Summary
The search and development of new quorum-sensing (QS) inhibitors are ongoing processes for biofilm control. Here, we present a protocol for in silico characterization of natural-based molecules as QS inhibitors. We describe steps for preparing models of protein receptors for virtual screening. We then detail procedures for construction and virtual screening of phytochemical libraries and hit picking to be experimentally validated by in vitro assays. This protocol allows exploration of a broad range of potential inhibitors for a specific target.
For complete details on the use and execution of this protocol, please refer to Fernandes et al.1
Subject areas: Health Sciences, Protein Biochemistry, Chemistry, Computer sciences
Graphical abstract

Highlights
-
•
Instructions for identifying QS inhibitors in a large phytochemical library
-
•
Guide for preparing and performing virtual screening with multiple docking programs
-
•
Steps for computationally predicting ligand-QS receptor binding affinities
Publisher’s note: Undertaking any experimental protocol requires adherence to local institutional guidelines for laboratory safety and ethics.
The search and development of new quorum-sensing (QS) inhibitors are ongoing processes for biofilm control. Here, we present a protocol for in silico characterization of natural-based molecules as QS inhibitors. We describe steps for preparing models of protein receptors for virtual screening. We then detail procedures for construction and virtual screening of phytochemical libraries and hit picking to be experimentally validated by in vitro assays. This protocol allows exploration of a broad range of potential inhibitors for a specific target.
Before you begin
Biofilms are responsible for more than 65% of all infections. Our current arsenal of antibiotics is not effective in controlling biofilm-related infections.2 Therefore, there is a need for drug discovery and development to control biofilms and target specific mechanisms. Quorum-sensing (QS) is probably the most attractive mechanism to target for biofilm control.1,2 A QS system is an intercellular chemical communication process based on cell-population density. Through the QS system, bacteria monitor the environment and coordinate gene expression responsible for several physiological functions, using diffusible extracellular signaling molecules, known as autoinducers (AIs), which increase in concentration with increasing cell-population density.3 QS systems can regulate biofilm formation, virulence secretion, swarming motility, sporulation, and protease production.3 In this way, QS inhibitors have been rising as a promising strategy to attenuate the expression of virulence factors and prevent biofilm formation.4 Among a wide variety of phytochemicals (compounds from the secondary metabolism of plants), many of them have been identified as QS inhibitors.5,6,7 The search for phytochemicals (as QS inhibitors) is an ongoing process.
In silico approaches have been applied to drug discovery and development processes. Instead of random selection and following tests of a large number of compounds (traditional approach), in silico screening reduces the run time and overall costs involved in the research of new bioactive compounds.8 Molecular docking and virtual screening have been widely used for the study of protein-ligand interactions, revealing high accuracy in the selection of bioactive compounds to be experimentally validated.9 In this way, this approach can be used to identify potential QS inhibitors among a large phytochemical library by identifying the binding affinities between ligands and specific QS receptors.4 For experimental validation (in vitro assays), only the most promising compounds able to interact with QS receptors are selected (top-scoring compounds). These compounds are more likely to have anti-QS activity against selected QS receptors.
Molecular docking identifies the most promising compounds from a large molecular database as a first attempt to guide the selection of a more limited set for experimental validation.10 Several compounds are docked into one specific target and a small set of the top scorers proceeds for experimental testing. Thus, molecular docking/virtual screening allows the selection of potential molecules with promising inhibitory activity against different receptors with application in distinct areas, such as medicine, biology, or industry.11,12,13,14
Regarding protein-ligand docking software, the number of alternatives available to the scientific community is extremely diverse.10,11,15,16 The performance of docking software was compared by several authors.10,17,18 Each docking software provides different accuracy in binding pose prediction and binding affinity estimation for different protein families.18 Scoring functions used in docking, in particular, can vary significantly in terms of performance between protein targets with different characteristics. Thus, researchers should consider evaluating more than one docking software and scoring function for the respective target receptor (molecular docking/virtual screening optimization).
Development of the protocol
Our group has been applying molecular docking and virtual screening for QS studies using distinct receptor targets. For example, Oliveira et al.19 corroborated the experimental results of QS inhibition through molecular docking simulations using the apo-protein AgrA LytTR domain from Staphylococcus aureus. Other studies identified potential QS inhibitors from large databases by molecular docking/virtual screening against CviR from Chromobacterium violaceum20 and MvfR (also known as PqsR)21,22 and LasR23 from Pseudomonas aeruginosa. Recently, Fernandes et al.1 screened QS inhibitors from a phytochemical library against both QS receptors LuxS of Bacillus subtilis and LasI/LasR of P. aeruginosa, followed by successful experimental validation of microbiological activity of picked phytochemicals. The main stages of molecular docking/virtual screening protocol for drug discovery were reviewed by Cerqueira et al.24
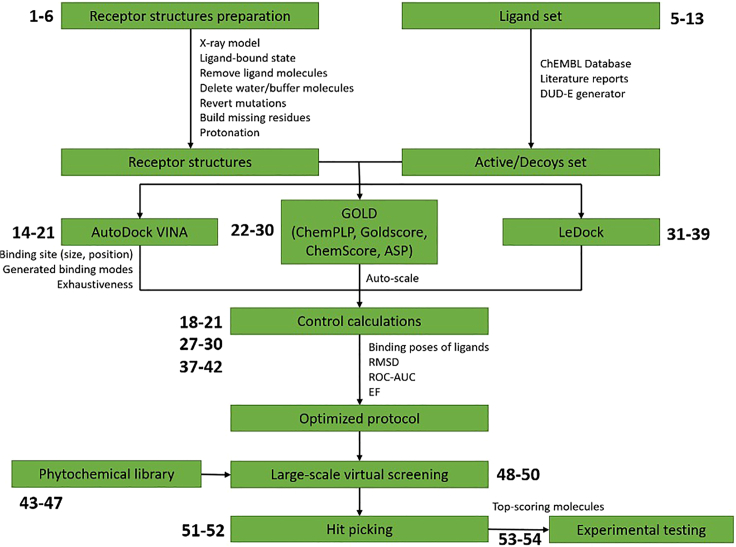
The present protocol (Figure 1) includes how to prepare a 3D model of protein receptor for molecular docking/virtual screening; molecular docking optimization by the comparison of docked and crystallographic ligand poses; optimization of a virtual screening protocol by maximizing the ability to discriminate between active ligands and decoy molecules; virtual screening of a large library of phytochemicals; selection of hits; and experimental validation by in vitro assays. While the protocol (Figure 2) should be general for QS-related proteins, we provide example data from a recent study against the LuxS receptor from B. subtilis. LuxS/AI-2 QS is considered the “universal QS system”, used for interspecies communication. LuxS is a particularly well-suited target for molecular docking/virtual screening: several crystallographic structures had been determined, the binding site is defined through ligand-bound crystallographic structures, there are several active ligands for control calculations, and in vitro assays to test identified hits are well established. For docking calculations, LuxS (PDB: 2FQT) is used as an example of a ligand-bound crystallographic structure. Separate docking simulations may be run for the other available conformations of the protein.
