,13 which prevents providers from learning from missed occlusions. Ultimately, this paradox highlights the severe form of incorporation bias14 upon which the STEMI paradigm is built: the STE is not only the index test but also the reference standard (regardless of how deadly or intervenable the patient’s culprit lesion was) (Figure 2).
Figure 2.
Incorporation Bias
Incorporation bias reflects a critical error in the diagnostic methodology, where the index test, which is intended for examination, is mirrored by the reference test, in whole or in part. In the context of myocardial infarction, such bias skews the diagnostic framework, obscuring false negative results and perpetuating the “no-false negative paradox” within the STEMI paradigm. Abbreviation as in Figure 1.
The dichotomized paradigm regards non-STEMIs as ineligible for emergent reperfusion, regardless of whether the patient has an ACO. This is a misreading of both the initial thrombolytic trials, and of subsequent studies on reperfusion. Most non-STEMIs are nonocclusive myocardial infarctions (NOMIs), so studies looking at all non-STEMI will miss the high-risk patients who do have ACO. Many non-STEMI trials also do not provide the kind of timely emergent reperfusion that would be required to show benefit. For example, the TIMACS (Timing of Intervention in Acute Coronary Syndromes) trial studied “early” invasive intervention in a group of non-STEMI and unstable angina patients, with a median time of 16 hours, by which any fully ischemic myocardium would have suffered irreversible infarction.15 The next largest trial (VERDICT [Very EaRly vs Deferred Invasive evaluation using Computerized Tomography])16 also included unstable angina and found a benefit for high-risk non-STEMI patients treated within 4.7 hours. The RIDDLE-NSTEMI (Randomized Study of Immediate Versus Delayed Invasive Intervention in Patients With Non–ST-Segment–Elevation Myocardial Infarction) study found lower mortality in patients who received immediate reperfusion.17 Non-STEMI trials also exclude patients with refractory ischemia or hemodynamic/electrical instability because current guidelines mandate immediate angiography in this group. Nevertheless, the “non-STEMI” designation of these patients leads to delayed reperfusion: a recent study found only 6.4% of very high-risk non-STEMI undergo guideline-recommended angiography within 2 hours.18
The risk of thrombolytics is far higher than the risk of angiography ± PCI. Using thrombolytic trials to guide the use of angiography/PCI ignores this risk/benefit difference. Emergent angiography also plays a key role in many acceptable false positive STEMI mimics that can only be diagnosed after ACO has been excluded, including myocarditis, takotsubo, coronary vasospasm, and spontaneous coronary artery dissection.6
STEMI anomalies
“Normal science often suppresses fundamental novelties because they are necessarily subversive of its basic commitments…The source of resistance is the assurance that the older paradigm will ultimately solve all its problems, that nature can be shoved into the box the paradigm provides.”1
Paradigms make normal science possible, but this inevitably leads to the discovery of anomalies that do not fit within the paradigm. When this happens, the normal response is to either reject these anomalies or try to absorb them into the prevailing paradigm. Even before the STEMI paradigm had fully emerged there were 3 early anomalies: anterior STD from posterior OMI,19 subtle inferior OMI with reciprocal STD in aVL,20 and Sgarbossa criteria for OMI in the presence of LBBB.21
A 1994 report on emergency department diagnosis of STEMI described the challenge of ECG interpretation for borderline STE, and differentiating STEMI from early repolarization, pericarditis, LBBB, and old MI with persisting STE.22 In other words, by dichotomizing ECGs by presence vs absence of STE, STEMI criteria cannot differentiate between different types of STE, or identify STE secondary to an abnormal QRS with superimposed primary ischemic elevation.
As a result of the vast research catalyzed by the STEMI paradigm, years of angiographic data have demonstrated the chasm between the STEMI/non-STEMI dichotomy and the underlying pathology of OMI (Figure 3).
Figure 3.
The STEMI/Non-STEMI False Dichotomy
STEMI criteria are supposed to differentiate patients with ACO from those without. But up to 25% of code STEMI have No ACO: these are appropriately termed false positives, receive a different discharge diagnosis, and are identified as a quality improvement issue. But at least 25% of “Non-STEMI” Have ACO on delayed angiography, with higher mortality. These should be recognized as false negative STEMI and identified as a quality improvement issue, but instead they are still diagnosed as “Non-STEMI.” This is what we call the “no false negative paradox”: In the STEMI paradigm, there cannot be a false negative diagnosis. ACO = acute coronary occlusion; ECG = electrocardiogram; other abbreviation as in Figure 1.
