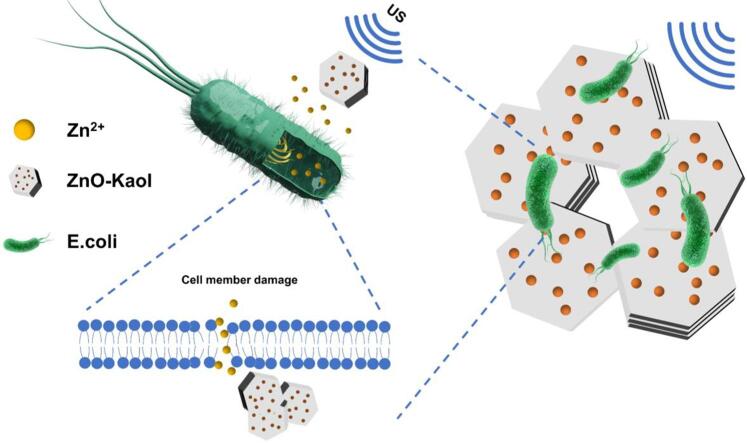
Graphical abstract
In this study, antimicrobial materials are treated by means of high-frequency ultrasound to release more metal ions and obtain more excellent antimicrobial effect, which provides a new idea for the development of antimicrobial field.
Keywords: Zinc oxide, Kaolinite nano-clay, Ultrasound, Antibacterial activity
Abstract
Bacterial infections pose considerable health risks, emphasising the critical need for effective and biocompatible antibacterial drugs. Considerably, we developed an efficient antimicrobial system incorporating the combined potential of high-frequency ultrasound and antimicrobial drugs against bacterial infections. A ZnO–kaolinite (Kaol) composite with antibacterial properties was synthesised by growing ZnO on the Kaol nano-clay surface using the co-precipitation method. High-frequency ultrasound efficiently promotes the release of Zn2+, which enhances the antibacterial properties. Furthermore, in-depth in vitro antibacterial studies and bacterial live/dead staining experiments validate the exceptionally high antibacterial performance of the composite. Therefore, owing to the synergistic effects of high-frequency ultrasound and antibacterial properties, the as-prepared novel antibacterial composite is a promising potential substitute for conventional antibacterial agents.
1. Introduction
Bacterial infections present a substantial hazard to human health and the global economy [1], [2], [3], [4], emphasising the vital importance of novel and effective antimicrobial interventions [5], [6]. ZnO nanoparticles have emerged as prominent inorganic antibacterial agents [7], [8], [9], with nano-ZnO demonstrating efficacy in sterilisation through the liberation of Zn2+ [10], [11], [12]. The release kinetics of Zn2+ are frequently enhanced via the size reduction of nano-ZnO particles [13], pH manipulation of the surrounding environment [14], [15] and utilisation of near-infrared light therapy [3]. These strategies potentially increase the release rate of Zn2+ by manipulating its release kinetics [16], [17]. However, research on the controlled release of Zn2+ remains limited.
Ultrasound (US) has gained increasing importance in the biomedical field [18], [19], [20]. However, its application in water disinfection [21] and food sterilisation [22] may not be suitable for biomedical purposes [23]. This is because owing to its strong cavitation effects [24], [25], low-frequency US can potentially disrupt cell membranes [26]. However, high-frequency US is known for its rapid [27], non-invasive [28] and controllable characteristics [29], and it is predominantly utilised in research fields such as ultrasonic imaging and drug delivery [30]. Although previous studies have demonstrated that high-frequency US can alter membrane permeability [31], [32] and produce reactive oxygen species (ROS) when combined with acoustic sensitisers to achieve antibacterial effects [33], [34], [35], the use of high-frequency US to control the release of antibacterial Zn2+ from nano-ZnO particles remains unexplored.
Herein, we prepared a highly dispersive, broad-spectrum and high-performing antibacterial composite of ZnO–kaolinite (Kaol) using the co-precipitation method. The composite was subjected to high-frequency US treatment to improve Zn2+ release and consequently enhance its antibacterial effectiveness. This study primarily (a) examines the physical and chemical properties, such as the crystal structure, particle size and chemical state, of ZnO–Kaol; (b) identifies the optimal frequency and duration of high-frequency US; (c) determines the mechanism underlying US-mediated ion release and (d) evaluates the antibacterial efficacy of ZnO–Kaol against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). This study also broadens the application scope of high-frequency US by facilitating the release of Zn2+, thereby enhancing the potential of collaborative utilisation of US and antibacterial materials.
2. Experimental
2.1. Reagents and materials
Kaol was sourced from Ganzhou, Jiangxi, and halloysite nanotubes (HNTs) were obtained from Guangzhou Runwo Materials Technology Co., Ltd. Medical-grade Kaol clay (Kaol(C)), Zn(NO3)2·6H2O and NaOH were purchased from Aladdin Reagent Co., Ltd. ZnO and hexametaphosphate sodium were obtained from Shandong Xiya Chemical Co., Ltd. Ethanol was purchased from China National Pharmaceutical Group Chemical Reagent Co., Ltd. E. coli (ATCC25922) and S. aureus (ATCC25922) were provided by the Xiangya Hospital of Central South University.
2.2. Purification of kaolin
Initially, 200 g Kaol was dispersed in 1500 mL deionised water, and 0.8 g of hexametaphosphate was subsequently added. Next, the mixture was stirred at 1000 rpm for 2 h using a stirrer and subsequently allowed to settle for 1 h to facilitate the precipitation of quartz. Thereafter, the precipitate was filtered, and the upper solution was collected. To ensure complete removal of the precipitate, the solution was centrifuged at 2000 rpm. The resulting upper solution was centrifuged at 7000 rpm to collect the precipitate. Finally, the collected precipitate was dried in an oven at 60 °C for 6 h to obtain purified Jiangxi Kaol clay.
