Abstract
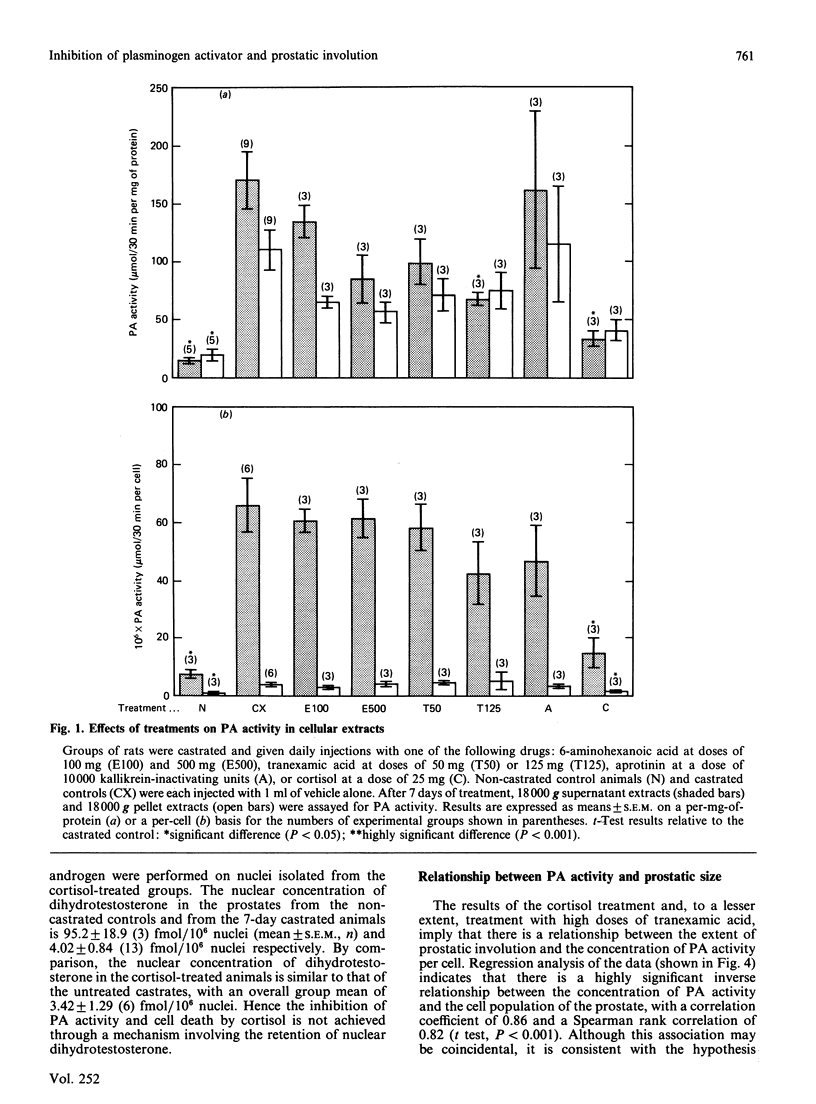
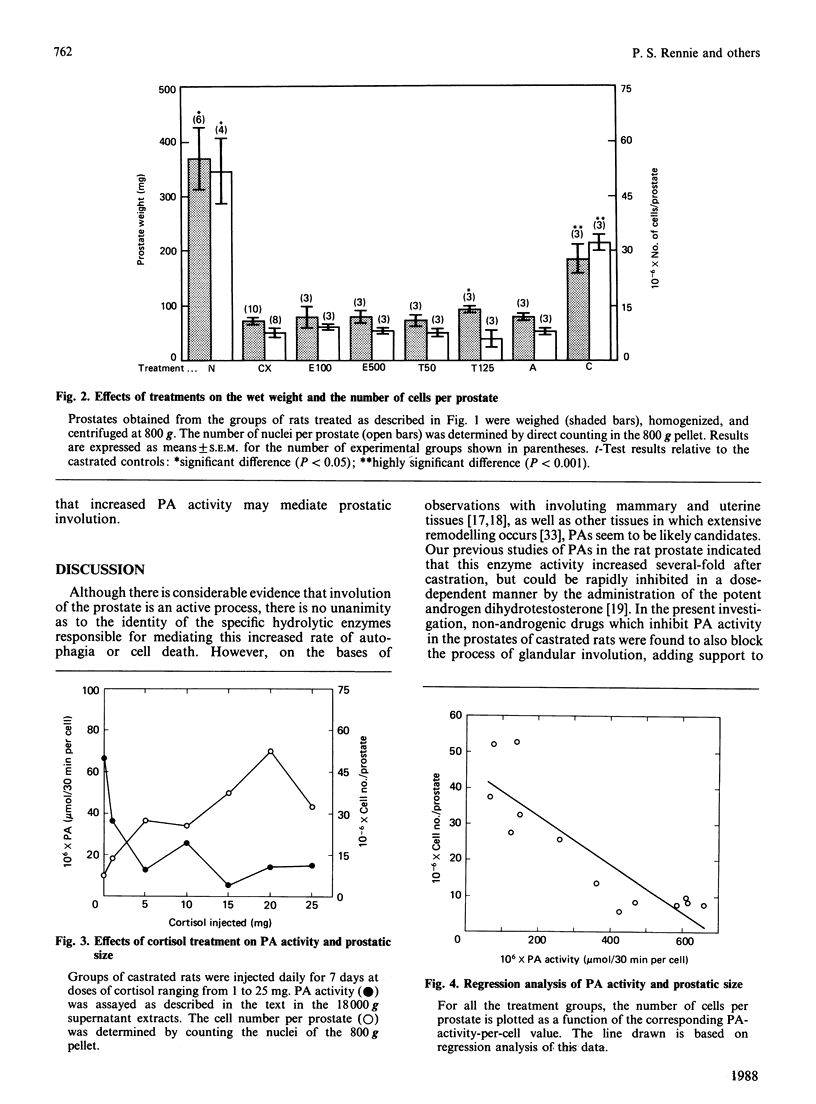
The role of plasminogen activators (PAs) as potential mediators of involution of the rat ventral prostate was investigated by using an approach involving the administration in vivo of anti-PA drugs. The prostates of castrated rats, which had been injected daily for 7 days with the anti-PA drugs 6-aminohexanoic acid, tranexamic acid, aprotinin and cortisol, were assayed for PA activity, weight and cell number. In the prostates from the castrated controls, there was a 10-fold increase in the mean PA activity and a 7-fold decrease in cell number relative to that of the non-castrated animals. Although this rise in enzyme activity could be decreased to some extent by all the drugs except aprotinin, only treatment with high doses of tranexamic acid or cortisol had a statistically significant effect. A similar pattern was observed with respect to the relative potency of the drugs in preventing the loss of prostatic weight and cell number after castration. The effects of cortisol were dose-dependent, with complete inhibition of both the rise in PA activity and cell loss occurring at a dose of about 15 mg/day. Since the concentration of the principal intranuclear androgen, dihydrotestosterone, was the same in the prostates from treated and untreated castrated rats, the effects of cortisol are not due to increased retention of this androgen. Rather, the high inverse correlation (r = 0.86) between the cellular concentration of PA activity and the cell population of the prostate implies that PAs are directly associated with prostatic involution and that cortisol, and to a lesser extent tranexamic acid, blocks the involution process through inhibition of PAs.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. W., 3rd, SCHADE A. L. The effects of castration and androgen replacement on the nucleic acid composition, metabolism, and enzymatic capacities of the rat ventral prostate. Endocrinology. 1958 Sep;63(3):271–279. doi: 10.1210/endo-63-3-271. [DOI] [PubMed] [Google Scholar]
- Beers W. H., Strickland S., Reich E. Ovarian plasminogen activator: relationship to ovulation and hormonal regulation. Cell. 1975 Nov;6(3):387–394. doi: 10.1016/0092-8674(75)90188-9. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Lesser B., Van Doorn E., Craven S. Hormonal effects on cell proliferation in rat prostate. Vitam Horm. 1975;33:61–102. doi: 10.1016/s0083-6729(08)60951-6. [DOI] [PubMed] [Google Scholar]