A prospective analysis of STEMI criteria on the initial ECG found they were only 35% sensitive for adjudicated STEMI23 (including serial ECGs) and 21% for OMI24 (including non-STEMI with OMI on angiogram) and the recent meta-analysis found a pooled sensitivity of only 43.6% of STEMI criteria for OMI.5 Large meta-analyses of non-STEMI patients have found that 25% have a totally occluded artery25 and 34% have Thrombolysis in Myocardial Infarction (TIMI) flow grade 0 to 1,26 with nearly double the mortality of non-STEMI without OMI, in spite of younger age and fewer comorbidities.
“Failure of existing rules is the prelude to a search for new ones.”1
Emergency physician Dr Stephen Smith led the search for new rules for occlusion, and answered the questions raised by the 1994 report: a formula to differentiate between early repolarization and subtle LAD occlusion, reciprocal STD in aVL to identify subtle inferior OMI and exclude pericarditis, T/QRS ratio to differentiate between old LV aneurysm and anterior STEMI with Q-wave, and the Smith-Modified Sgarbossa criteria to identify OMI in LBBB and paced rhythms.27
Throughout the STEMI paradigm, cardiologists and emergency physicians have continued to find patterns of occlusion and reperfusion that do not fit into the STEMI box28: Wellens reperfusion T-wave inversion at risk for reocclusion, de Winter T waves indicating LAD occlusion, “South African flag sign” to identify first diagonal occlusion, Aslanger pattern of inferior OMI with concomitant high-grade stenosis, ischemic STD maximum V1-V4 to identify posterior OMI and differentiate it from subendocardial ischemia. These advances reveal the limitations of the STEMI paradigm (Tables 1 and 2).
Table 1.
STEMI Criteria Vs Evidence-Based OMI Findings
| STEMI Criteria | OMI |
|---|---|
| Defined by ECG | Clinical: ACS with refractory ischemia, hemodynamic or electrical instability is indication for immediate angiography regardless of ECG Echo: bedside ultrasound can identify new regional wall motion abnormalities |
| Must have ST-segment elevation | Ischemic STDmaxV1-4 identifies posterior OMI with 97% specificity |
| Hyperacute T waves (large relative to QRS, look inflated with large area under the curve) | |
| de Winter T waves (ST-segment depression rising into hyperacute T waves) | |
| Wellens waves (reperfusion T-wave inversion) identifies spontaneous reperfusion with risk of reocclusion | |
| ST-segment elevation in at least 2 contiguous leads | South African flag pattern (STE in noncontiguous leads V2 and aVL, with inferior reciprocal STD) identifies first diagonal occlusion |
| Aslanger pattern (STE in III with reciprocal STD I/aVL, ST in V1>V2 and STD V5-V6) indicates inferior OMI with concomitant critical stenosis | |
| ST-segment elevation must be at least 1 mm | Pericarditis results in false positive STEMI while many inferior OMI are false negative STEMI, but primary STD-aVL is 99% sensitive for inferior OMI and excludes pericarditis |
| ST-segment elevation in V2-V3 must be at least 1.5 mm in women, 2 mm in men >40, and 2.5 in men <40 | Early repolarization results in false positive STEMI while many LAD occlusion are false negative STEMI. Any ischemic abnormality (Q wave in V2-V3, terminal QRS distortion in V2-V3, convex ST-segment, precordial STD or TWI, or inferior reciprocal change) excludes BER as a cause of anterior STE. The 4 variable formula (using QRS in V2, STE in V3, R in V4, and QTc) differentiates subtle LAD occlusion from BER |
| STEMI defined as ST-segment elevation in the absence of LBBB | Smith-Modified Sgarbossa criteria (any lead with concordant STE, concordant STD V1-V3, or discordant STE/S >25%) identifies OMI in LBBB or ventricular paced rhythm |
| Q waves regarded as old or completed infarcts | Anterior QS waves with ST-segment elevation can be from LV aneurysm (resulting in false positive STEMI) or acute LAD occlusion (resulting in false negative STEMI), but any lead V1-V4 with T/QRS >0.36 identifies acute LAD occlusion and differentiates it from LV aneurysm |
BER = benign early repolarization; ECG = electrocardiogram; LBBB = left bundle branch block; OMI = occlusion myocardial infarction; QTc = corrected QT interval; STD = ST-segment depression; STEMI = ST-segment elevation myocardial infarction.