Figure 1.
Workflow of molecular docking/virtual screening protocol for the identification of anti-QS compounds (with a phytochemical nature)
In silico approach comprises the preparation of an atomistic tri-dimensional model of a protein receptor, protocol optimization (molecular docking and virtual screening), virtual screening of a phytochemical library, and hit picking of top-scoring molecules for following experimental validation.
Figure 2.
General flow diagram of molecular docking/virtual screening optimization for phytochemical selection
The method comprises the preparation of receptor structures and ligand set, optimization of molecular docking/virtual screening of different docking software/scoring functions (AutoDock VINA, GOLD, and LeDOck), and preparation of a phytochemical library for large-scale virtual screening. At the end, the top-scoring molecules are selected for the following experimental testing.
For QS inhibition studies, the target for molecular docking/virtual screening can be a protein involved in the production or recognition/detection of AIs. The KEGG PATHWAY database (https://www.genome.jp/kegg/pathway.html, assessed on Fev 2023) and the Biofilms Structural Database (https://biofilms.biosim.pt/, assessed on Fev 2023) contain the information about different proteins/enzymes related to QS system for specific microorganisms. The target structure can be obtained from Protein Data Bank (PDB) (https://www.rcsb.org/, assessed on Fev 2023). High-resolution ligand-bound crystallographic structures are preferred since the binding site is well-defined in the bound state and it will outperform ligand-free structures. Furthermore, the amino acid side chains around the binding pocket are already pre-organized into a better conformation to accommodate a putative inhibitor. However, ligand-free crystallographic structures are also useful for virtual screening validation, but the risk of failure increases. Strategies to circumvent these problems involve the use of molecular dynamics simulations before docking or the use of flexible protein docking, with a great increase in computational cost.
The binding site can be identified by a co-crystallized structure of ligand and target protein (ligand-bound structure), or according to published works with detailed binding site residues. Typically, several (if not all) crystallographic structures of the target protein (receptor) are prepared for molecular docking. It involves the reversion of mutations to the wild type (especially if they are close or within the binding site); the removal of water and buffer molecules (components involved in crystallization conditions); the removal of ligand molecules; the addition of hydrogen atoms; the setting up bond orders and formal charges; and the definition of amino acid protonation states. All these aspects are relevant for the success of molecular docking to depict the interactions more accurately on the binding site.
The optimization of the molecular docking protocol is performed by re-docking and cross-docking studies. Re-docking aims to assess the quality of the molecular docking software/scoring function protocol in reproducing the known experimental binding pose of the crystallographic ligands in their specific target. For that, different docking software/scoring functions can be optimized: AutoDock VINA,25 GOLD (ChemPLP, GoldScore, ChemScore, and ASP),26 and LeDock27 are popular choices. Other docking software/scoring functions can be implemented with some protocol adjustments but using the same workflow. The ability of each docking software/scoring function to predict crystallographic ligand pose for each specific ligand-bound structure is assessed by both visual inspection of the reported interaction between receptor/ligand and quantitatively by calculating the minimum Root Mean Square Deviation (RMSD, Å) between docked and crystallographic ligand poses. Cross-docking allows assessing the ability of each structure to correctly accommodate ligands from other structures (or from literature) and measure its general usefulness, in terms of potential for virtual screening of a large diversity of ligands. The average binding energy score from each molecular docking software/scoring function for all ligands is also calculated for each target protein. The protocol can be optimized by changing the center and dimensions of the binding pocket regions explored in docking and testing different docking parameters in each software to increase the ability to reproduce the experimentally confirmed binding poses and improve the average binding energy scores.
The optimization of the virtual screening protocol is performed by the ability to discriminate between real ligands (actives) and non-ligands (decoys). Actives molecules are compounds with known experimental activity against the target protein. The ChEMBL Database (https://www.ebi.ac.uk/chembl, assessed on Fev 2023) reports active compounds against selected receptors. Decoys are compounds randomly generated with similar 1D physicochemical properties to actives (such as molecular mass, number of rotatable binds, and logP), but distinct 2D topology that makes them inactive. The Directory of Useful Decoys Enhanced (DUD-E, http://dude.docking.org/, assessed on Fev 2023) retrieves a set of 50 decoys for each active compound. The previously optimized molecular docking protocols are applied to the active/decoys set.
The virtual screening protocol is validated based on control calculations to minimize the risk of failure. In this way, prepared receptor structures and molecular docking parameters should prioritize known ligands over decoys. The performance of different scoring functions in discriminating between actives/decoys can be evaluated by the calculation of the area under (AU) Receiver Operator Characteristic (ROC) curve. ROC curve is the plot of the true positive rate (TPR = TP/P) as a function of the false positive rate (FPR = FP/N), where TP is the number of true positives, FP is the number of false positives, P and N is the total number of positives (actives) and negatives (decoys), respectively. The higher the AU-ROC values, the better the discrimination between TP and FP. A random ranking corresponds to the equality line TPR = FPR, and AU-ROC of 0.5 (50%). The best scoring function should rank ligands early on a large score list. The enrichment factor at 1% (EF1%) and 20% (EF20%) measured the number of active ligands recovered at 1% and 20% of the active/decoys database, respectively, over the expected number of recovered active compounds using random scores. A high EF value in the first 1% means that the scoring function is better at discerning true active compounds early.28 Ranked lists of active/decoys based on binding energy scores obtained with previous optimized scoring functions (VINA, GOLD, and LeDock) are used to determine AU-ROC, EF1%, and EF20%.29
At the end of this stage, an optimized virtual screening protocol will be selected using the best-performing molecular docking software/scoring function and protein structures (receptor), that predict reasonable ligand poses and discern ligands with a higher rank than decoys. For a ligand-free crystallographic structure, RMSD calculations are impossible, and the performance of molecular docking software/scoring function and protein structure can be evaluated based on the ability to discriminate known actives from a background of decoys.
Once molecular docking/virtual screening protocol is optimized and validated, large libraries of molecules can be virtually screened against selected target proteins. These molecules must be readily available for in vitro testing. From virtual screening, molecules are ranked according to the docking score (binding energy score).
The phytochemical library can be developed by combining several online libraries: Analyticon (4561 molecules – https://ac-discovery.com/), Molport (1471 molecules – https://www.molport.com/shop/index), and PhytoHub (1532 molecules – http://phytohub.eu/), all accessed in July 2024. After the removal of duplicates, the phytochemical library comprised about 7564 molecules (only from plant sources). In addition, the library can be limited by selecting molecules with specific properties associated with other drug-like molecules – Lipinski's Rule of Five: a) the number of hydrogen bond donors ≤ 5; b) the number of hydrogen bond acceptors ≤ 10; c) molecular weight < 500 Da; d) partition coefficient (logP) < 5.30 This restriction is facultative.
