Abstract
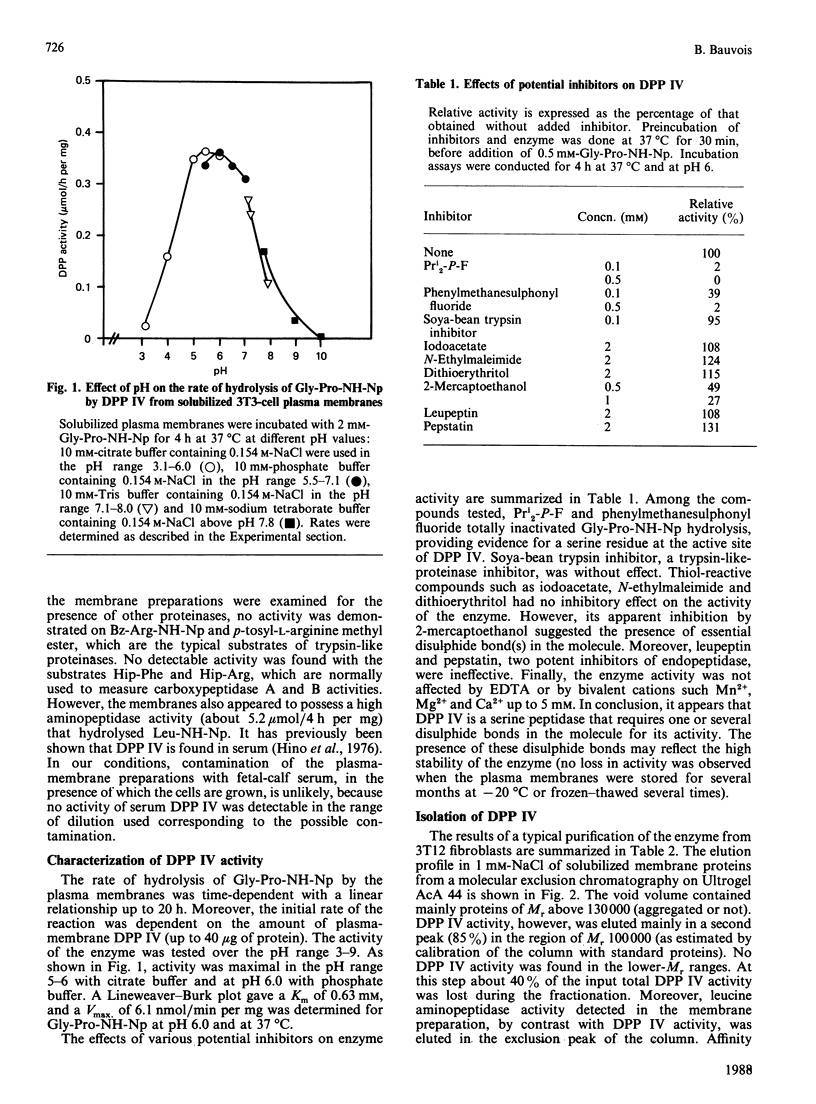
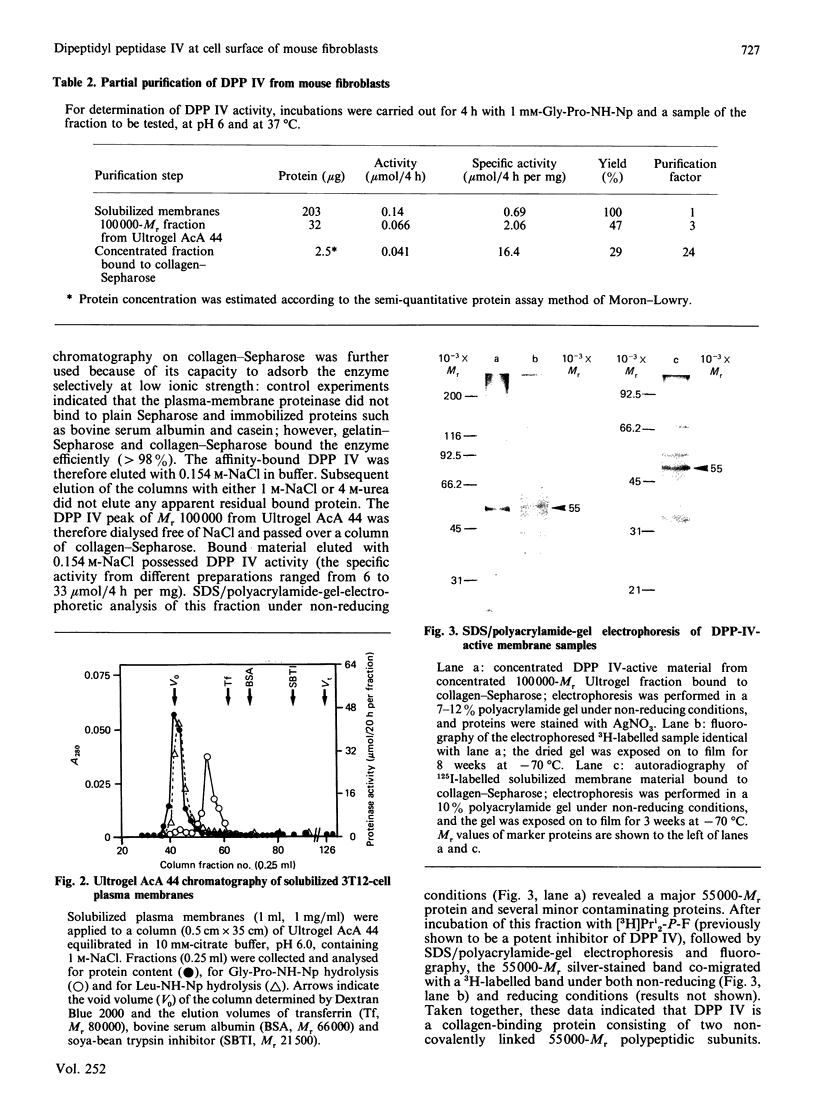
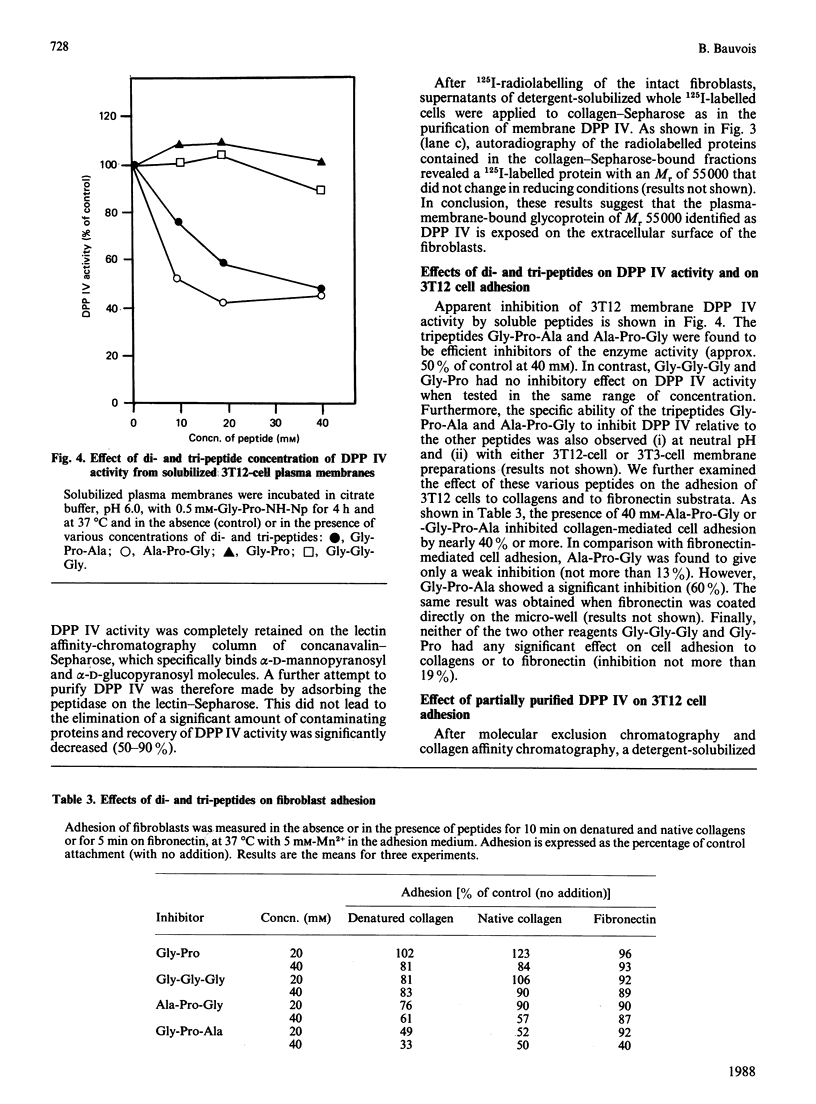
A dipeptidyl aminopeptidase (DPP) was detected in plasma membranes from normal (3T3) and transformed (3T12) mouse fibroblasts. This enzyme was active in cleaving the prolyl bond in the synthetic dipeptide nitroanilide Gly-Pro-NH-Np, which is a specific substrate for DPP IV (Km 0.63 mM and Vmax 6.1 nmol/min per mg at pH 6.0 and 37 degrees C). However, it did not degrade Pro-NH-Np or other dipeptide nitroanilides such as Gly-Arg-NH-Np or Val-Ala-NH-Np. The enzyme was totally inhibited by di-isopropyl phosphorofluoridate (Pri2-P-F) and by phenylmethanesulphonyl fluoride, indicating a serine catalytic site for the proteinase. DPP IV is a glycoprotein that specifically recognized immobilized gelatin and type I collagen. Upon molecular exclusion chromatography, the proteinase exhibited an apparent Mr of 100,000. SDS/polyacrylamide-gel electrophoresis under non-reducing and reducing conditions revealed that the [3H]Pri2-P-protein was exclusively represented by a polypeptide of Mr 55,000. This suggested that DPP IV consists of two non-covalently linked 55,000-Mr subunits. Fibroblast adhesion to native or denatured collagen was significantly inhibited by the two dipeptide inhibitors of DPP IV, Gly-Pro-Ala and Ala-Pro-Gly, but not by the peptides Gly-Pro and Gly-Gly-Gly, which are not inhibitors of the proteinase. Moreover, preliminary fractionation of DPP IV by molecular exclusion chromatography and affinity chromatography indicated that this material was active in disrupting cell adhesion to collagens. Taken together, the above data suggest that a fibroblast membrane-associated collagen-binding glycoprotein, DPP IV, may play a role in cell attachment to collagen.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Yamada S. S., Yamada K. M. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986 Feb;102(2):442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aumailley M., Timpl R. Attachment of cells to basement membrane collagen type IV. J Cell Biol. 1986 Oct;103(4):1569–1575. doi: 10.1083/jcb.103.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauvois B., Roth S. A collagen:glucosyltransferase at the surface of malignant fibroblasts. J Cell Physiol. 1985 Aug;124(2):213–218. doi: 10.1002/jcp.1041240207. [DOI] [PubMed] [Google Scholar]