2.3. Preparation of composite materials
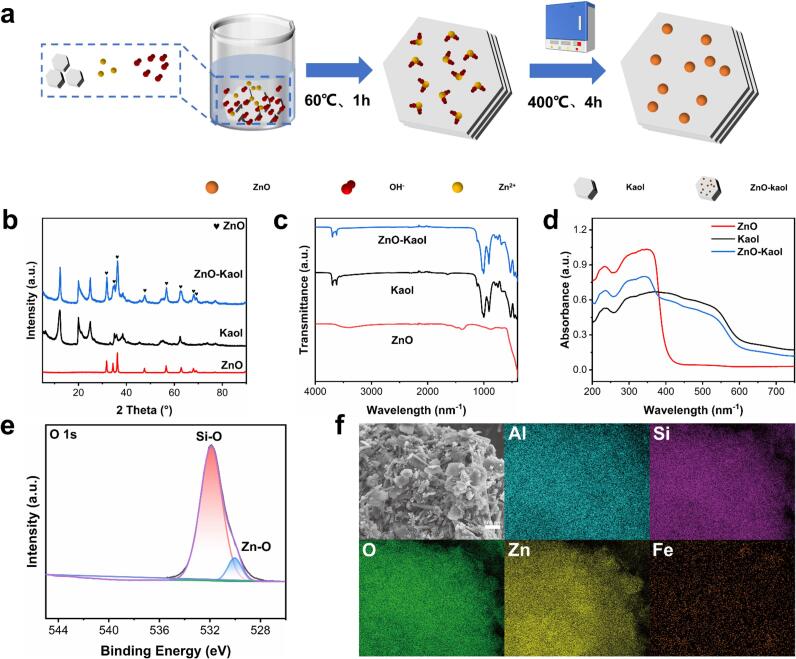
In total, 0.8 g of each mineral (Kaol, Kaol(C) and HNTs) and 0.735 g of Zn(NO3)2·6H2O were dispersed in 80 mL of an ethanol solution. Next, the mixture was stirred at 60 °C using a magnetic stirrer to form a stable suspension. Subsequently, 200 mL of an ethanol solution containing 0.247 g NaOH was gradually added dropwise to the suspension over 1 h, followed by continuous stirring for an additional 1 h. The resulting mixture was washed several times with deionised water, and the washed solution was dried at 60 °C for 6 h. The dried composite material was ground and subsequently calcined in a muffle furnace at 400 °C for 4 h. Afterwards, the sample was ground to obtain the final material. Herein, the pink Kaol clay loaded with ZnO is denoted as ZnO–Kaol. The Kaol(C) loaded with ZnO was denoted by ZnO–Kaol(C), and the HNTs loaded with ZnO were labelled ZnO–HNTs.
2.4. Synthesis of nano-zinc
Initially, 2.4487 g Zn(NO3)2·6H2O was dispersed in a 100 mL ethanol solution. The mixture was stirred at 60 °C using a magnetic stirrer to form a stable suspension. Subsequently, a 250 mL ethanol solution containing 0.8231 g NaOH was added dropwise to the suspension over 1 h, and the reaction was continued for an additional 1 h. The resulting mixture was washed several times with deionised water, and the washed solution was dried at 60 °C for 6 h. The dried solid sample was ground and collected to obtain nano-ZnO.
2.5. Characterization
The crystal phase composition of the samples was determined via X-ray diffraction (XRD, D8 ADVANCE) analysis at 5°–90° at a scanning speed of 5°/min. Fourier transform infrared (FT-IR, Nicolet iS50) spectroscopy was employed to study the composition and changes in the functional groups at 4000–400 cm−1. The morphology of the composite materials was analysed using scanning electron microscopy (SEM, GeminiSEM 300) and transmission electron microscopy (TEM, Talos F200x G2). The elemental distribution on the material surfaces was determined via energy-dispersive X-ray spectroscopy. The elemental variance states of the composite materials were scrutinised using X-ray photoelectron spectroscopy (XPS, Thermo Scientific K-Alpha). The ultraviolet–visible (UV–vis, Hitachi) absorption spectra of the composite materials were examined using a solid-state UV–vis spectrophotometer. ROS detection was performed using electron spin resonance (ESR, EMXplus-6/1) spectroscopy.
2.6. Device design
A uniquely designed acoustic generation device was utilized. This device primarily consisted of a circular piezoelectric transducer (PZT), cylindrical shell–based device foundation, detachable sample cuvette and threaded lid. It was crafted using three-dimensional (3D) printing materials and was composed of a circular piece of piezoelectric ceramic (Changzhou Ultrasonic Electronic, PZT 51, D: 15 mm), resin-based elements (30 × 30 × 42 mm3) and several shell-like structures with distinct dimensions and openings. The assembly includes a rectangular base featuring dual openings, which encloses a cylindrical shell of an inner diameter (ID), outer diameter (OD), and height (H) of 15.4 mm, 18 mm, and 5 mm respectively. Atop the base, there's another cylindrical shell with ID, OD, and H of 16 mm, 20 mm, and 30 mm. This shell is filled with an impedance-matching layer to facilitate the entry of acoustic waves into the cuvette, which has an ID, OD, and H of 14 mm, 15 mm, and 40 mm. The cylindrical shell also features a threaded ring on its outer wall. This ring is used alongside a threaded lid (with an ID, OD, and H of 18.7 mm, 26 mm, and 22 mm) to secure the cuvette. All components of this assembly were produced through 3D printing. Fig. S1 provides physical and structural illustrations of the device.
2.7. Device operation using traveling wave
The upper cylindrical shell was filled with 700 μL water to ensure the smooth entry of acoustic waves into the cuvette. The transducer was driven by a signal generator (Keysight 33500B). To generate acoustic waves in the acoustic generation device, the transducers were driven at 137 kHz, 2.16 MHz, 4.5 MHz and 6.9 MHz (Fig. S2), measured using a frequency response analyser (CleverScope, CS320A-FRA, New Zealand) at a peak–peak voltage of 20 V.