Among the top-ranked molecules from virtual screening of the phytochemical library, molecules that are likely ligands will be present, but also with identified false positives due to inherent docking limitations. Thus, based on chemical properties, availability, and cost, phytochemicals are selected for the following experimental validation. The anti-QS activity of selected phytochemicals is validated by in vitro assays. The success of virtual screening is evaluated by its ability to reveal novel active phytochemicals against selected QS targets.
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| AutoDock Vina (version 1.0) | Scripps Research Institute, San Diego | https://vina.scripps.edu |
| GOLD (version 2021.0.1) | CCDC | https://www.ccdc.cam.ac.uk/solutions/software/gold/ |
| LeDock | Lephar | http://www.lephar.com/download.htm |
| Pymol (version 1.7.6.0) | Schrodinger | https://pymol.org/ |
| Open Babel (version 3.1.1) | Open Babel | http://openbabel.org/docs/Installation/install.html |
| DockRMSD | Zhang Lab | https://zhanggroup.org/DockRMSD/ |
| DataWarrior 5.0.0 | Open Molecules | https://openmolecules.org/datawarrior/ |
| Vim (text editor) | Open-source | https://www.vim.org/ |
| Microsoft Excel | Microsoft | https://www.microsoft.com/en/microsoft-365/excel?market=af |
| Other | ||
| Computer with Linux operating system and internet access | N/A | N/A |
| KEGG PATHWAY database | Human Genome Program | https://www.genome.jp/kegg/pathway.html |
| Biofilms Structural Database | BioSIM Lab | https://biofilms.biosim.pt |
| Protein Data Bank | RCSB PDB | https://www.rcsb.org/ |
| ChEMBL database | EMBL | https://www.ebi.ac.uk/chembl |
| Directory of Useful Decoys Enhanced | Shoichet Lab | http://dude.docking.org/ |
| AnalytiCon | AnalytiCon Discovery | https://ac-discovery.com/ |
| Molport | Molport | https://www.molport.com/shop/index |
| PhytoHub | PhytoHub | http://phytohub.eu/ |
Step-by-step method details
Set up protein receptors for molecular docking
Timing: 15 min
This section is aimed at preparing the protein structure files for the molecular docking experiments.
-
1.Download the crystallographic structures of the selected target (LuxS from B. subtilis) from the PDB (.pdb file).
-
a.Preference should be given to structures of high resolution (better than 2.5 Å) and with a ligand bound in the binding site. Structures without ligands are useable but tend to produce poorer results due to unconstrained binding site geometries.
-
b.In this protocol, structure 2FQT (https://www.rcsb.org/structure/2FQT) was used. Troubleshooting 1.
-
a.
-
2.Visualize the crystallographic protein structure (receptor) and binding site in PyMOL software (or VMD as an alternative):
-
a.Delete components from the PDB that do not contribute to ligand binding (lipids, water, and buffer molecules). To remove water molecules in PyMOL, press A (Action) next to the structure name on the right column, and select “remove waters”. To remove other molecules, select them and then press A next to the selection and select “remove atoms”. Troubleshooting 2.
-
b.Protein cofactors and metal ions should be kept or discarded depending on their relevance to ligand binding.
-
c.Examine mutations and revert or rebuild as necessary.
-
d.Add hydrogen atoms. To do this in PyMOL, press A (Action) next to the structure name, go to hydrogens and press “add”. I.Note: If the protein is represented in ribbon or cartoon, this will not make a visual change. In order to see the added hydrogen, the representation should be changed to lines or licorice.
-
e.All crystallographic structures should be aligned. To align the structures in PyMOL, on the command line, write “align structure_1, structure_2”.
-
a.
-
3.
Save the protein prepared in the previous step as all_filename.pdb (for example, all_2FQT.pdb).
-
4.
The only information that is needed from the PDB file are the atom coordinates. Therefore remove lines that do not start with “ATOM”, “HETATM”, “TER” or “END” using Linux shell command lines. For this, you can do it manually with a text editor, or with the following command:
grep -E 'ˆ(ATOM|HETATM|TER|END)' input_file > output_file
-
5.
Extract ligand molecules for another file. Troubleshooting 3.
-
6.
Also save crystallographic protein (receptor) and ligand as separated PDB file as receptor_filename.pdb and ligand_filename.pdb (for example, receptor_2FQT.pdb and ligand_H1D.pdb).
Collect and build the active/decoys set
Timing: 30 min
In this section, a selection of active molecule will be downloaded and prepared for virtual screening. Additionally, decoys molecules will be generated and prepared as well.
-
7.
Search and download active molecules against selected receptors from the ChEMBL Database (https://www.ebi.ac.uk/chembl) or from literature reports.
Note: Ligands with the best IC50 and Ki values should be selected, however the chosen molecules are always dependent on the experimental data available for each system and how many known actives are available.
-
8.Prepare the library with Datawarrior.
-
a.Open the downloaded CSV file with Datawarrior.
-
b.Delete all unwanted molecules and generate 3D conformers by selecting Chemistry on the toolbar and choosing the Generate Conformers option.
-
c.Save the file as a Version 2 sdf file (Save Special -> SD-File. Choose SD-File Version 2 and 3D Atom coordinates).
-
a.
-
9.
Use the Open Babel software to protonate ligands for docking. For that, use the following script:
obabel -isdf Library.sdf -osdf -O Library_protonated.sdf -p 7
-
10.
Generate decoys for each active molecule using DUDE-Z (https://tldr.docking.org/).
Note: For that upload SMILES of active molecules to the input section and start the decoys generation. A final set of 50 decoys per active will be calculated. In some cases, it is possible that the DUDE-Z will not be able to generate 50 different decoys for all actives, so the final number of decoys may not be exactly what was expected (nº of actives ∗ 50).
-
11.Convert the generated file (decoys.smi) is into an sdf file.
-
a.Open the generated SMILES file with a text editor, copy and paste all compounds into the DataWarrior software.
-
b.Generate conformers (Chemistry -> Generate Conformers) – 3D structure of molecules – and save this data in decoys.sdf file. Finally, protonate the decoys, as done above.
-
a.
-
12.
Combine all protonated active, and decoy molecules.
cat ∗.sdf >> DecoyActives.sdf
-
13.Finally, Decoys_protonated.sdf and Library_protonated.sdf file should be converted and separated into .pdbqt files for use with AutoDock Vina.
-
a.Create a new directory named “pdbqt”. This is done to avoid having multiple files in the same directory.
-
b.Place the generated .pdbqt files this new directory.
-
c.Repeat the following command for both the actives (Library_protonated.sdf) and the decoys (Decoys_protonated.sdf). Change the name of the output accordingly.
-
a.
mkdir pdbqt
obabel -isdf Library_protonated.sdf -opdbqt -O pdbqt/Actives.pdbqt -m
obabel -isdf Library_protonated.sdf -opdbqt -O pdbqt/Decoys.pdbqt -m
Optimization of molecular docking protocol
Timing:At least 5 days
This section is aimed at testing various variables in order to optimize the molecular docking protocol.