Table 2.
Examples of Evidence-Based OMI Findings
| STEMI(−) OMI from first diagonal occlusion, with South African flag pattern |  |
| STEMI(−) OMI from RCA occlusion with concomitant LAD stenosis, with Aslanger pattern. | 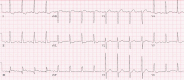 |
| STEMI(−)OMI with de Winter pattern | 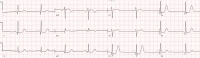 |
| STEMI(−)OMI. There are anterior hyperacute T waves and early Q waves, from LAD occlusion |  |
| STEMI(−)OMI interpreted as normal, but there’s primary STD-aVL reciprocal to inferior hyperacute T waves, and STD V2, from RCA occlusion. | 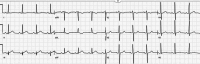 |
| False positive STEMI. Cath lab activated for anterior STEMI, but there’s QS waves with small T/QRS ratio from old LV aneurysm |  |
| STEMI(−)OMI in paced rhythm, with, Smith-Modified Sgarbossa (STE/S >25%) in III from RCA occlusion | 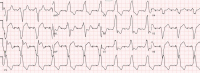 |
| STEMI(−)OMI. Patient with prior MI presented with chest pain, without any changes on serial ECG. But there was refractory ischemia and new regional wall motion abnormality, from LAD occlusion with huge infarct (peak troponin I 225,000 ng/L) |
 
|
LAD = left anterior descending; RCA = right coronary artery; abbreviations as in Table 1.
STEMI crisis
“By proliferating versions of the paradigm, crisis loosens the rules of normal puzzle-solving in ways that ultimately permits a new paradigm to emerge…The proliferation of competing articulations, the willingness to try anything, the expression of explicit discontent, the recourse to philosophy and to debate over fundamentals, all these are symptoms of a transition from normal to extraordinary research.”1
The increasing anomalies in the STEMI paradigm have given rise to proliferating versions of the paradigm, with terms like “subtle STEMI,” “semi-STEMI,” and “STEMI-equivalents.”6 Now this crisis is reflected in official guidelines: the 2018 Fourth Universal Definition of MI summarized multiple advances in ECG interpretation, including lesser degrees of STE, reciprocal STD, hyperacute T waves, acute Q waves, de Winter T waves, and anterior STD.29 But the conclusion of this document dares not deviate from the paradigm: “it is essential to integrate the ECG findings with the aim of classifying type 1 MI into STEMI or non-STEMI in order to establish the appropriate treatment according to current guidelines.”29 Similarly, for the first time, the 2023 European Society of Cardiology acute coronary syndromes guidelines discuss STEMI and non-STEMI within the same document because of the shared pathophysiology. But these new guidelines still dichotomize acute coronary syndromes by STEMI vs non-STEMI, not only as the initial “working diagnosis” but also as the “final diagnosis.”30 This will ensure that non-STEMI with OMI will continue to be classified, even retrospectively, as “non-STEMI” rather than being identified as missed STEMI(−)OMI.13
In response to the STEMI crisis, we can see debate over fundamentals. In 2018, Smith and Meyers published OMI Manifesto31 which coined the term Occlusion MI and in subsequent articles with Aslanger called for the “OMI paradigm shift.”32,33 There are competing articulations in multiple cardiology journals questioning the STEMI/non-STEMI dichotomy and the need to shift focus to ACOs.3,34,35
We can also see the new science of OMI emerging from the study of STEMI anomalies. OMI represents acute vessel occlusion or near occlusion without sufficient collateral circulation, which puts myocardium at imminent risk of infarction. Because 15% to 20% of true STEMI have TIMI flow grade 3 by the time of angiogram due to spontaneous reperfusion, a common research definition of OMI also includes acute culprit lesions with very high peak troponins, defined as fourth-generation troponin I >10 ng/mL. Therefore OMI has been defined2: as 1) acute culprit lesion with reduced flow; 2) acute culprit lesion with normal TIMI flow grade 3 and highly elevated peak troponin; and for patients who do not undergo angiogram and; 3) highly elevated peak troponin and new regional wall motion abnormality. As with the STEMI literature, there are some variations across studies36,37 in what TIMI scores are used for reduced flow, and what constitutes “highly elevated” troponin, but the concept is consistent and the outcome measure far more accurate and patient-centered than falsely dichotomizing by STE millimeter criteria.
