Abstract
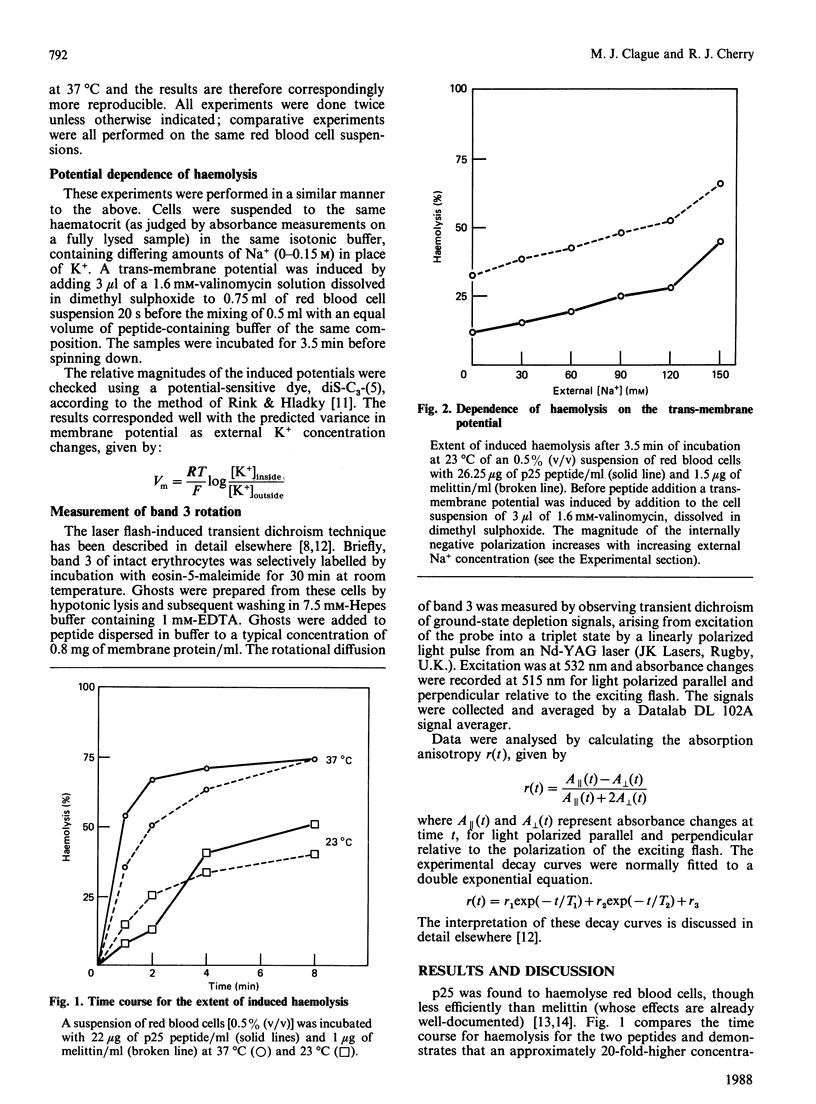
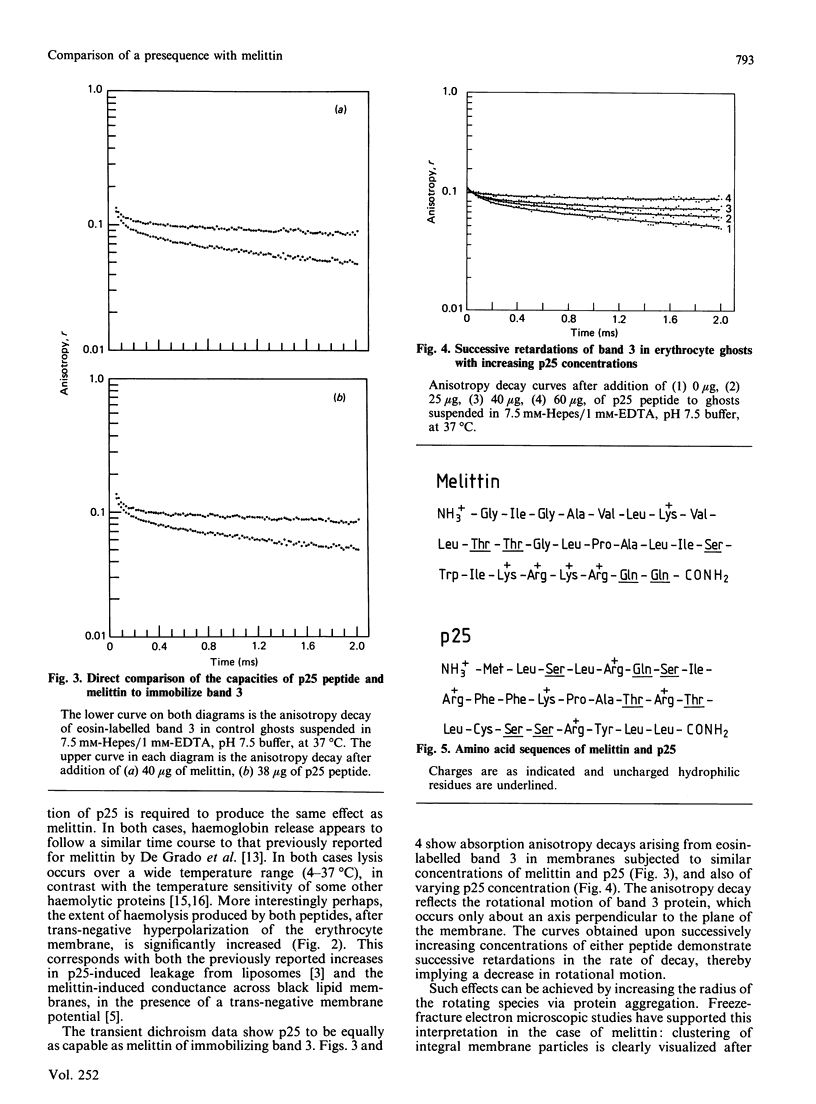
The 25 residue presequence (p25) for subunit IV of yeast cytochrome oxidase had previously been shown to possess structural and behavioural characteristics in common with the bee venom polypeptide, melittin. The present study extends the results of leakage experiments on model-membrane systems to the haemolysis of human erythrocytes, which both peptides are shown to accomplish in a manner sensitive to membrane potential. In addition, the laser flash-induced transient dichroism technique for measuring protein rotational diffusion has been used to show that both peptides aggregate band 3, the major integral membrane protein of the erythrocyte. Aggregation cannot be reversed by high ionic strength; this serves to differentiate these peptides from other positively charged species such as polylysine that aggregate band 3 at low ionic strength. These results suggest that aggregation of membrane proteins may possibly prove to be a feature of the interaction of p25 signal peptide with mitochondrial membranes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs M. S., Gierasch L. M. Molecular mechanisms of protein secretion: the role of the signal sequence. Adv Protein Chem. 1986;38:109–180. doi: 10.1016/s0065-3233(08)60527-6. [DOI] [PubMed] [Google Scholar]
- Cabantchik Z. I., Knauf P. A., Rothstein A. The anion transport system of the red blood cell. The role of membrane protein evaluated by the use of 'probes'. Biochim Biophys Acta. 1978 Sep 29;515(3):239–302. doi: 10.1016/0304-4157(78)90016-3. [DOI] [PubMed] [Google Scholar]