AutoDock Vina.
-
14.Receptor and ligand input files should be in .pdbqt format.
-
a.Use obabel to convert the ligand .pdb into .pdbqt file and define the protonation state at pH 7. Save the ligand as ligand_H1D.pdbqt.
-
b.For the receptor use the prepare_receptor4 script from MGLtools. Troubleshooting 4.
-
a.
prepare_receptor4 -r receptor_2FQT.pdb -o receptor_2FQT.pdbqt -A checkhydrogens
-
15.
Adjust position, size, and visualization properties using the Autodock/Vina plugin from the PyMOL software.
Note: The binding site (center and size) is normally defined using a crystallographic ligand-bound receptor as a box around the reference ligand.
-
16.
Create the configuration file as file.conf according to the following instructions.
receptor = receptor_2FQT.pdbqt
center_x = -22.82
center_y = 30.63
center_z = -16.50
size_x = 19
size_y = 17
size_z = 18
exhaustiveness = 8
num_modes = 9
-
17.
Finally, start the molecular docking calculations.
./vina --config file.conf –-ligand H1D.pdbqt -–log H1D_docked.log –-out H1D_docked.pdbqt
-
18.
Verify the position of docked ligands inside the binding site by visual inspection using PyMOL software.
-
19.Compare generated docked ligand poses (file_output_docking.pdbqt, for example, H1D_docked.pdbqt) to the crystallographic pose for each ligand-bound crystallographic protein (ligand_filename.pdbqt, for example, ligand_H1D.pdbqt).
-
a.Compare with visual inspection in the PyMOL software.
-
b.Compare with RMSD calculations using DockRMSD, according to following script.
-
a.
Note: This script can also be used for different types of files (.pdbqt or .pdb format), but the authors recommend comparing .mol2 format.
obabel -ipdbqt ligand_H1D.pdbqt -omol2 -O H1D.mol2 -p 7
obabel -ipdbqt 2FQT_H1D_docked.pdbqt -omol2 -O 2FQT_H1D_docked.mol2 -p 7
DockRMSD [H1D.mol2] [2FQT_H1D_docked.mol2]
-
20.Optimize protocol by adjusting parameters for VINA including the box size and position of the binding site, maximum number of generated binding modes, and exhaustiveness of the global search (roughly proportional to time).
-
a.The position of the binding site should include all important residues for the interaction with the ligands, while not being too large that it would negatively affect the docking procedure.
-
b.The exhaustiveness should allow the exploration of many binding conformations while still being quick and not too computationally expensive.
-
c.The user can test multiple conditions and see when there is not any significant gain in docking accuracy compared to the increased calculation time.
-
a.
Note: The best-optimized conditions for VINA should allow high predicted binding affinity/ negative binding energy (kcal/mol) and low RMSD values in the comparison with the crystallographic pose. Predicted binding energy of more negative than −7 kcal/mol is indicative of a good interaction, while RMSD values below 2 are indicative of a good pose.
-
21.
For the active/decoys VS, run VINA for each of the active and decoy molecules.
Note: Running the program one by one is not the most practical option. In order to automatize the process, use the following script. In order to run this script, the user must have all .pdbqt files and file.conf in the same directory as the script.
#!/bin/bash
mkdir -p LOGS
mkdir -p OUTS
for x in ∗.pdbqt; do
basename_x=$(basename "$x" .pdbqt)
vina --config file.conf --ligand "$x" --log LOGS/"$basename_x".log --out OUTS/"$basename_x".pdbqt
done
-
22.
The final step is to generate a file with best scores from all docked ligands. That can be done with the following command:
grep “RESULT” -m 1 OUTS/∗pdbqt > best_scores.txt
GOLD (ChemPLP, GoldScore, ChemScore, ASP).
-
23.
Ligand input files should be in .mol2 or .sdf format. To convert .pdb into .sdf or .mol2 files and define protonation state at pH 7, the following scripts should be used:
obabel -ipdb ligand_H1D.pdb -osdf -O ligand_H1D.sdf -p 7
obabel -ipdb ligand_H1D.pdb -omol2 -O ligand_H1D.mol2 -p 7
-
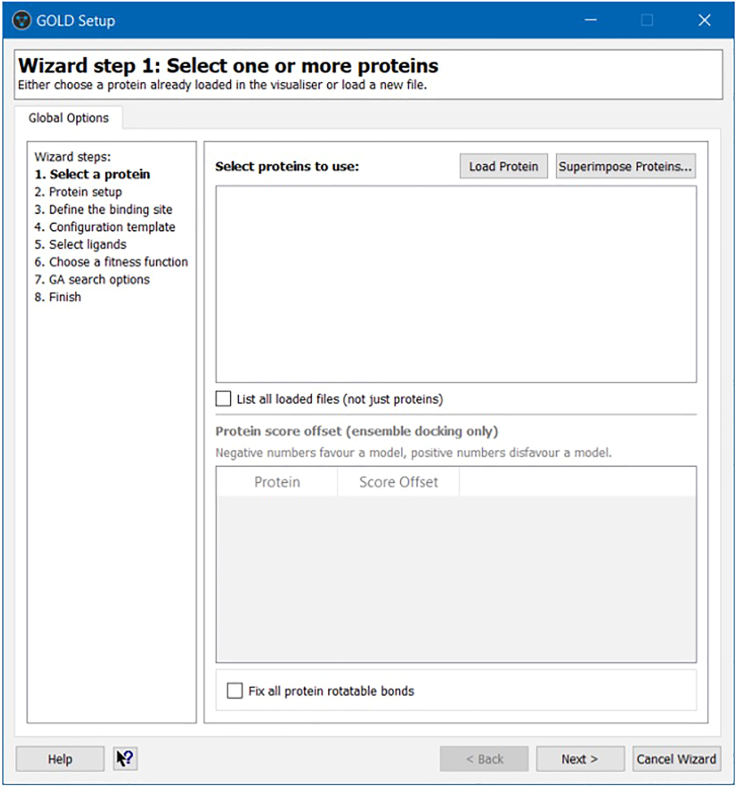
24.Open GOLD Setup window and create a new GOLD configuration file in Wizard menu, that guides through the essential configuration steps (Figure 3).
-
a.The first step is to load protein structures (.pdb or .mol2 files) - 2FQT.pdb.
-
b.Click on the Add Hydrogens button to protonate the protein.
-
c.Save the protein in .mol2 format (File -> Save As).
-
d.Define the binding site in the gold.conf file.
-
a.
CRITICAL: The point of center of binding site is the same used and optimized with VINA. The radius is chosen to guarantee an overall volume consistent with the box size previously used with VINA. Alternatively, the crystallographic ligand can be used directly as a reference for the docking. In the repository, gold.conf files with both options are available. In the ligand_data_file section, add the crystallographic ligand in .mol2 or .sdf format. The number in front is the number of runs the program will perform. A higher number means a more exhaustive search, but also a higher computational cost.