- Bauvois B., Roth S. Initial adhesion of murine fibroblasts to collagen and fibronectin occurs by two mechanisms. Cell Biochem Funct. 1987 Oct;5(4):281–287. doi: 10.1002/cbf.290050407. [DOI] [PubMed] [Google Scholar]
- Becker U., Fietzek P. P., Furthmayr H., Timpl R. Non-helical sequences of rabbit collagen. Correlation with antigenic determinants detected by rabbit antibodies in homologous regions of rat and calf collagen. Eur J Biochem. 1975 Jun;54(2):359–366. doi: 10.1111/j.1432-1033.1975.tb04146.x. [DOI] [PubMed] [Google Scholar]
- Charonis A. S., Tsilibary E. C., Yurchenco P. D., Furthmayr H. Binding of laminin to type IV collagen: a morphological study. J Cell Biol. 1985 Jun;100(6):1848–1853. doi: 10.1083/jcb.100.6.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang T. M., Kang A. H. Isolation and purification of collagen alpha 1(I) receptor from human platelet membrane. J Biol Chem. 1982 Jul 10;257(13):7581–7586. [PubMed] [Google Scholar]
- Dedhar S., Ruoslahti E., Pierschbacher M. D. A cell surface receptor complex for collagen type I recognizes the Arg-Gly-Asp sequence. J Cell Biol. 1987 Mar;104(3):585–593. doi: 10.1083/jcb.104.3.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elovson J. Biogenesis of plasma membrane glycoproteins. Purification and properties of two rat liver plasma membrane glycoproteins. J Biol Chem. 1980 Jun 25;255(12):5807–5815. [PubMed] [Google Scholar]
- Fietzek P. P., Kühn K. The primary structure of collagen. Int Rev Connect Tissue Res. 1976;7:1–60. doi: 10.1016/b978-0-12-363707-9.50007-1. [DOI] [PubMed] [Google Scholar]
- Geiger P. J., Bessman S. P. Protein determination by Lowry's method in the presence of sulfhydryl reagents. Anal Biochem. 1972 Oct;49(2):467–473. doi: 10.1016/0003-2697(72)90450-2. [DOI] [PubMed] [Google Scholar]
- Grinnell F., Minter D. Attachment and spreading of baby hamster kidney cells to collagen substrata: effects of cold-insoluble globulin. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4408–4412. doi: 10.1073/pnas.75.9.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannan G. N., McAuslan B. R. Rapid purification of collagen binding glycoproteins secreted by cultured endothelial cells. Biochem Int. 1983 Mar;6(3):375–384. [PubMed] [Google Scholar]
- Hanski C., Huhle T., Reutter W. Involvement of plasma membrane dipeptidyl peptidase IV in fibronectin-mediated adhesion of cells on collagen. Biol Chem Hoppe Seyler. 1985 Dec;366(12):1169–1176. doi: 10.1515/bchm3.1985.366.2.1169. [DOI] [PubMed] [Google Scholar]