Comparing the STEMI vs OMI paradigms, Meyers2 found that STEMI criteria missed 40% of OMI (defined as acute culprit with TIMI flow grade 0-2, or TIMI flow grade 3 flow with troponin T >1.0 ng/L), and that STEMI(−)OMI patients experienced preventable delayed to reperfusion and peak troponins similar to STEMI(+)OMIs. Abusharekh found 29% of non-STEMI had OMI (defined as TIMI flow grade 0-1 flow or TIMI flow grade 2-3 flow with troponin >100-fold upper reference limit), with rates of cardiogenic shock, no reflow and long-term mortality similar to STEMI and much worse than non-STEMI without OMI.37
OMI paradigm shift
“The decision to reject one paradigm is always simultaneously the decision to accept another, and the judgment leading to that decision involves the comparison of both paradigms with nature and with each other.”1
The study of STEMI anomalies has given rise to the new OMI paradigm (Figure 4). The STEMI and OMI paradigms are now being directly compared with nature and with each other. The OMI timeline summarizes this growing body of literature.38
Figure 4.
Occlusion MI Paradigm
The OMI paradigm classifies MI by the presence or absence of occlusion in the patient. As with other paradigm shifts, this incorporates the previous paradigm (eg, STEMI(+)OMI) while providing evidence-based advances for STEMI(−)OMI that not only add to ECG interpretation but change the diagnostic focus from ECG to patient. Abbreviations as in Figures 1 and 3.
Aslanger published the DIFOCCULT (DIagnostic accuracy oF electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction) trial,36 in which 28% of non-STEMI were reclassified as “OMI” by blinded ECG interpretation alone. These non-STEMI-OMI patients had much larger infarcts and higher 800-day mortality (10.6% vs 4.4%). Similarly, Meyers39 found that emergency physicians trained in the OMI paradigm could identify OMI with twice the sensitivity and preserved specificity as STEMI criteria, and significantly earlier.
Citing the work of Meyers,2 the new American College of Cardiology expert consensus on acute chest pain warns that STEMI criteria “will miss a significant minority of patients with ACO” and that providers need to look for subtle “ECG signs of vessel occlusion”—like hyperacute T waves, subtle STE with reciprocal change, anterior depression from posterior OMI, and Smith-Modified Sgarbossa criteria.4 This is a crucial acknowledgment of the difference between the limited surrogate marker of STEMI criteria and the underlying pathology of OMI, and promotion of evidence-based ECG advances.
While STEMI criteria are outdated, the paradigm built a reperfusion network and research infrastructure that can be harnessed for the OMI paradigm. Just as the STEMI paradigm took time to implement,12 the OMI paradigm requires a process of interdisciplinary collaboration guided by reperfusion goals, quality improvement (QI) interventions, new technology, and ongoing research (Central Illustration).
Central Illustration.
From ST-Segment Elevation MI to occlusion MI
The new paradigm shift in acute myocardial infarction. AI = artificial intelligence; CT = computed tomography; NOMI = nonocclusive myocardial infarction; other abbreviations as in Figure 1.