-
25.
Finally, start the molecular docking calculations, where gold.conf is the name of a configuration file:
gold_auto gold.conf &
-
26.
Verify the position of docked ligands inside the binding site by visual inspection using PyMOL software.
-
27.Compare generated docked ligand poses to the crystallographic pose for each ligand-bound crystallographic protein.
-
a.Compare by visual inspection in PyMOL software.
-
b.Compare with RMSD calculations. Convert the files from .sdf to .mol2 using obabel before running DockRMSD.
-
a.
DockRMSD [H1D.mol2] [gold_soln_ligand.mol2]
-
28.
Optimize protocol by adjusting parameters for GOLD including the number of runs.
Note: The best optimized conditions for GOLD should allow high binding affinity/high binding score (dimensionless) and low RMSD values. The different scoring functions of GOLD have scores with different scales. A good score for ChemPLP is at least in the 80–100 range. As for the other scoring functions, they are usually lower, with ASP and ChemScore being in the 30–50 range and GoldScore being in the 50–70 range.
-
29.
For the active/decoys vs., simply open the gold.conf file and replace the ligand_data_file with the DecoysActives.sdf file that was generated on step 12.
Figure 3.
Wizard menu of GOLD setup window
The wizard steps include: the selection of one or more receptor proteins; protein setup; definition of the binding site from an atom, a point, or a reference ligand; configuration template; selection of ligands; selection of a fitness function (ChemPLP, Goldscore, ChemScore, and ASP); GA search options; and finish.
LeDock.
-
30.
Generate a list of the ligand names with the following command:
obabel -ipdb H1D.pdb -omol2 -O H1D.mol2 -p 7
ls ∗mol2> list_ligands
CRITICAL: Ligand input files should be in .mol2 format and in the same directory the docking will be run. Ligands list is a text file (without any extension) with a list of the names of the ligands to dock.
-
31.
Use LePro to prepare the protein file (automatically add hydrogen atoms by considering the protonation state of histidine, remove water, ions, small ligands, and cofactors).
Note: The present version Lepro is only compatible with Zn, Mn, Ca and Mg ions. Since 2FQT has a Co ion, Lepro removes it. It is important to always check the importance of any co-factor before performing docking and virtual screening experiments. If it is essential for binding, one should choose a structure with one of the compatible ions or use a program that is able to accurately consider the interaction with the metal ion, such as GOLD.
-
32.
Calculate the binding site from previously adjusted dimensions with VINA according to the following calculations.
xmin=center_x−size_x/2.
xmax=center_x+size_x/2.
ymin=center_y−size_y/2.
ymax=center_y+size_y/2.
zmin=center_z−size_z/2.
zmax=center_z+size_y/2.
-
33.
The configuration file (dock.in) contains the following information. The RMSD 1.0 is a recommended number. The number of binding poses 20 is by default.
Receptor
receptor_2FQT.pdb
RMSD
1.0
Binding pocket
-32.52 -13.32
22.13 39.13
-25.50 -7.50
Number of binding poses
20
Ligands list
list_ligands
END
-
34.
Run LeDock:
ledock dock.in
-
35.
The output is a .dok file with all the docked poses and energies generated for each ligand. Dok2mol2 must be downloaded and compiled before it can be used. That information is converted to .mol2 file according to the following command, where “numb” should be an integer corresponding to the docking pose number:
dok2mol2 filename.dok original.mol2 numb >file.mol2
-
36.
Verify the position of docked ligands inside the binding site by visual inspection using PyMOL software.
-
37.Compare the generated docked ligand poses (file.mol2) to crystallographic pose for each ligand-bound crystallographic protein.
-
a.Compare by visual inspection in PyMOL software.
-
b.Compare with RMSD calculations with DockRMSD with the following command:
-
a.
DockRMSD [H1D.mol2] [file.mol2]
Note: The best performance of LeDock should allow high predicted binding energy/negative binding energy (kcal/mol) and low RMSD values. A good predicted binding energy for LeDock is more negative than −6 kcal/mol.
-
38.
To run the actives/decoys virtual screening, the protocol is similar to what was described above. The difference is that all actives and decoys must be added to the list of ligands.
-
39.
To obtain a summary of the best scores, use the ledock_anal script, also available in the Ledock website.
Virtual screening optimization
Timing:At least 1 day
In this section, the actives and decoys libraries will be used to optimize the virtual screening protocol.
-
40.Use the previous optimized molecular docking software/scoring function to analyze an active/decoys set, typically in the proportion 1:50. From each molecular docking/scoring function, it is given a list of active/decoy molecules ranked according to binding energy scores that are used to determine the area under (AU) Receiver Operator Characteristic (ROC) curve, enrichment factor at 1% and 20% (EF1% and EF20%, respectively)29:
-
a.The performance of each molecular docking software/scoring function in discriminating between active/decoys can be evaluated by the calculation of AU-ROC curve. ROC curve (Figure 4) is the plot of the true positive rate (TPR) versus the false positive rate (FPR).In which TP is the number of true positives, FP is the number of false positives, P and N is the total number of positives (actives) and negatives (decoys), respectively.In which i corresponds to the number of hit of active/decoys set screened.
-
b.The EF1% and EF20% measured the number of active molecules recovered at 1% and 20% of the active/decoys set, respectively, over the expected number of recovered active compounds using random scores:In which Ha and Ht is the number of active hits and total hits in a percentage of the active/decoys set screened (1% or 20%).Note: An AU-ROC value of 50 would mean that the protocol is random, while a value of 100 would mean all the actives are scored higher than the first decoy. Ideally, AU-ROC values of at least 80 would be considered good and above 90 are excellent. Additionally, the best molecular docking/scoring function should have high EF values in the first 1%, ranking actives early on a large score list. As for EF, EF1% values above 10 are generally considered good, while values above 20 are excellent. Finally, for EF 20, values higher than 5 are considered good and above 10 are excellent.
-
a.
-
41.
At the end of this stage, an optimized virtual screening protocol was selected using the best-performing molecular docking software/scoring function and protein structures. Troubleshooting 5.
Figure 4.
Example of ROC curve
The gray line represents a random ranking, corresponding to the equality line TPR = FPR, and AU-ROC of 0.5 (50%).
Virtual screening of a phytochemical library
Timing:Depends on the computational performance but can take more than1week
This section is aimed at using the optimized protocol to screen a created phytochemical library.
-
42.
Create a phytochemical library by combining several online libraries, for example, Analyticon (4561 molecules - https://ac-discovery.com/), Molport (1471 molecules - https://www.molport.com/shop/index), and PhytoHub (1532 molecules - http://phytohub.eu/) - all accessed in July 2024.
-
43.
Use the DataWarrior software, remove duplicates, estimate chemical properties from SMILES information (Chemistry -> From chemical structure -> Calculate properties), and generate conformers (Chemistry -> Generate Conformers).