2.8. Acoustic pressure measurements
The acoustic pressure in the custom-built acoustic trapping device was measured using a fibre optic hydrophone (FOH; Precision Acoustics, Dorchester, UK) consisting of an interferometer and a sensor. The sensor was mounted on a 3D displacement stage (PDV PP110-75), and a raster scan with a complimentary scan step of 20 μm was performed to measure the acoustic pressure in a 2D plane. The voltage signal was displayed on an oscilloscope (Keysight EDUX1002A) to visualise the acoustic pressure.
2.9. Simulation of the interaction between acoustic wave and microparticles
Simulations were conducted using the finite element method. To simulate ZnO nanoparticles, independent spheres (10 nm in diameter, made of ZnO) were used. For kaolinite-ZnO composites, square sheets (800 nm × 800 nm × 25 nm, made of kaolinite) with a ZnO sphere (10 nm in diameter) on the surface were employed. Each model was placed in a cylindrical water environment. A plane wave radiation condition was set on the cylinder's bottom surface, with the radiation pressure set to 0.1 MPa based on hydrophone measurements. Subsequently, acoustic and structural interaction studies were performed.
2.10. Zn2+ release translation
A 3 mL solution of the composite material (1 mg/mL) was subjected to US treatment using a self-constructed device. Different US frequencies of 137 kHz, 2.16 MHz, 4.5 MHz and 6.9 MHz were employed by changing the specifications of the piezoelectric ceramics and the output frequency of the signal generator. Different treatment durations of 1, 3, 5 and 10 min were achieved by controlling the duration of the US treatment. Following the US treatment, the composite material solution stood for 30 min under specified conditions. Subsequently, it was transferred to a centrifuge tube and centrifuged at 8000 rpm for 10 min. The supernatant was collected using a 0.22 μm filter head. The Zn2+ concentration in the supernatant was determined via inductively coupled plasma–optical emission spectrometry (ICP–OES; Avio200).
2.11. Particle size analysis
In total, 1 mg of the composite material was disaggregated in 1 mL of deionised water and mixed for 5 s using a homogeniser. The solution was diluted step-by-step 10−3 times its original concentration. The diluted solution (5 μL) was subsequently dropped onto a TEM copper grid and air-dried. The composite material solution (1 mg/mL) was treated using US (2.16 MHz; 1.5 V) for 5 min and diluted to 10−3 times its original concentration. Subsequently, 5 μL of the diluted solution was dropped onto a TEM copper grid and air-dried. Thereafter, TEM determined the morphology of the composite material. The resulting image was used for statistical analysis of the ZnO particle size.
2.12. Antibacterial experiment
The plate counting method was employed for bacterial detection. Initially, 50 μL of the original bacterial solution was added to 5 mL of the liquid medium and incubated in a constant-temperature incubator for 12 h. The resuscitated bacterial solution was diluted to achieve an OD 600 value of 0.10 ± 0.01. Subsequent dilutions were performed to 10−2 times. Next, 4 mg of the material was added to 4 mL of the liquid medium, followed by 40 μL of the diluted bacterial solution. The mixture was sonicated for 5 min and incubated at a constant temperature for 4 h. The incubated bacteria were diluted to 10−3 times their initial concentration and applied to agar plates, which were subsequently incubated at 37 °C for 24 h. The inhibition rate was calculated by counting the number of colonies in the experimental and blank groups. All plate experiments were simultaneously performed in triplicate.
2.13. Bacterial viability staining
To assess bacterial viability, laser confocal microscopy was employed in conjunction with a live/dead bacterial viability kit. Calcein xanthophyll/propidium iodide was chosen as the fluorescent dye. Calcium xanthophyll–stained live bacteria fluoresce in green, whereas propidium iodide–stained dead bacteria exhibit red fluorescence. E. coli and S. aureus bacteria were divided into four groups for culturing: blank, US, material-added and material + US groups. After centrifugation, the samples were stained with propidium iodide and calcein (1 and 10 µg/mL) for 30 min. After washing with phosphate buffer solution thrice, bacterial growth was observed using laser confocal microscopy.
3. Results and discussion
3.1. Characterization of ZnO-Kaol
The ZnO–Kaol composites were obtained by loading ZnO onto the Kaol surface via the co-precipitation method using Kaol as the substrate (Fig. 1a). XRD analysis was performed to identify the composition and crystal structure of the composites. ZnO nanoparticles have a hexagonal wurtzite structure (JCPDS Card No. 36-1451) [36], which was also observed for the ZnO–Kaol material (Fig. 1b). Furthermore, all the peaks matched those of Kaol, with the exception of ZnO's diffraction peak. This suggests that ZnO was successfully added to the Kaol surface without changing its crystal lattice [37], [38], [39], [40]. Importantly, FT-IR analysis shows that ZnO-Kaol has characteristic bands (Si-O, Al-OH, and Si-O-Al resonance bands), similar to those of Kaol. This indicates that the Kaol structure was not altered during this modification (Fig. 1c) [41]. The UV–vis analysis (Fig. 1d) revealed strong absorption bands at approximately 350 nm for ZnO–Kaol and ZnO, which were attributed to the fundamental bandgap energy of ZnO crystals resulting from the electron jumps from the valence band to the conduction band. The remaining absorption peaks observed for ZnO–Kaol closely resemble those of Kaol, indicating that the composite retains the UV absorption properties of ZnO and Kaol.
Fig. 1.
Fabrication process of ZnO–Kaol and characterisation results; (a) schematics of the synthetic process of ZnO–Kaol; (b) XRD, (c) FT-IR and (d) UV–vis profiles of ZnO, Kaol and ZnO–Kaol; (e) high-resolution O 1s spectrum of ZnO–Kaol; (f) SEM image (scale bar = 500 nm) and elemental mapping of ZnO–Kaol.