- Hanski C., Reutter W., Evans W. H. Turnover of the protein and carbohydrate moieties of a 105kD glycoprotein of plasma membranes from mouse liver determined by immunoprecipitation. Eur J Cell Biol. 1984 Jan;33(1):123–126. [PubMed] [Google Scholar]
- Hino M., Fuyamada H., Nagatsu T., Kurokawa S., Okuyama S. Glycylprolyl beta-naphthylamidase activities in serum, liver and kidney of rats in chronic carbon tetrachloride intoxication. Clin Chim Acta. 1976 Feb 16;67(1):103–105. doi: 10.1016/0009-8981(76)90223-0. [DOI] [PubMed] [Google Scholar]
- Hixson D. C., DeLourdes Ponce M., Allison J. P., Walborg E. F., Jr Cell surface expression by adult rat hepatocytes of a non-collagen glycoprotein present in rat liver biomatrix. Exp Cell Res. 1984 Jun;152(2):402–414. doi: 10.1016/0014-4827(84)90641-4. [DOI] [PubMed] [Google Scholar]
- Horwitz A., Duggan K., Greggs R., Decker C., Buck C. The cell substrate attachment (CSAT) antigen has properties of a receptor for laminin and fibronectin. J Cell Biol. 1985 Dec;101(6):2134–2144. doi: 10.1083/jcb.101.6.2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato T., Nagatsu T., Fukasawa K., Harada M., Nagatsu I., Sakakibara S. Successive cleavage of N-terminal Arg1--Pro2 and Lys3-Pro4 from substance P but no release of Arg1-Pro2 from bradykinin, by X-Pro dipeptidyl-aminopeptidase. Biochim Biophys Acta. 1978 Aug 7;525(2):417–422. doi: 10.1016/0005-2744(78)90237-1. [DOI] [PubMed] [Google Scholar]
- Kleinman H. K., Klebe R. J., Martin G. R. Role of collagenous matrices in the adhesion and growth of cells. J Cell Biol. 1981 Mar;88(3):473–485. doi: 10.1083/jcb.88.3.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler J. K., Nudelman E. D., Hakomori S. A collagen-binding protein on the surface of ejaculated rabbit spermatozoa. J Cell Biol. 1980 Aug;86(2):529–536. doi: 10.1083/jcb.86.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotite N. J., Cunningham L. W. Specific adsorption of a platelet membrane glycoprotein by human insoluble collagen. J Biol Chem. 1986 Jun 25;261(18):8342–8347. [PubMed] [Google Scholar]
- Kreisel W., Heussner R., Volk B., Büchsel R., Reutter W., Gerok W. Identification of the 110000 Mr glycoprotein isolated from rat liver plasma membrane as dipeptidylaminopeptidase IV. FEBS Lett. 1982 Oct 4;147(1):85–88. doi: 10.1016/0014-5793(82)81016-8. [DOI] [PubMed] [Google Scholar]