-
44.Filter the phytochemical library by selecting compounds with specific properties associated with other drug-like compounds (3479 compounds) – Lipinski’s Rule of Five: a) the number of hydrogen bond donors ≤ 5; b) the number of hydrogen bond acceptors ≤ 10; c) molecular weight < 500 Da; d) partition coefficient (logP) < 5.30
-
a.To filter the compounds based on the desired properties, use the options on the right side of the screen. Then, delete the filtered rows (Data -> Delete Rows -> Invisible Rows).
-
a.
-
45.
Save the phytochemical library as phyto.sdf.
-
46.
Finally, phyto.sdf file should be converted and separated into .pdbqt or .mol2 files. The following command is an example to convert .sdf into .mol2 file. It is important to create the directory where the files are going to be saved before executing the command.
obabel -isdf phyto.sdf -omol2 mol2/phyto.mol2 -m -p 7
-
47.
For large-scale virtual screening, use the best performing molecular docking software/scoring function and protein structures as previously detailed for ligands and active/decoys set.
-
48.
Record a phytochemical list ranked according to binding energy scores.
-
49.
Convert the result .pdbqt or .mol2 file into a phyto_docked.sdf file.
Hit picking and experimental validation
Timing:At least 30 days
This section is aimed at selecting hit compounds and proceed to their experimental validation.
-
50.The selection of phytochemicals is based on high binding score against the QS receptor. Other selection criteria are high availability and low cost.
-
a.Open phyto_docked.sdf file in the DataWarrior software to rank all molecules according to increased binding score.
-
b.Open the list of ranked phytochemicals in Microsoft Excel software and the search for availability/cost of each compound should be performed for the initial set.
-
a.
-
51.
Finally, the picked hits can be now experimentally tested. For the LuxS study, a previous report detailed the experiments.1 The present protocol can be applied to other QS targets, and experimental validation should be reliable for that.
-
52.
Examples of protocols for in vitro validation of QS inhibitors (not limited to that) are detailed in the following articles. QS system of Gram-negative bacteria that is typically LuxI/LuxR type QS system.31,32,33 For P. aeruginosa, LasI/LasR QS system34; RhlI/RhlR QS system35; Pseudomonas Quinolone System (Pqs) QS system targeting PqsR (MvfR)36; and Las-Rhl-Pqs QS system hierarchy.37 For S. aureus, accessory gene regulator (Agr) QS system.38 For Enterococcus spp., Fsr QS system.39
Expected outcomes
At the end of this protocol, a QS receptor will have been selected and prepared for docking simulation, molecular docking/virtual screening protocol will have been optimized based on control calculations (i.e., RMSD, AU-ROC, and EF), and a large phytochemical library will have been screened to pick the most promising molecules (phytochemicals) as QS inhibitors. The first optimization steps are essential to minimize the risk of failure. The virtual screening will allow the docking and ranking of thousands of phytochemicals against a QS target of interest. The main goal of this protocol is to select a small set of phytochemicals from a large library with a better chance to develop an anti-QS activity. LuxS was used as an example of a QS target that provided successful results. Moreover, this protocol has been applied to different QS targets demonstrating its broad applicability.9,19,20,21,22 In addition, different molecular libraries have been screened not limited to phytochemicals. Depending on the QS target of interest and the analysis and selection criteria of hits, different final molecules will be attained to be experimentally tested. Our experience in the field by now suggests that the in silico approach is likely enough to succeed to be worth the investment. At the same time, the novel ligands (potential QS inhibitors) that result can bring new biological insight into the field of structure-based enterprise and understanding of QS receptor-ligand interactions for the design of new active molecules.
Limitations
The present protocol demands for a broad researcher’s background in several areas (including computation, chemistry, and biology) to perform all the steps of the process, namely for the correct selection and preparation of QS targets and ligands and the evaluation of the obtained results. Selected docking software assumes ligand conformation as flexible while receptor structure remains rigid, the flexibility of the target protein will require more computational power.40 Molecular docking/virtual screening approaches exhibit an inherent limitation related to the generation of false positives and false negatives. False positives can be identified in the first steps of experimental validation by in vitro screening. In contrast, false negatives correspond to potentially valuable compounds that will not be considered for following tests.8 Hence, dedicating particular attention into validating the initial protocol with known experimentally confirmed active molecules (typically against decoy molecules) is important to ensure a minimization of the number of false negatives in the search of the large virtual databases. Nevertheless, in silico approaches enable the consideration of a large diversity of molecules, ensuring an extended exploration of the chemical space for potential compounds with chance of being active, reducing the time and cost of drug discovery and development processes compared to the traditional process. Molecular docking/virtual screening requires ideally the availability of a protein structure obtained by X-ray crystallography, NMR or Cryo-EM, with a good resolution, preferably already with a bound-ligand or inhibitor. The prediction of tri-dimensional structure of receptor by homology-modeling methods can increase the number of false positives, reducing the approach accuracy (high risk of failure).8
Troubleshooting
Problem 1
No available experimental structure of target protein.
Possible reason
Crystallographic, NMR or Cryo-EM structure is not reported in PDB database.
Potential solution
Homology-modeling methods should be used to build the 3D-protein structure; AlphaFold database for AI-generated models should be checked.
Problem 2
Water molecules contribute to binding site.
Possible reason
Some water molecules establish important interactions between protein and ligand.
Potential solution
Critical water molecules should be identified by analyzing their role inside the binding site and include them as part of the grid that defines the binding site.
Problem 3
Identification of binding site.
Possible reason
Unavailable ligand-bound crystallographic structure.
Potential solution
Binding site can be inferred from literature, in which research articles can provide clues about amino acid residues involved in binding site; or using computation tools to identify and characterize potential binding site (POCKET, LIGSITE, SURFNET, SPHGEN, etc).
Problem 4
Prepare_receptor4.py does not work.
Possible reason
Incompatibilities caused by Prepare_receptor.py being and old Python2 script.
Potential solution
Run the following commands.
virtualenv -p python2 venv
source venv/bin/activate
Navigate to the MGLTools install directory (specified in install.sh).
Copy the prepare_receptor4.py script to the top level of the install directory.
Finally, execute the script.
python prepare_receptor4 -r receptor_2FQT.pdb -o receptor_2FQT.pdbqt -A checkhydrogens
Problem 5
High RMSD values for the best scoring ligands.
Possible reason
Incorrect docked pose.
Potential solution
Visual inspection of docked ligand poses for good scoring ligands.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Manuel Simões (mvs@fe.up.pt).
Technical contact
Technical questions on executing this protocol should be directed to and will be answered by the technical contact, Sérgio F. Sousa (sergiofsousa@med.up.pt).
Materials availability
This study did not generate new unique reagents.
Data and code availability
The datasets and code supporting the current study have been deposited to Zenodo: https://github.com/biosim-fm/In-silico-characterization (https://doi.org/10.5281/zenodo.13273453).