First, the shared goal needs to shift from identifying STEMI criteria on ECG to identifying patients with OMI, to avoid the resource strain of false positive STEMI and the myocardial loss due to failure to identify OMI because of absence of STEMI criteria.25,26
Secondly, this new goal requires a shift in patient-centered outcome measures. Rather than classifying patients as STEMI vs non-STEMI, the medical record needs to reflect actual patient outcome of OMI vs NOMI—based on available angiogram, echocardiogram, and peak troponin results.2,36,37 For QI and education, “false positive STEMI” would remain similar: absence of occlusion6; but cases of OMI which do not get emergent reperfusion, which at the present time receive a discharge diagnosis of “non-STEMI” would instead be identified as “false negative STEMI,” or missed OMI.13
Thirdly, audit and feedback on all these cases requires a shift in QI interventions to reflect the new paradigm.40 While the language of “code STEMI” has ingrained protocols based on STE false dichotomy, a “code OMI” would shift the clinical decision-making to include advanced ECG interpretation,27,28 clinical signs such as refractory ischemia,18 point of care ultrasound for regional wall motion abnormalities,41 or advanced imaging including emergent CT coronary angiogram.42
Fourth, deploying these new diagnostic techniques requires not only widespread educational interventions but also a technological shift in automation. Conventional computer automation can even apply the interpretation of “normal” to ECGs that are diagnostic of OMI.43 But learning subtle ECG signs of occlusion and reperfusion is challenging, and these advances need to be made widely available to paramedics, nurses, emergency physicians, and cardiologists. This is where new generation artificial intelligence plays a key role, which has already been shown to be superior to STEMI criteria, with double the sensitivity and preserved specificity of STEMI criteria.44,45 Table 3 summarizes studies comparing paradigms.
Table 3.
Comparison of STEMI and OMI Paradigms
| First Author | OMI Interpretation of ECG | Patient Outcome |
|---|---|---|
| Aslanger et al36 | OMI expert: blinded interpretation identify 28.2% of non-STEMI identified by as having ACO | Non-STEMI identified as ACO had higher myocardial damage, in-hospital and long-term mortality than non-STEMI without ACO |
| Meyers et al39 | OMI expert: blinded interpretation for OMI double sensitivity compared with STEMI (86% vs 41%) and preserved specificity (94% vs 91%) | STEMI(+) and STEMI(−)OMI similar outcomes STEMI(−) OMI could be identified an average of 3 h earlier using OMI criteria |
| Al-Zaiti et al44 | AI: 86% sensitivity and 98% specificity for OMI, superior to practicing clinicians (58% and 93%) and conventional computer (79% and 80%) | AI could reclassify one in three patients with chest pain |
| Herman et al45 | AI: 80.6% sensitivity and 93.7% specificity for OMI, superior to STEMI criteria (32.5% sensitivity) with preserved specificity (97.7%) | AI could detect OMI 3 hours earlier |
ACO = acute coronary occlusion; AI = artificial intelligence; other abbreviations as in Table 1.
Fifth, a shift in research will be required to reflect and accelerate the new paradigm. Current STEMI databases and national registries do not include non-STEMI patients with OMI and are a barrier to QI interventions for STEMI(−)OMI. For instance, it is impossible to research the sensitivity of the ECG for OMI using these databases, and studies of the sensitivity for STEMI show exaggerated sensitivities because the test is the definition. But a shift to OMI/NOMI classification and trials on emergent reperfusion for all OMI would result in a new generation of databases that can generate new and evolving research questions.
Conclusions
The STEMI paradigm was a major step forward a generation ago but has revealed increasing anomalies that cannot be solved within the paradigm itself. The study of these anomalies has given rise to the OMI paradigm. Just as the STEMI paradigm once reshaped emergency cardiology, the OMI paradigm offers the next opportunity to revolutionize patient care.