The valence states of each component of the material were analysed using full-range XPS. Al, O and Si detected in the wide scan (Fig. S3) were related to the surface composition of Kaol. In the Zn 2p spectrum, the peaks at 1021.88 and 1044.9 eV were attributed to the Zn 2p2/3 and Zn 2p1/2 electronic states, respectively, with a d-value of 23.02 eV—which is consistent with the binding energy of ZnO. In the O 1 s spectrum, the peaks at 533.1, 532.1 and 530.8 eV were attributed to Si–O, Al–OH and Zn–O, respectively (Fig. 1e). Furthermore, the morphology of the ZnO–Kaol composites and their elemental distribution were characterised (Fig. 1f). The uniform particle size distribution of ZnO loaded on the Kaol surface (particle size of 13 nm) indicated that Kaol effectively prevents the agglomeration of ZnO nanoparticles, and a strong interaction exists between ZnO and Kaol, which renders the tight loading of these spherical particles on Kaol. Moreover, the energy spectrum analysis demonstrated a uniform distribution of ZnO on the Kaol surface [42]. All the characterisation results effectively indicated that ZnO was successfully loaded on the Kaol surface.
3.2. Ultrasonic promotion of Zn2+ release
The US waves with the aforementioned frequencies generated using a signal generator were transmitted through the impedance-matching layer between the cylindrical shell and sample cuvette, eventually reaching the solution containing the samples. Thereafter, the acoustic pressure within the sample cuvette was detected using an FOH, obtaining characteristic curves with the corresponding wavelengths (Fig. 2a–c and Fig. S4). Because the lowest frequency of the US used was 137 kHz, the corresponding acoustic pressure was the highest owing to the cavitation effect. Notably, the highest acoustic pressure of the high-frequency US of 2.16 MHz was 0.075 MPa. Without the matching layer, the sound field was undetectable because an air layer hindered sound transmission between the detachable sample pool and the device foundation (Fig. S4). The US device was continuously, sustainably operated for 10 min, and the thermal changes obtained by the device were noted. During this short period, US did not induce considerable thermal changes in the sample, avoiding any substantial impact of thermal effects on the release of Zn2+ (Fig. 2d and e).
Fig. 2.
(a–d) Relation between the acoustic pressure and axial displacement at the frequencies of (a) 2.16, (b) 4.45 and (c) 6.9 MHz with (black) and without (red) the impedance-matching layer; (d) relation between the temperature and operational time during the operation of the US device; (e) graph of the temperature measurement of the US device during the operational state of 10 min.
The Kaol material possesses a combination of lamellar and tubular structures. Considering the effects of the material structure, lamellar Kaol(C) (Figs. S5 and S6) and tubular HNTs (Figs. S7 and S8) loaded with ZnO were utilised to assess the effects of US on Zn2+ release. Initially, the particle sizes of ZnO–Kaol, ZnO–Kaol(C) and ZnO–HNTs were determined as 3.072, 3.638 and 1.655 μm, respectively (Fig. S9). To demonstrate that US cannot remove ZnO from ZnO–Kaol, FT-IR analyses were performed on ZnO, ZnO dispersed in water, and the ZnO–Kaol supernatant obtained after US treatment. No Zn–O energy bands were detected in the ZnO–Kaol supernatant (Fig. S10). The 2 mL samples of the sonicated material solution were freeze-dried, then re-dispersed in 200 μL and 20 μL of pure water for TEM tests. These tests did not detect any ZnO nanoparticles (Figs. S11–S13). These findings confirm that the ICP test is specific to Zn2+. The experimental results proved that the ICP test exclusively targeted Zn2+. Subsequently, an ICP test was conducted on the untreated material solution, revealing that the Zn2+ release did not considerably change within 10 min (Fig. 3a); this indicates that the composite can stabilise Zn2+ release in an aqueous environment. Ultrasonic mechanical effects are affected by three primary factors: voltage, frequency and time; therefore, these three factors should be investigated. The experiment involving the ultra-sonication of the material solution at 1.5, 3 and 4.5 V revealed that the voltage variations did not exert a substantial effect on Zn2+ release (Fig. 3b). Therefore, the voltage was 1.5 V for subsequent experiments. Next, US waves with frequencies of 137 kHz, 2.16 MHz, 4.45 MHz and 6.9 MHz were used to treat the three structures of the materials, and the results revealed that the high- and low-frequency US treatments enhanced the release of Zn2+. The largest release of Zn2+ was observed during the treatment at 2.16 MHz (Fig. 3c), which was consistent with the previous observation of the highest US acoustic pressure at 2.16 MHz. Therefore, the composites were subsequently treated at the US frequency of 2.16 MHz.
Fig. 3.
Zn2+ solubility versus time for ZnO–Kaol, ZnO–HNTs and ZnO–Kaol(C); release of Zn2+ at different ultrasonic (b) voltages, (c) frequencies and (d) times.
The time-gradient ultra-sonication of the materials revealed that ultra-sonication did not considerably promote the release of Zn2+ from ZnO nanoparticles, probably because the wavelength of US was substantially larger than the particle size of ZnO nanoparticles (20 nm), and therefore, US could not affect the ZnO nanoparticles. The maximum release was observed after 1 min of the ultra-sonication of ZnO–Kaol. However, the release of ZnO–HNTs and ZnO–Kaol(C) reached saturation at the treatment times of 3 and 5 min, respectively (Fig. 3d). The morphology of the Kaol lamellae interspersed with tubular materials provides a complex environment for US scattering and reflection. The simulation results suggest a significant increase in the stress at the location of the attachment point, which is conducive to the US-promoted release (Figs. S14 and S15). Whereas, the wavelength of ultrasound (2.16 MHz, 694 μm) is much larger than the diameter of nanoparticles (10 nm), the ultrasound has almost no effect on ZnO nanoparticles.