- Bruchovsky N., Rennie P. S., Doorn E. V., Noble R. L. Pathological growth of androgensensitive tissues resulting from latent actions of steroid hormones. J Toxicol Environ Health. 1978 Mar-May;4(2-3):391–408. doi: 10.1080/15287397809529667. [DOI] [PubMed] [Google Scholar]
- Busso N., Belin D., Failly-Crépin C., Vassalli J. D. Glucocorticoid modulation of plasminogen activators and of one of their inhibitors in the human mammary carcinoma cell line MDA-MB-231. Cancer Res. 1987 Jan 15;47(2):364–370. [PubMed] [Google Scholar]
- Camiolo S. M., Markus G., Englander L. S., Siuta M. R., Hobika G. H., Kohga S. Plasminogen activator content and secretion in explants of neoplastic and benign human prostate tissues. Cancer Res. 1984 Jan;44(1):311–318. [PubMed] [Google Scholar]
- Coffey D. S., Shimazaki J., Williams-Ashman H. G. Polymerization of deoxyribonucleotides in relation to androgen-induced prostatic growth. Arch Biochem Biophys. 1968 Mar 20;124(1):184–198. doi: 10.1016/0003-9861(68)90319-6. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danø K., Andreasen P. A., Grøndahl-Hansen J., Kristensen P., Nielsen L. S., Skriver L. Plasminogen activators, tissue degradation, and cancer. Adv Cancer Res. 1985;44:139–266. doi: 10.1016/s0065-230x(08)60028-7. [DOI] [PubMed] [Google Scholar]
- De Larminat M. A., Rennie P. S., Bruchovsky N. Radioimmunoassay measurements of nuclear dihydrotestosterone in rat prostate. Relationship to androgen receptors and androgen-regulated responses. Biochem J. 1981 Dec 15;200(3):465–474. doi: 10.1042/bj2000465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel G., Lee C., Grayhack J. T. Acid ribonuclease in rat prostate during castration-induced involution. Biol Reprod. 1980 May;22(4):827–831. doi: 10.1095/biolreprod22.4.827. [DOI] [PubMed] [Google Scholar]
- Frenette G., Dubé J. Y., Tremblay R. R. Effect of castration and steroid treatments on the activity of some hydrolytic enzymes in dog prostate. Prostate. 1983;4(4):383–390. doi: 10.1002/pros.2990040408. [DOI] [PubMed] [Google Scholar]
- Gelehrter T. D., Sznycer-Laszuk R., Zeheb R., Cwikel B. J. Dexamethasone inhibition of tissue-type plasminogen activator (tPA) activity: paradoxical induction of both tPA antigen and plasminogen activator inhibitor. Mol Endocrinol. 1987 Jan;1(1):97–101. doi: 10.1210/mend-1-1-97. [DOI] [PubMed] [Google Scholar]