Acknowledgments
This work received financial support from FCT/MCTES (UIDB/50006/2020 DOI 10.54499/UIDB/50006/2020) through national funds. This work was also supported by Project InnovAntiBiofilm (ref. 101157363) financed by the European Commission (Horizon-Widera 2023-Acess-02/Horizon-CSA) and national funds through FCT/MCTES (PIDDAC): LEPABE, UIDB/00511/2020 (https://doi.org/10.54499/UIDB/00511/2020) and UIDP/00511/2020 (https://doi.org/10.54499/UIDP/00511/2020), and ALiCE, LA/P/0045/2020 (https://doi.org/10.54499/LA/P/0045/2020). This work was also supported by the FCT PhD scholarship attributed to S.F. (FCT/SFRH/BD/147276/2019) and to M. Sousa (2023.00337.BD). S.F.S. thanks FCT for grant 2020.01423.CEECIND.
Author contributions
S.F. designed the experiments, analyzed data, and wrote the manuscript with additional input from all authors. M. Sousa and F.G.M. tested the protocol. S.F.S. provided computational resources. M. Simões and S.F.S supervised the research.
Declaration of interests
The authors declare no competing interests.
Contributor Information
Manuel Simões, Email: mvs@fe.up.pt.
Sérgio F. Sousa, Email: sergiofsousa@med.up.pt.
References
- 1.Fernandes S., Borges A., Gomes I.B., Sousa S.F., Simões M. Curcumin and 10-undecenoic acid as natural quorum sensing inhibitors of LuxS/AI-2 of Bacillus subtilis and LasI/LasR of Pseudomonas aeruginosa. Food Res. Int. 2023;165 doi: 10.1016/j.foodres.2023.112519. [DOI] [PubMed] [Google Scholar]
- 2.Gonçalves A.S.C., Leitão M.M., Simões M., Borges A. The action of phytochemicals in biofilm control. Nat. Prod. Rep. 2023;40:595–627. doi: 10.1039/D2NP00053A. [DOI] [PubMed] [Google Scholar]
- 3.Hawver L.A., Jung S.A., Ng W.L. Specificity and complexity in bacterial quorum-sensing systems. FEMS Microbiol. Rev. 2016;40:738–752. doi: 10.1093/femsre/fuw014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu L., Li M., Yi G., Liao L., Cheng Q., Zhu J., Zhang B., Wang Y., Chen Y., Zeng M. Screening strategies for quorum sensing inhibitors in combating bacterial infections. J. Pharm. Anal. 2022;12:1–14. doi: 10.1016/j.jpha.2021.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bali E.B., Türkmen K.E., Erdönmez D., Sağlam N. Comparative study of inhibitory potential of dietary phytochemicals against quorum sensing activity of and biofilm formation by Chromobacterium violaceum 12472, and swimming and swarming behaviour of Pseudomonas aeruginosa PAO1. Food Technol. Biotechnol. 2019;57:212–221. doi: 10.17113/ftb.57.02.19.5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakraborty P., Dastidar D.G., Paul P., Dutta S., Basu D., Sharma S.R., Basu S., Sarker R.K., Sen A., Sarkar A., Tribedi P. Inhibition of biofilm formation of Pseudomonas aeruginosa by caffeine: a potential approach for sustainable management of biofilm. Arch. Microbiol. 2020;202:623–635. doi: 10.1007/s00203-019-01775-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhong L., Ravichandran V., Zhang N., Wang H., Bian X., Zhang Y., Li A. Attenuation of Pseudomonas aeruginosa quorum sensing by natural products: virtual screening, evaluation and biomolecular interactions. Int. J. Mol. Sci. 2020;21:2190. doi: 10.3390/ijms21062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maia E.H.B., Assis L.C., de Oliveira T.A., da Silva A.M., Taranto A.G. Structure-based virtual screening: from classical to artificial intelligence. Front. Chem. 2020;8:343. doi: 10.3389/fchem.2020.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Puertas-Martín S., Banegas-Luna A.J., Paredes-Ramos M., Redondo J.L., Ortigosa P.M., Brovarets' O.O., Pérez-Sánchez H. Is high performance computing a requirement for novel drug discovery and how will this impact academic efforts? Expert Opin. Drug Discov. 2020;15:981–986. doi: 10.1080/17460441.2020.1758664. [DOI] [PubMed] [Google Scholar]
- 10.Vieira T.F., Sousa S.F. Comparing AutoDock and VINA in ligand/decoy discrimination for virtual screening. Appl. Sci. 2019;9:4538. doi: 10.3390/app9214538. [DOI] [Google Scholar]
- 11.Fernandes M.J.G., Pereira R.B., Rodrigues A.R.O., Vieira T.F., Fortes A.G., Pereira D.M., Sousa S.F., Gonçalves M.S.T., Castanheira E.M.S. Liposomal formulations loaded with a eugenol derivative for application as insecticides: encapsulation studies and in silico identification of protein targets. Nanomaterials. 2022;12:3583. doi: 10.3390/nano12203583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lapaillerie D., Charlier C., Guyonnet-Dupérat V., Murigneux E., Fernandes H.S., Martins F.G., Magalhães R.P., Vieira T.F., Richetta C., Subra F., et al. Selection of bis-indolyl pyridines and triphenylamines as new inhibitors of SARS-CoV-2 cellular entry by modulating the spike protein/ACE2 interfaces. Antimicrob. Agents Chemother. 2022;66 doi: 10.1128/aac.00083-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vieira T.F., Martins F.G., Moreira J.P., Barbosa T., Sousa S.F. In silico identification of possible inhibitors for protein kinase B (PknB) of Mycobacterium tuberculosis. Molecules. 2021;26:6162. doi: 10.3390/molecules26206162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Natal C.M., Fernandes M.J.G., Pinto N.F.S., Pereira R.B., Vieira T.F., Rodrigues A.R.O., Pereira D.M., Sousa S.F., Fortes A.G., Castanheira E.M.S., T Gonçalves M.S. New carvacrol and thymol derivatives as potential insecticides: synthesis, biological activity, computational studies and nanoencapsulation. RSC Adv. 2021;11:34024–34035. doi: 10.1039/d1ra05616f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sousa S.F., Ribeiro A.J.M., Coimbra J.T.S., Neves R.P.P., Martins S.A., Moorthy N.S.H.N., Fernandes P.A., Ramos M.J. Protein-ligand docking in the new millennium – a retrospective of 10 years in the field. Curr. Med. Chem. 2013;20:2296–2314. doi: 10.2174/0929867311320180002. [DOI] [PubMed] [Google Scholar]
- 16.Zhang B., Li H., Yu K., Jin Z. Molecular docking-based computational platform for high-throughput virtual screening. CCF Trans. High Perform. Comput. 2022;4:63–74. doi: 10.1007/s42514-021-00086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dickerhoff J., Warnecke K.R., Wang K., Deng N., Yang D. Evaluating molecular docking software for small molecule binding to G-quadruplex DNA. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms221910801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Z., Sun H., Yao X., Li D., Xu L., Li Y., Tian S., Hou T. Comprehensive evaluation of ten docking programs on a diverse set of protein-ligand complexes: the prediction accuracy of sampling power and scoring power. Phys. Chem. Chem. Phys. 2016;18:12964–12975. doi: 10.1039/c6cp01555g. [DOI] [PubMed] [Google Scholar]