To investigate the effects of US on Zn2+ release, time-series SEM and TEM analyses were conducted (Fig. 4). In the absence of US, ZnO maintained its spherical morphology, exhibiting negligible changes in the particle size. However, with increasing US treatment time, the particle size of ZnO gradually decreased. The ZnO particle size analysis using TEM revealed a reduction in the ZnO particle size of ZnO–Kaol from 13 nm to 10 nm. Similar time-series experiments performed on ZnO–Kaol(C) revealed similar results, with a notable reduction in the ZnO particle size from 16 nm to 10 nm (Fig. S16). Therefore, ultra-sonication promoted the release of Zn2+ by facilitating the dissolution of ZnO.
Fig. 4.
SEM images (scale bar = 300 nm), TEM images (scale bar = 100 nm) and particle sizes of the ZnO–Kaol composites at different ultrasonic times.
3.3. Antibacterial activity and mechanism
The antibacterial activity of the ZnO–Kaol composites was confirmed against S. aureus and E. coli using the plate counting method (Fig. 5a). Upon the treatment of the bacteria exclusively with US, S. aureus bacterial cells minimally decreased whereas that of E. coli cells increased, probably because US alters the permeability of the cell membrane. Moreover, E. coli exhibits better growth than S. aureus because E. coli can obtain nutrients from the environment owing to the protection of its cell wall. The survival rate of E. coli was 29.28 % whereas that of S. aureus was 46.20 % in the 0.6 mg/mL material group without sonication (Fig. 5b). Results demonstrated that ZnO–Kaol exhibited bacteriostatic activity without high-frequency ultra-sonication; however, it could not be sterilised. After 5 min of ultra-sonication at 2.16 MHz, the survival rate of bacteria in 0.6 mg/mL material group decreased to 0 due to the increase in the concentration of Zn2+ in the material after high-frequency ultra-sonication, which further altered the permeability of the bacterial membrane, leading to the release of bacterial contents and consequently the death of bacteria. Therefore, the introduction of high-frequency US can effectively kill the bacteria and decrease the amount of ZnO–Kaol, reducing their toxicity in practical applications. Similarly, we used the aforementioned method to verify the antimicrobial activity of ZnO–Kaol(C) and ZnO–HNT composites (Figs. S17 and S18). In all the cases, antimicrobial activity considerably increased after the US treatment.
Fig. 5.
(a) Viable colony units that grew on the plates after treatment; (b) antibacterial rates of the samples against E.coli and S. aureus; (c) fluorescence of live/dead bacteria after treatment; (d) ESR spectra of Kaol, ZnO and ZnO–Kaol.
To confirm the damage caused by ZnO–Kaol and US on bacteria, we performed live/dead staining of bacteria treated under different conditions (Fig. 5c). Live and dead bacteria were stained green and red, respectively [43]. In the absence of the ZnO–Kaol composite, the bacteria were not dead. After incubation with ZnO–Kaol (without sonication), the intensity of the red fluorescence of the bacteria increased, indicating that ZnO–Kaol possessed a considerable bactericidal effect. However, after the simultaneous use of ZnO–Kaol and sonication, the presence of viable bacteria was negligible, which is consistent with the results of in vitro plate counting. Furthermore, we utilised ESR spectroscopy (Fig. 5d) to detect the generation of hydroxyl radicals in the ZnO–Kaol composites. There was no detectable ESR signal for Kaol, suggesting that kaolinite couldn't produce ·OH radicals. The 1:2:2:1 signal characteristic peaks were present in both ZnO and ZnO-Kaol assays, showing that both ZnO and ZnO-Kaol could produce ·OH radicals. However, the signal from ZnO-Kaol was weaker than that from ZnO, likely due to the interaction of Kaol with ZnO, indicating that the antimicrobial performance of the composites did not depend on ROS generation and that the action of high-frequency US could promote the release of Zn2+ and effectively enhance the antimicrobial ability of the material. Therefore, these findings emphasise that the accelerated release of Zn2+ is the primary antimicrobial mechanism of the synthesised materials.
Based on the aforementioned antimicrobial activity and characterisation results, a potential antimicrobial mechanism involving high-frequency US and the ZnO–Kaol composites was proposed. High-frequency US alters the permeability of the bacterial membrane and concurrently accelerates the release of Zn2+ from ZnO–Kaol. Subsequently, Zn2+ ions destroy the bacterial cell membranes, permeates the bacterial cells, kills the bacteria and inhibits the bacterial growth, achieving a highly efficient antimicrobial activity.
4. Conclusions
ZnO–Kaol composites were prepared using the co-precipitation method. The fundamental physico–chemical properties of the composites were determined, revealing the successful loading of ZnO on the Kaol surface. Furthermore, ZnO was uniformly dispersed on the Kaol surface, which mitigated the agglomeration of ZnO nanoparticles. Moreover, the combination of the as-synthesised composite with high-frequency US improved the Zn2+ release ability and considerably enhanced the antimicrobial performance of the material. Herein, natural clay minerals were used to modulate the physico–chemical properties of oxides, and the prepared composites were used in combination with high-frequency US to create an efficient and safe antibacterial system, providing novel concepts for the future development of the field of antimicrobials.
CRediT authorship contribution statement
Han Yi: Writing – original draft, Data curation. Xingyu Jiang: Writing – original draft, Data curation. Li Feng: Data curation. Liangfei Tian: Writing – review & editing, Supervision, Funding acquisition, Conceptualization. Huaming Yang: Writing – review & editing, Supervision, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Huaming Yang reports financial support was provided by University of Geosciences. Huaming Yang reports a relationship with University of Geosciences that includes: funding grants. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by the National Key R&D Program of China (2022YFC2904804), the CUG Scholar Scientific Research Funds at China University of Geosciences (Wuhan) (2019152) and the Fundamental Research Funds for the Central Universities at China University of Geosciences (Wuhan).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ultsonch.2024.107096.