- Isaacs J. T. Antagonistic effect of androgen on prostatic cell death. Prostate. 1984;5(5):545–557. doi: 10.1002/pros.2990050510. [DOI] [PubMed] [Google Scholar]
- KIRCHHEIM D., SCOTT W. W. THE EFFECTS OF CASTRATION AND SEX HORMONES UPON AMINOPEPTIDASES AND PHOSPHATASES OF THE RAT PROSTATE. Invest Urol. 1965 Jan;2:393–404. [PubMed] [Google Scholar]
- Katz J., Troll W., Levy M., Filkins K., Russo J., Levitz M. Estrogen-dependent trypsin-like activity in the rat uterus. Localization of activity in the 12,000g pellet and nucleus. Arch Biochem Biophys. 1976 Mar;173(1):347–354. doi: 10.1016/0003-9861(76)90269-1. [DOI] [PubMed] [Google Scholar]
- Kessner A., Troll W. Fluorometric microassay of plasminogen activators. Arch Biochem Biophys. 1976 Oct;176(2):411–416. doi: 10.1016/0003-9861(76)90183-1. [DOI] [PubMed] [Google Scholar]
- Lesser B., Bruchovsky N. Effect of duration of the period after castration on the response of the rat ventral prostate to androgens. Biochem J. 1974 Aug;142(2):429–431. doi: 10.1042/bj1420429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesser B., Bruchovsky N. The effects of testosterone, 5 -dihydrotestosterone and adenosine 3',5'-monophosphate on cell proliferation and differentiation in rat prostate. Biochim Biophys Acta. 1973 May 18;308(3):426–437. doi: 10.1016/0005-2787(73)90336-5. [DOI] [PubMed] [Google Scholar]
- Littlefield B. A., Johnston L. J., Manzer D. S., Roche P. C. Glucocorticoid inhibition of urokinase-like plasminogen activators in cultured human lymphoblasts. Endocrinology. 1985 Sep;117(3):1100–1109. doi: 10.1210/endo-117-3-1100. [DOI] [PubMed] [Google Scholar]
- Mira-y-Lopez R., Reich E., Stolfi R. L., Martin D. S., Ossowski L. Coordinate inhibition of plasminogen activator and tumor growth by hydrocortisone in mouse mammary carcinoma. Cancer Res. 1985 May;45(5):2270–2276. [PubMed] [Google Scholar]
- Montpetit M. L., Lawless K. R., Tenniswood M. Androgen-repressed messages in the rat ventral prostate. Prostate. 1986;8(1):25–36. doi: 10.1002/pros.2990080105. [DOI] [PubMed] [Google Scholar]
- Ossowski L., Biegel D., Reich E. Mammary plasminogen activator: correlation with involution, hormonal modulation and comparison between normal and neoplastic tissue. Cell. 1979 Apr;16(4):929–940. doi: 10.1016/0092-8674(79)90108-9. [DOI] [PubMed] [Google Scholar]