Funding support and author disclosures
Dr Meyers has been a paid consultant to Rapid AI and Baxter/Veritas and holds stocks from Powerful Medical. Dr Smith has received personal fees from Cardiologs, HEARTBEAM, Rapid AI, and Baxter/Veritas; and holds stocks from Powerful Medical and Pulse AI. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
References
- 1.Kuhn T.S. University of Chicago Press; 1970. The Structure of Scientific Revolutions. [Google Scholar]
- 2.Meyers H.P., Bracey A., Lee D., et al. Comparison of the ST-elevation myocardial infarction (STEMI) vs. NSTEMI and occlusion MI (OMI) vs. NOMI paradigms of acute MI. J Emerg Med. 2021;60:273–284. doi: 10.1016/j.jemermed.2020.10.026. [DOI] [PubMed] [Google Scholar]
- 3.Sankardas M.A., Ramakumar V., Farooqui F.A. Of occlusions, inclusions, and exclusions: time to reclassify infarctions? Circulation. 2021;144:333–335. doi: 10.1161/CIRCULATIONAHA.121.055827. [DOI] [PubMed] [Google Scholar]
- 4.Kontos M.C., de Lemos J.A., Deitelzweig S.B., et al. 2022 ACC expert consensus decision pathway on the evaluation and disposition of acute chest pain in the emergency department: a report of the American College of cardiology solution set oversight committee. J Am Coll Cardiol. 2022;80:1925–1960. doi: 10.1016/j.jacc.2022.08.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Alencar J.N., Scheffer M.K., Correia B.P., Franchini K.G., Felicioni S.P., Marchi M.F.N.D. Systematic review and meta-analysis of diagnostic test accuracy of ST-segment elevation for acute coronary occlusion. Int J Cardiol. 2024;402:131889. doi: 10.1016/j.ijcard.2024.131889. [DOI] [PubMed] [Google Scholar]
- 6.Rokos I.C., French W.J., Mattu A., et al. Appropriate cardiac cath lab activation: optimizing electrocardiogram interpretation and clinical decision-making for acute ST-elevation myocardial infarction. Am Heart J. 2010;160:995–1003. doi: 10.1016/j.ahj.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 7.Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomised trials of more than 1000 patients. Fibrinolytic Therapy Trialists’ (FTT) Collaborative. Lancet. 1994;343:311–322. [PubMed] [Google Scholar]
- 8.Ryan T.J., Anderson J.L., Antman E.M., et al. ACC/AHA guidelines for the management of patients with acute myocardial infarction. A report of the American College of cardiology/American heart association task force on practice guidelines (committee on management of acute myocardial infarction) J Am Coll Cardiol. 1996;28:1328–1428. doi: 10.1016/s0735-1097(96)00392-0. [DOI] [PubMed] [Google Scholar]
- 9.Ryan T.J., Antman E.M., Brooks N.H., et al. 1999 update: ACC/AHA guidelines for the management of patients with acute myocardial infarction: executive summary and recommendations: a report of the American College of cardiology/American heart association task force on practice guidelines (committee on management of acute myocardial infarction) Circulation. 1999;100:1016–1030. doi: 10.1161/01.cir.100.9.1016. [DOI] [PubMed] [Google Scholar]
- 10.Braunwald E., Antman E.M., Beasley J.W., et al. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: executive summary and recommendations. A report of the American College of Cardiology/American Heart Association task force on prac. Circulation. 2000;102:1193–1209. doi: 10.1161/01.cir.102.10.1193. [DOI] [PubMed] [Google Scholar]
- 11.Macfarlane P.W., Browne D., Devine B., et al. Modification of ACC/ESC criteria for acute myocardial infarction. J Electrocardiol. 2004;37(Suppl):98–103. doi: 10.1016/j.jelectrocard.2004.08.032. [DOI] [PubMed] [Google Scholar]
- 12.Krumholz H.M., Bradley E.H., Nallamothu B.K., et al. A campaign to improve the timeliness of primary percutaneous coronary intervention: door-to-Balloon: an Alliance for Quality. JACC Cardiovasc Interv. 2008;1:97–104. doi: 10.1016/j.jcin.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 13.McLaren J.T.T., El-Baba M., Sivashanmugathas V., Meyers H.P., Smith S.W., Chartier L.B. Missing occlusions: quality gaps for ED patients with occlusion MI. Am J Emerg Med. 2023;73:47–54. doi: 10.1016/j.ajem.2023.08.022. [DOI] [PubMed] [Google Scholar]