- Kreisel W., Volk B. A., Büchsel R., Reutter W. Different half-lives of the carbohydrate and protein moieties of a 110,000-dalton glycoprotein isolated from plasma membranes of rat liver. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1828–1831. doi: 10.1073/pnas.77.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurkinen M., Taylor A., Garrels J. I., Hogan B. L. Cell surface-associated proteins which bind native type IV collagen or gelatin. J Biol Chem. 1984 May 10;259(9):5915–5922. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lesot H., Kühl U., Mark K. Isolation of a laminin-binding protein from muscle cell membranes. EMBO J. 1983;2(6):861–865. doi: 10.1002/j.1460-2075.1983.tb01514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malinoff H. L., Wicha M. S. Isolation of a cell surface receptor protein for laminin from murine fibrosarcoma cells. J Cell Biol. 1983 May;96(5):1475–1479. doi: 10.1083/jcb.96.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Czech M. P. The subunit structures of two distinct receptors for insulin-like growth factors I and II and their relationship to the insulin receptor. J Biol Chem. 1982 May 10;257(9):5038–5045. [PubMed] [Google Scholar]
- Massagué J. Epidermal growth factor-like transforming growth factor. II. Interaction with epidermal growth factor receptors in human placenta membranes and A431 cells. J Biol Chem. 1983 Nov 25;258(22):13614–13620. [PubMed] [Google Scholar]
- McDonald J. K., Hoisington A. R., Eisenhauer D. A. Partial purification and characterization of an ovarian tripeptidyl peptidase: a lysosomal exopeptidase that sequentially releases collagen-related (Gly-Pro-X) triplets. Biochem Biophys Res Commun. 1985 Jan 16;126(1):63–71. doi: 10.1016/0006-291x(85)90571-6. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Mollenhauer J., Bee J. A., Lizarbe M. A., von der Mark K. Role of anchorin CII, a 31,000-mol-wt membrane protein, in the interaction of chondrocytes with type II collagen. J Cell Biol. 1984 Apr;98(4):1572–1579. doi: 10.1083/jcb.98.4.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mollenhauer J., von der Mark K. Isolation and characterization of a collagen-binding glycoprotein from chondrocyte membranes. EMBO J. 1983;2(1):45–50. doi: 10.1002/j.1460-2075.1983.tb01378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Pierschbacher M. D., Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties expected of a fibronectin receptor. Cell. 1985 Jan;40(1):191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- Rao N. C., Barsky S. H., Terranova V. P., Liotta L. A. Isolation of a tumor cell laminin receptor. Biochem Biophys Res Commun. 1983 Mar 29;111(3):804–808. doi: 10.1016/0006-291x(83)91370-0. [DOI] [PubMed] [Google Scholar]