Contributor Information
Liangfei Tian, Email: liangfei.tian@zju.edu.cn.
Huaming Yang, Email: hm.yang@cug.edu.cn.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
XPS spectra of ZnO-Kaol, Kaol, ZnO. O 1s spectra of ZnO-Kaol, Kaol, ZnO. Plot of the relationship between the driving frequency and both the impedance and phase of PZTs, Schematic diagrams of the cylindrical shell on the device base, XRD patterns of ZnO-Kaol(C), Kaol and ZnO. FTIR spectra of the ZnO-Kaol(C) and Kaol(C). XPS survey spectrum of ZnO-Kaol(C). XRD patterns of ZnO-HNTs, HNTs and ZnO. FTIR spectra of the ZnO-HNTs and HNTs. XPS survey spectrum of ZnO-HNTs. SEM spectra of Kaol, ZnO-Kaol(C), HNTs and ZnO-HNTs. The particle size of ZnO, ZnO-HNTs, ZnO-Kaol, ZnO-Kaol(C). TEM spectra of ZnO dispersed solution and the sonicated material solution. Simulation of stress distribution on the ZnO surface under acoustic wave. Antibacterial test of ZnO-Kaol(C) and ZnO-HNTs.
References
- 1.Cheng S., Qi M., Li W., Sun W., Li M., Lin J., Bai X., Sun Y., Dong B., Wang L. Dual-responsive nanocomposites for synergistic antibacterial therapies facilitating bacteria-infected wound healing. Adv. Healthc. Mater. 2023;12 doi: 10.1002/adhm.202202652. [DOI] [PubMed] [Google Scholar]
- 2.Wu H., Meng Y., Yu M., Yang H. Modulating the antibacterial activity of ZnO/talc by balancing the monodispersity of ZnO nanoparticles. Appl. Clay Sci. 2023;242 doi: 10.1016/j.clay.2023.107024. [DOI] [Google Scholar]
- 3.Yu H., Xu X., Xie Z., Huang X., Lin L., Jiao Y., Li H. High-efficiency near-infrared light responsive antibacterial system for synergistic ablation of bacteria and biofilm. ACS Appl. Mater. Interfaces. 2022;14:36947–36956. doi: 10.1021/acsami.2c08406. [DOI] [PubMed] [Google Scholar]
- 4.Xie B.-X., Wang H.-S., Zheng H.-Q., Xu J., Chen L., Zhang F.-Z., Wang Y.-L., Lin Z.-J., Lin R.-G. Boosting antibacterial photodynamic therapy in a nanosized Zr MOF by the combination of Ag NP encapsulation and porphyrin doping. Inorg. Chem. 2023;62:13892–13901. doi: 10.1021/acs.inorgchem.3c01785. [DOI] [PubMed] [Google Scholar]
- 5.Wu M., Zhao S., Jing R., Shao Y., Liu X., Lv F., Hu X., Zhang Q., Meng Z., Liu A. Competitive adsorption of antibiotic tetracycline and ciprofloxacin on montmorillonite. Appl. Clay Sci. 2019;180 doi: 10.1016/j.clay.2019.105175. [DOI] [Google Scholar]
- 6.Imani I.M., Kim B., Xiao X., Rubab N., Park B.-J., Kim Y.-J., Zhao P., Kang M., Kim S.-W. Ultrasound-driven on-demand transient triboelectric nanogenerator for subcutaneous antibacterial activity. Adv. Sci. 2023;10 doi: 10.1002/advs.202204801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X., Du W., Xu W., Ling G., Zhang P. Dissolving microneedles based on ZnO nanoparticles and an ionic liquid as synergistic antibacterial agents. J. Mater. Chem. B. 2023;11:4354–4364. doi: 10.1039/D3TB00127J. [DOI] [PubMed] [Google Scholar]
- 8.La D.D., Nguyen-Tri P., Le K.H., Nguyen P.T.M., Nguyen M.D.-B., Vo A.T.K., Nguyen M.T.H., Chang S.W., Tran L.D., Chung W.J., Nguyen D.D. Effects of antibacterial ZnO nanoparticles on the performance of a chitosan/gum arabic edible coating for post-harvest banana preservation. Prog. Organ. Coat. 2021;151 doi: 10.1016/j.porgcoat.2020.106057. [DOI] [Google Scholar]
- 9.Pasquet J., Chevalier Y., Pelletier J., Couval E., Bouvier D., Bolzinger M.-A. The contribution of zinc ions to the antimicrobial activity of zinc oxide. Colloids Surf. A. 2014;457:263–274. doi: 10.1016/j.colsurfa.2014.05.057. [DOI] [Google Scholar]
- 10.Ju Y., Zeng H., Ye X., Dai M., Fang B., Liu L. Zn2+ incorporated composite polysaccharide microspheres for sustained growth factor release and wound healing. Mater. Today Bio. 2023;22 doi: 10.1016/j.mtbio.2023.100739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao L., Wu X., Wu S., Pan X., Tu J., Chen M., Al-Bishari A.M., Al-Baadani M.A., Yao L., Shen X., Liu J. Atomic layer deposition of zinc oxide on microrough zirconia to enhance osteogenesis and antibiosis. Ceram. Int. 2019;45:24757–24767. doi: 10.1016/j.ceramint.2019.08.216. [DOI] [Google Scholar]
- 12.Mendes C.R., Dilarri G., Forsan C.F., de M.R. Sapata V., Lopes P.R.M., de Moraes P.B., Montagnolli R.N., Ferreira H., Bidoia E.D. Antibacterial action and target mechanisms of zinc oxide nanoparticles against bacterial pathogens. Sci. Rep. 2022;12:2658. doi: 10.1038/s41598-022-06657-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dziewiątka K., Matusik J., Trenczek-Zając A., Cempura G. TiO2-loaded nanotubular kaolin group minerals: the effect of mineral support on photodegradation of dyes as model pollutants. Appl. Clay Sci. 2023;245 doi: 10.1016/j.clay.2023.107123. [DOI] [Google Scholar]