- 14.Kea B., Hall M.K., Wang R. Recognising bias in studies of diagnostic tests part 2: interpreting and verifying the index test. Emerg Med J. 2019;36:501–505. doi: 10.1136/emermed-2019-208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta S.R., Granger C.B., Boden W.E., et al. Early versus delayed invasive intervention in acute coronary syndromes. N Engl J Med. 2009;360:2165–2175. doi: 10.1056/NEJMoa0807986. [DOI] [PubMed] [Google Scholar]
- 16.Kofoed K.F., Kelbæk H., Hansen P.R., et al. Early versus standard care invasive examination and treatment of patients with non-ST-segment elevation acute coronary syndrome. Circulation. 2018;138:2741–2750. doi: 10.1161/CIRCULATIONAHA.118.037152. [DOI] [PubMed] [Google Scholar]
- 17.Milosevic A., Vasiljevic-Pokrajcic Z., Milasinovic D., et al. Immediate versus delayed invasive intervention for non-STEMI patients: the RIDDLE-NSTEMI study. JACC Cardiovasc Interv. 2016;9:541–549. doi: 10.1016/j.jcin.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 18.Lupu L., Taha L., Banai A., et al. Immediate and early percutaneous coronary intervention in very high-risk and high-risk non-ST segment elevation myocardial infarction patients. Clin Cardiol. 2022;45:359–369. doi: 10.1002/clc.23781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boden W.E., Kleiger R.E., Gibson R.S., et al. Electrocardiographic evolution of posterior acute myocardial infarction: importance of early precordial ST-segment depression. Am J Cardiol. 1987;59:782–787. doi: 10.1016/0002-9149(87)91091-5. [DOI] [PubMed] [Google Scholar]
- 20.Birnbaum Y., Sclarovsky S., Mager A., Strasberg B., Rechavia E. ST segment depression in a VL: a sensitive marker for acute inferior myocardial infarction. Eur Heart J. 1993;14:4–7. doi: 10.1093/eurheartj/14.1.4. [DOI] [PubMed] [Google Scholar]
- 21.Sgarbossa E.B., Pinski S.L., Barbagelata A., et al. Electrocardiographic diagnosis of evolving acute myocardial infarction in the presence of left bundle-branch block. N Engl J Med. 1996;334:481–487. doi: 10.1056/NEJM199602223340801. [DOI] [PubMed] [Google Scholar]
- 22.Emergency department: rapid identification and treatment of patients with acute myocardial infarction. National heart attack alert program coordinating committee, 60 minutes to treatment working group. Ann Emerg Med. 1994;23:311–329. [PubMed] [Google Scholar]
- 23.Hillinger P., Strebel I., Abächerli R., et al. APACE Investigators Prospective validation of current quantitative electrocardiographic criteria for ST-elevation myocardial infarction. Int J Cardiol. 2019;292:1–12. doi: 10.1016/j.ijcard.2019.04.041. [DOI] [PubMed] [Google Scholar]
- 24.Meyers H.P., Smith S.W. Prospective, real-world evidence showing the gap between ST elevation myocardial infarction (STEMI) and occlusion MI (OMI) Int J Cardiol. 2019;293:P48–P49. doi: 10.1016/j.ijcard.2019.07.043. [DOI] [PubMed] [Google Scholar]
- 25.Khan A.R., Golwala H., Tripathi A., et al. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017;38:3082–3089. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 26.Hung C.-S., Chen Y.-H., Huang C.-C., et al. Prevalence and outcome of patients with non-ST segment elevation myocardial infarction with occluded “culprit” artery - a systemic review and meta-analysis. Crit Care. 2018;22:34. doi: 10.1186/s13054-018-1944-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miranda D.F., Lobo A.S., Walsh B., et al. New insights into the use of the 12-lead electrocardiogram for diagnosing acute myocardial infarction in the emergency department. Can J Cardiol. 2018;34(2):132–145. doi: 10.1016/j.cjca.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Aslanger E.K., Meyers H.P., Smith S.W. Recognizing electrocardiographically subtle occlusion myocardial infarction and differentiating it from mimics: ten steps to or away from cath lab. Turk Kardiyol Dernegi Arsivi. 2021;49:488–500. doi: 10.5543/tkda.2021.21026. [DOI] [PubMed] [Google Scholar]