- 19.Oliveira D., Borges A., Ruiz R.M., Negrín Z.R., Distinto S., Borges F., Simões M. 2-(2-Methyl-2-nitrovinyl)furan but not furvina interfere with Staphylococcus aureus Agr quorum-sensing system and potentiate the action of fusidic acid against biofilms. Int. J. Mol. Sci. 2021;22:613. doi: 10.3390/ijms22020613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martins F.G., Melo A., Sousa S.F. Identification of new potential inhibitors of quorum sensing through a specialized multi-level computational approach. Molecules. 2021;26:2600. doi: 10.3390/molecules26092600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vieira T.F., Magalhães R.P., Simões M., Sousa S.F. Drug repurposing targeting Pseudomonas aeruginosa MvfR using docking, virtual screening, molecular dynamics, and free-energy calculations. Antibiotics (Basel) 2022;11:185. doi: 10.3390/antibiotics11020185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira T.F., Magalhães R.P., Cerqueira N.M.F.S.A., Simões M., Sousa S.F. Targeting Pseudomonas aeruginosa MvfR in the battle against biofilm formation: a multi-level computational approach. Mol. Syst. Des. Eng. 2022;7:1294–1306. doi: 10.1039/D2ME00088A. [DOI] [Google Scholar]
- 23.Magalhães R.P., Vieira T.F., Melo A., Sousa S.F. Identification of novel candidates for inhibition of LasR, a quorum-sensing receptor of multidrug resistant Pseudomonas aeruginosa, through a specialized multi-level in silico approach. Mol. Syst. Des. Eng. 2022;7:434–446. doi: 10.1039/d2me00009a. [DOI] [Google Scholar]
- 24.Cerqueira N.M.F.S.A., Gesto D., Oliveira E.F., Santos-Martins D., Brás N.F., Sousa S.F., Fernandes P.A., Ramos M.J. Receptor-based virtual screening protocol for drug discovery. Arch. Biochem. Biophys. 2015;582:56–67. doi: 10.1016/j.abb.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 25.Trott O., Olson A.J. AutoDock VINA: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verdonk M.L., Cole J.C., Hartshorn M.J., Murray C.W., Taylor R.D. Improved protein-ligand docking using GOLD. Proteins. Proteins. 2003;52:609–623. doi: 10.1002/prot.10465. [DOI] [PubMed] [Google Scholar]
- 27.Zhang N., Zhao H. Enriching screening libraries with bioactive fragment space. Bioorg. Med. Chem. Lett. 2016;26:3594–3597. doi: 10.1016/j.bmcl.2016.06.013. [DOI] [PubMed] [Google Scholar]
- 28.Miller B.R., McGee T.D., Swails J.M., Homeyer N., Gohlke H., Roitberg A.E. MMPBSA.py: an efficient program for end-state free energy calculations. J. Chem. Theory Comput. 2012;8:3314–3321. doi: 10.1021/ct300418h. [DOI] [PubMed] [Google Scholar]
- 29.Empereur-Mot C., Zagury J.F., Montes M. Screening explorer - an interactive tool for the analysis of screening results. J. Chem. Inf. Model. 2016;56:2281–2286. doi: 10.1021/acs.jcim.6b00283. [DOI] [PubMed] [Google Scholar]
- 30.Benet L.Z., Hosey C.M., Ursu O., Oprea T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016;101:89–98. doi: 10.1016/j.addr.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bell E.W., Zhang Y. DockRMSD: an open-source tool for atom mapping and RMSD calculation of symmetric molecules through graph isomorphism. J. Cheminform. 2019;11:40. doi: 10.1186/s13321-019-0362-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McClean K.H., Winson M.K., Fish L., Taylor A., Chhabra S.R., Camara M., Daykin M., Lamb J.H., Swift S., Bycroft B.W., et al. Quorum sensing and Chromobacterium violaceum: explitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 33.Borges A., Serra S., Cristina Abreu A., Saavedra M.J., Salgado A., Simões M. Evaluation of the effects of selected phytochemicals on quorum sensing inhibition and in vitro cytotoxicity. Biofouling. 2014;30:183–195. doi: 10.1080/08927014.2013.852542. [DOI] [PubMed] [Google Scholar]
- 34.Massai F., Imperi F., Quattrucci S., Zennaro E., Visca P., Leoni L. A multitask biosensor for micro-volumetric detection of N-3-oxo-dodecanoyl-homoserine lactone quorum sensing signal. Biosens. Bioelectron. 2011;26:3444–3449. doi: 10.1016/j.bios.2011.01.022. [DOI] [PubMed] [Google Scholar]
- 35.Eibergen N.R., Moore J.D., Mattmann M.E., Blackwell H.E. Potent and selective modulation of the RhlR quorum sensing receptor by using mon-native ligands: an emerging target for virulence control in Pseudomonas aeruginosa. Chembiochem. 2015;16:2348–2356. doi: 10.1002/cbic.201500357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fletcher M.P., Diggle S.P., Cámara M., Williams P. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat. Protoc. 2007;2:1254–1262. doi: 10.1038/nprot.2007.158. [DOI] [PubMed] [Google Scholar]
- 37.Welsh M.A., Eibergen N.R., Moore J.D., Blackwell H.E. Small molecule disruption of quorum sensing cross-regulation in Pseudomonas aeruginosa causes major and unexpected alterations to virulence phenotypes. J. Am. Chem. Soc. 2015;137:1510–1519. doi: 10.1021/ja5110798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiong Y.-Q., Wamel W.V., Nast C.C., Yeaman M.R., Cheung A.L., Bayer A.S. Activaction and transcriptional interaction between agr RNAII and RNAIII in Staphylococcus aureus in vitro and in an experimental endocarditis model. J. Infect. Dis. 2002;186:668–677. doi: 10.1086/342046. [DOI] [PubMed] [Google Scholar]
- 39.Nakayama J., Tanaka E., Kariyama R., Nagata K., Nishiguchi K., Mitsuhata R., Uemura Y., Tanokura M., Kumon H., Sonomoto K. Siamycin attenuates fsr quorum sensing mediated by a gelatinase biosynthesis-activating pheromone in Enterococcus faecalis. J. Bacteriol. 2007;189:1358–1365. doi: 10.1128/JB.00969-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Agrawal P., Singh H., Srivastava H.K., Singh S., Kishore G., Raghava G.P.S. Benchmarking of different molecular docking methods for protein-peptide docking. BMC Bioinform. 2019;19:426. doi: 10.1186/s12859-018-2449-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets and code supporting the current study have been deposited to Zenodo: https://github.com/biosim-fm/In-silico-characterization (https://doi.org/10.5281/zenodo.13273453).