- 14.Liang W., Cheng J., Zhang J., Xiong Q., Jin M., Zhao J. pH-responsive on-demand alkaloids release from core-shell ZnO@ZIF-8 nanosphere for synergistic control of bacterial wilt disease. ACS Nano. 2022;16:2762–2773. doi: 10.1021/acsnano.1c09724. [DOI] [PubMed] [Google Scholar]
- 15.Wu P., Cui P., Du H., Alves M.E., Zhou D., Wang Y. Long-term dissolution and transformation of ZnO in soils: the roles of soil pH and ZnO particle size. J. Hazard. Mater. 2021;415 doi: 10.1016/j.jhazmat.2021.125604. [DOI] [PubMed] [Google Scholar]
- 16.Wang R., Xu X., Li X., Zhang N., Jiang W. pH-responsive ZnO nanoprobe mediated DNAzyme signal amplification strategy for sensitive detection and live cell imaging of multiple microRNAs. Sens. Actuators B. 2019;293:93–99. doi: 10.1016/j.snb.2019.05.002. [DOI] [Google Scholar]
- 17.Min H., Li K., Wang Q., Gao X., Xie L., Tian W. A novel filler of biocomposites for long-term self-regulated delivery of immunomodulatory and antibacterial components to accelerate bone regeneration. Compos. B Eng. 2022;238 doi: 10.1016/j.compositesb.2022.109942. [DOI] [Google Scholar]
- 18.Liao M., Du J., Chen L., Huang J., Yang R., Bao W., Zeng K., Wang W., Aphan B.C., Wu Z., Ma L., Lu Q. Sono-activated materials for enhancing focused ultrasound ablation: design and application in biomedicine. Acta Biomater. 2024;173:36–50. doi: 10.1016/j.actbio.2023.11.004. [DOI] [PubMed] [Google Scholar]
- 19.Huang D., Wang J., Song C., Zhao Y. Ultrasound-responsive matters for biomedical applications. The Innovation. 2023;4 doi: 10.1016/j.xinn.2023.100421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu Y., Liu Y., Khan K., Arkin G., Tareen A.K., Xie Z., He T., Su L., Guo F., Lai X., Xu J., Zhang J. Ultrasound combined with nanomaterials for cancer therapy. Materi. Today Adv. 2023;17 doi: 10.1016/j.mtadv.2022.100330. [DOI] [Google Scholar]
- 21.Zhou X., Li Z., Lan J., Yan Y., Zhu N. Kinetics of inactivation and photoreactivation of Escherichia coli using ultrasound-enhanced UV-C light-emitting diodes disinfection. Ultrason. Sonochem. 2017;35:471–477. doi: 10.1016/j.ultsonch.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 22.Evelyn F.V.M. Silva, Ultrasound assisted thermal inactivation of spores in foods: pathogenic and spoilage bacteria, molds and yeasts. Trends Food Sci. Technol. 2020;105:402–415. doi: 10.1016/j.tifs.2020.09.020. [DOI] [Google Scholar]
- 23.Yuan Z., Aweya J.J., Li J., Wang Z., Huang S., Zheng M., Shi L., Deng S., Yang S. Synergistic antibacterial effects of low-intensity ultrasound and peptide LCMHC against Staphylococcus aureus. Int. J. Food Microbiol. 2022;373 doi: 10.1016/j.ijfoodmicro.2022.109713. [DOI] [PubMed] [Google Scholar]
- 24.Liu C., Cao Z., He S., Sun Z., Chen W. The effects and mechanism of phycocyanin removal from water by high-frequency ultrasound treatment. Ultrason. Sonochem. 2018;41:303–309. doi: 10.1016/j.ultsonch.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Wu Y., Li W., Martin G.J.O., Ashokkumar M. Mechanism of low-frequency and high-frequency ultrasound-induced inactivation of soy trypsin inhibitors. Food Chem. 2021;360 doi: 10.1016/j.foodchem.2021.130057. [DOI] [PubMed] [Google Scholar]
- 26.Guo M., Zhang X., Ismail B.B., He Q., Yang Z., Xianyu Y., Liu W., Zhou J., Ye X., Liu D. Super antibacterial capacity and cell envelope-disruptive mechanism of ultrasonically grafted N-halamine PBAT/PBF films against Escherichia coli. ACS Appl. Mater. Interfaces. 2023;15:38910–38929. doi: 10.1021/acsami.3c05378. [DOI] [PubMed] [Google Scholar]
- 27.Xu J., Wang N., Chu T., Yang B., Jian X., Cui Y. A high-frequency mechanical scanning ultrasound imaging system. Biosensors. 2023;13:32. doi: 10.3390/bios13010032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shan D., Wu N., Wang Q., Ren W., Zhu A., Wang L., Liu Y., Sun L., Guo L., Xu H. Value of pseudopod sign on high-frequency ultrasound in predicting the pathological invasion of extramammary Paget’s disease lesions. J. Eur. Acad. Dermatol. Venereol. 2022;36:1235–1245. doi: 10.1111/jdv.18104. [DOI] [PubMed] [Google Scholar]
- 29.Dimming control and characteristics of high-frequency operated metal halide lamps | IEEE J. Mag. | IEEE Xplore, (n.d.). https://ieeexplore.ieee.org/document/1296762 (accessed April 2, 2024).