- Rauvala H., Hakomori S. I. Studies on cell adhesion and recognition. III. The occurrence of alpha-mannosidase at the fibroblast cell surface, and its possible role in cell recognition. J Cell Biol. 1981 Jan;88(1):149–159. doi: 10.1083/jcb.88.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseman S. The synthesis of complex carbohydrates by multiglycosyltransferase systems and their potential function in intercellular adhesion. Chem Phys Lipids. 1970 Oct;5(1):270–297. doi: 10.1016/0009-3084(70)90024-1. [DOI] [PubMed] [Google Scholar]
- Rubin K., Gullberg D., Borg T. K., Obrink B. Hepatocyte adhesion to collagen. Isolation of membrane glycoproteins involved in adhesion to collagen. Exp Cell Res. 1986 May;164(1):127–138. doi: 10.1016/0014-4827(86)90460-x. [DOI] [PubMed] [Google Scholar]
- Rubin K., Hök M., Obrink B., Timpl R. Substrate adhesion of rat hepatocytes: mechanism of attachment to collagen substrates. Cell. 1981 May;24(2):463–470. doi: 10.1016/0092-8674(81)90337-8. [DOI] [PubMed] [Google Scholar]
- Schor S. L., Court J. Different mechanisms in the attachment of cells to native and denatured collagen. J Cell Sci. 1979 Aug;38:267–281. doi: 10.1242/jcs.38.1.267. [DOI] [PubMed] [Google Scholar]
- Shur B. D. Embryonal carcinoma cell adhesion: the role of surface galactosyltransferase and its 90K lactosaminoglycan substrate. Dev Biol. 1983 Oct;99(2):360–372. doi: 10.1016/0012-1606(83)90286-5. [DOI] [PubMed] [Google Scholar]
- Urushihara H., Yamada K. M. Evidence for involvement of more than one class of glycoprotein in cell interactions with fibronectin. J Cell Physiol. 1986 Mar;126(3):323–332. doi: 10.1002/jcp.1041260302. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Rao C. N., Hassell J. R., Liotta L. A., Martin G. R., Kleinman H. K. Interactions of basement membrane components. Biochim Biophys Acta. 1983 Dec 27;761(3):278–283. doi: 10.1016/0304-4165(83)90077-6. [DOI] [PubMed] [Google Scholar]
- Yamada K. M. Cell surface interactions with extracellular materials. Annu Rev Biochem. 1983;52:761–799. doi: 10.1146/annurev.bi.52.070183.003553. [DOI] [PubMed] [Google Scholar]