- 29.Thygesen K., Alpert J.S., Jaffe A.S., et al. Fourth universal definition of myocardial infarction (2018) Eur Heart J. 2018;40:237–269. doi: 10.1093/eurheartj/ehy462. [DOI] [PubMed] [Google Scholar]
- 30.Byrne R.A., Rossello X., Coughlan J.J., ESC Scientific Document Group 2023 ESC Guidelines for the management of acute coronary syndromes. Eur Heart J. 2023;44:3720–3826. doi: 10.1093/eurheartj/ehad191. [DOI] [PubMed] [Google Scholar]
- 31.Dr Smith’s ECG blog: the OMI Manifesto. https://hqmeded-ecg.blogspot.com/2018/04/the-omi-manifesto.html
- 32.Aslanger E.K., Meyers H.P., Bracey A., Smith S.W. The STEMI/NonSTEMI Dichotomy needs to be replaced by Occlusion MI vs. Non-Occlusion MI. Int J Cardiol. 2021;330:15. doi: 10.1016/j.ijcard.2021.02.015. [DOI] [PubMed] [Google Scholar]
- 33.Aslanger E.K., Meyers H.P., Smith S.W. STEMI: a transitional fossil in MI classification? J Electrocardiol. 2021;65:163–169. doi: 10.1016/j.jelectrocard.2021.02.001. [DOI] [PubMed] [Google Scholar]
- 34.Tziakas D., Chalikias G., Al-Lamee R., Kaski J.C. Total coronary occlusion in non ST elevation myocardial infarction: time to change our practice? Int J Cardiol. 2021;329:1–8. doi: 10.1016/j.ijcard.2020.12.082. [DOI] [PubMed] [Google Scholar]
- 35.Zoni C.R., Mukherjee D., Gulati M. Proposed new classification for acute coronary syndrome: acute coronary syndrome requiring immediate reperfusion. Cathet Cardiovasc Interv. 2023;101:1177–1181. doi: 10.1002/ccd.30667. [DOI] [PubMed] [Google Scholar]
- 36.Aslanger E.K., Yıldırımtürk Ö., Şimşek B., et al. DIagnostic accuracy oF electrocardiogram for acute coronary OCClUsion resuLTing in myocardial infarction (DIFOCCULT Study) Int J Cardiol Heart Vasc. 2020;30 doi: 10.1016/j.ijcha.2020.100603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abusharekh M., Kampf J., Dykun I., et al. Acute coronary occlusion with vs. without ST elevation: impact on procedural outcomes and long-term all-cause mortality. Eur Heart J Qual Care Clinical Outcomes. 2024;10:402–410. doi: 10.1093/ehjqcco/qcae003. [DOI] [PubMed] [Google Scholar]
- 38.OMI Literature Timeline [Internet] Dr Smith’s ECG Blog. https://hqmeded-ecg.blogspot.com/p/omi-literature-timeline.html
- 39.Meyers H.P., Bracey A., Lee D., et al. Accuracy of OMI ECG findings versus STEMI criteria for diagnosis of acute coronary occlusion myocardial infarction. Int J Cardiol Heart Vasc. 2021;33 doi: 10.1016/j.ijcha.2021.100767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McLaren J.T.T., Meyers H.P., Smith S.W., Chartier L.B. From STEMI to occlusion MI: paradigm shift and ED quality improvement. CJEM. 2022;24:250–255. doi: 10.1007/s43678-021-00255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracey A., Massey L., Pellet A.C., et al. FOCUS may detect wall motion abnormalities in patients with ACS. Am J Emerg Med. 2023;69:17–22. doi: 10.1016/j.ajem.2023.03.056. [DOI] [PubMed] [Google Scholar]
- 42.Linde J.J., Kelbæk H., Hansen T.F., et al. Coronary CT angiography in patients with non-ST-segment elevation acute coronary syndrome. J Am Coll Cardiol. 2020;75:453–463. doi: 10.1016/j.jacc.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 43.McLaren J.T.T., Meyers H.P., Smith S.W., Chartier L.B. Emergency department Code STEMI patients with initial electrocardiogram labeled “normal” by computer interpretation: a 7-year retrospective review. Acad Emerg Med. 2024;31:296–300. doi: 10.1111/acem.14795. [DOI] [PubMed] [Google Scholar]
- 44.Al-Zaiti S.S., Martin-Gill C., Zègre-Hemsey J.K., et al. Machine learning for ECG diagnosis and risk stratification of occlusion myocardial infarction. Nat Med. 2023;29:1804–1813. doi: 10.1038/s41591-023-02396-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herman R., Meyers H.P., Smith S.W., et al. International evaluation of an artificial intelligence-powered ecg model detecting acute coronary occlusion myocardial infarction. Eur Heart J Digital Health. 2023;5:123–133. doi: 10.1093/ehjdh/ztad074. [DOI] [PMC free article] [PubMed] [Google Scholar]