- 30.Li C.-H., Chang Y.-C., Hsiao M., Chan M.-H. Ultrasound and nanomedicine for cancer-targeted drug delivery: screening, cellular mechanisms and therapeutic opportunities. Pharmaceutics. 2022;14:1282. doi: 10.3390/pharmaceutics14061282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang S., Yuan Z., Aweya J.J., Huang S., Deng S., Shi L., Zheng M., Zhang Y., Liu G. Low-intensity ultrasound enhances the antimicrobial activity of neutral peptide TGH2 against Escherichia coli. Ultrason. Sonochem. 2021;77 doi: 10.1016/j.ultsonch.2021.105676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y., Shi X., Zhang Z., Peng Y. Enhanced coagulation by high-frequency ultrasound in Microcystis aeruginosa-laden water: strategies and mechanisms. Ultrason. Sonochem. 2019;55:232–242. doi: 10.1016/j.ultsonch.2019.01.022. [DOI] [PubMed] [Google Scholar]
- 33.Escoffre J.-M., Campomanes P., Tarek M., Bouakaz A. New insights on the role of ROS in the mechanisms of sonoporation-mediated gene delivery. Ultrason. Sonochem. 2020;64 doi: 10.1016/j.ultsonch.2020.104998. [DOI] [PubMed] [Google Scholar]
- 34.Wang Z., Wang M., Qian Y., Xie Y., Sun Q., Gao M., Li C. Dual-targeted nanoformulation with Janus structure for synergistic enhancement of sonodynamic therapy and chemotherapy. Chin. Chem. Lett. 2023;34 doi: 10.1016/j.cclet.2022.107853. [DOI] [Google Scholar]
- 35.Sun X., Wei M., Pang X., Lin L., Gao Q., Su L., Liu T., Yao Y., Song J., Wang W., Yan X. Sonodynamic bacterial inactivation enhanced by an actuator-integrated mechanism. Adv. Funct. Mater. 2023;33 doi: 10.1002/adfm.202214619. [DOI] [Google Scholar]
- 36.Faisal M., Rashed M.A., Ahmed J., Alsaiari M., Alkorbi A.S., Jalalah M., Alsareii S.A., Harraz F.A. Rapid photodegradation of linezolid antibiotic and methylene blue dye over Pt nanoparticles/polypyrrole-carbon black/ZnO novel visible light photocatalyst. J. Environ. Chem. Eng. 2021;9 doi: 10.1016/j.jece.2021.106773. [DOI] [Google Scholar]
- 37.Batistela V.R., Fogaça L.Z., Fávaro S.L., Caetano W., Fernandes-Machado N.R.C., Hioka N. ZnO supported on zeolites: Photocatalyst design, microporosity and properties. Colloids Surf. A. 2017;513:20–27. doi: 10.1016/j.colsurfa.2016.11.023. [DOI] [Google Scholar]
- 38.Freitas W.A., Soares B.E.C.F., Rodrigues M.S., Trigueiro P., Honorio L.M.C., Peña-Garcia R., Alcântara A.C.S., Silva-Filho E.C., Fonseca M.G., Furtini M.B., Osajima J.A. Facile synthesis of ZnO-clay minerals composites using an ultrasonic approach for photocatalytic performance. J. Photochem. Photobiol. A Chem. 2022;429 doi: 10.1016/j.jphotochem.2022.113934. [DOI] [Google Scholar]
- 39.Zyoud A.H., Zubi A., Zyoud S.H., Hilal M.H., Zyoud S., Qamhieh N., Hajamohideen A., Hilal H.S. Kaolin-supported ZnO nanoparticle catalysts in self-sensitized tetracycline photodegradation: zero-point charge and pH effects. Appl. Clay Sci. 2019;182 doi: 10.1016/j.clay.2019.105294. [DOI] [Google Scholar]
- 40.Liang H., Wang H., Sun X., Xu W., Meng N., Zhou N. Development of ZnO/Ag nanoparticles supported polydopamine-modified montmorillonite nanocomposites with synergistic antibacterial performance. Appl. Clay Sci. 2023;244 doi: 10.1016/j.clay.2023.107112. [DOI] [Google Scholar]
- 41.Alswat A.A., Ahmad M.B., Saleh T.A., Hussein M.Z.B., Ibrahim N.A. Effect of zinc oxide amounts on the properties and antibacterial activities of zeolite/zinc oxide nanocomposite. Mater. Sci. Eng. C. 2016;68:505–511. doi: 10.1016/j.msec.2016.06.028. [DOI] [PubMed] [Google Scholar]
- 42.Motshekga S.C., Ray S.S., Onyango M.S., Momba M.N.B. Microwave-assisted synthesis, characterization and antibacterial activity of Ag/ZnO nanoparticles supported bentonite clay. J. Hazard. Mater. 2013;262:439–446. doi: 10.1016/j.jhazmat.2013.08.074. [DOI] [PubMed] [Google Scholar]
- 43.Wen Z., Shi X., Li X., Liu W., Liu Y., Zhang R., Yu Y., Su J. Mesoporous TiO2 coatings regulate ZnO nanoparticle loading and Zn2+ release on titanium dental implants for sustained osteogenic and antibacterial activity. ACS Appl. Mater. Interfaces. 2023;15:15235–15249. doi: 10.1021/acsami.3c00812. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
XPS spectra of ZnO-Kaol, Kaol, ZnO. O 1s spectra of ZnO-Kaol, Kaol, ZnO. Plot of the relationship between the driving frequency and both the impedance and phase of PZTs, Schematic diagrams of the cylindrical shell on the device base, XRD patterns of ZnO-Kaol(C), Kaol and ZnO. FTIR spectra of the ZnO-Kaol(C) and Kaol(C). XPS survey spectrum of ZnO-Kaol(C). XRD patterns of ZnO-HNTs, HNTs and ZnO. FTIR spectra of the ZnO-HNTs and HNTs. XPS survey spectrum of ZnO-HNTs. SEM spectra of Kaol, ZnO-Kaol(C), HNTs and ZnO-HNTs. The particle size of ZnO, ZnO-HNTs, ZnO-Kaol, ZnO-Kaol(C). TEM spectra of ZnO dispersed solution and the sonicated material solution. Simulation of stress distribution on the ZnO surface under acoustic wave. Antibacterial test of ZnO-Kaol(C) and ZnO-HNTs.