- Peterson H. Fibrinolysis and antifibrinolytic drugs in the growth and spread of tumours. Cancer Treat Rev. 1977 Sep;4(3):213–217. doi: 10.1016/s0305-7372(77)80025-x. [DOI] [PubMed] [Google Scholar]
- Rennie P. S., Bouffard R., Bruchovsky N., Cheng H. Increased activity of plasminogen activators during involution of the rat ventral prostate. Biochem J. 1984 Jul 1;221(1):171–178. doi: 10.1042/bj2210171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Hook S. L. Androgenic regulation of a tissue specific isoenzyme of acid phosphatase in rat ventral prostate. J Steroid Biochem. 1978 Jul;9(7):585–593. doi: 10.1016/0022-4731(78)90167-x. [DOI] [PubMed] [Google Scholar]
- Rennie P. S., Bruchovsky N., Mo S., de Jong G., Cheng H. Plasminogen activator activity in human prostate and breast tumors: relationship to steroid receptors. Prostate. 1982;3(5):483–492. doi: 10.1002/pros.2990030507. [DOI] [PubMed] [Google Scholar]
- Seifert S. C., Gelehrter T. D. Mechanism of dexamethasone inhibition of plasminogen activator in rat hepatoma cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6130–6133. doi: 10.1073/pnas.75.12.6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada H., Okamura H., Espey L. L., Mori T. Increase in plasminogen activator in the involuting uterus of the postpartum rat. J Endocrinol. 1985 Feb;104(2):295–298. doi: 10.1677/joe.0.1040295. [DOI] [PubMed] [Google Scholar]
- Stanisic T., Sadlowski R., Lee C., Grayhack J. T. Elevated rate of 3H-uridine incorporation in regressing rat ventral prostate. Invest Urol. 1978 Jul;16(1):15–18. [PubMed] [Google Scholar]
- Tanabe E. T., Lee C., Grayhack J. T. Activities of cathepsin D in rat prostate during castration induced involution. J Urol. 1982 Apr;127(4):826–828. doi: 10.1016/s0022-5347(17)54059-8. [DOI] [PubMed] [Google Scholar]
- Verstraete M. Clinical application of inhibitors of fibrinolysis. Drugs. 1985 Mar;29(3):236–261. doi: 10.2165/00003495-198529030-00003. [DOI] [PubMed] [Google Scholar]
- Wilkin R. P., Bruchovsky N., Shnitka T. K., Rennie P. S., Comeau T. L. Stromal 5 alpha-reductase activity is elevated in benign prostatic hyperplasia. Acta Endocrinol (Copenh) 1980 Jun;94(2):284–288. doi: 10.1530/acta.0.0940284. [DOI] [PubMed] [Google Scholar]