- Cherry R. J. Measurement of protein rotational diffusion in membranes by flash photolysis. Methods Enzymol. 1978;54:47–61. doi: 10.1016/s0076-6879(78)54007-x. [DOI] [PubMed] [Google Scholar]
- DeGrado W. F., Musso G. F., Lieber M., Kaiser E. T., Kézdy F. J. Kinetics and mechanism of hemolysis induced by melittin and by a synthetic melittin analogue. Biophys J. 1982 Jan;37(1):329–338. doi: 10.1016/S0006-3495(82)84681-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake A. F., Hider R. C. The structure of melittin in lipid bilayer membranes. Biochim Biophys Acta. 1979 Aug 7;555(2):371–373. doi: 10.1016/0005-2736(79)90178-0. [DOI] [PubMed] [Google Scholar]
- Dufton M. J., Hider R. C., Cherry R. J. The influence of melittin on the rotation of band 3 protein in the human erythrocyte membrane. Eur Biophys J. 1984;11(1):17–24. doi: 10.1007/BF00253854. [DOI] [PubMed] [Google Scholar]
- Garnier J., Gaye P., Mercier J. C., Robson B. Structural properties of signal peptides and their membrane insertion. Biochimie. 1980;62(4):231–239. doi: 10.1016/s0300-9084(80)80397-x. [DOI] [PubMed] [Google Scholar]
- Gillespie L. L., Argan C., Taneja A. T., Hodges R. S., Freeman K. B., Shore G. C. A synthetic signal peptide blocks import of precursor proteins destined for the mitochondrial inner membrane or matrix. J Biol Chem. 1985 Dec 25;260(30):16045–16048. [PubMed] [Google Scholar]
- Habermann E. Bee and wasp venoms. Science. 1972 Jul 28;177(4046):314–322. doi: 10.1126/science.177.4046.314. [DOI] [PubMed] [Google Scholar]
- Hu K. S., Dufton M. J., Morrison I. E., Cherry R. J. Protein rotational diffusion measurements on the interaction of bee venom melittin with bacteriorhodopsin in lipid vesicles. Biochim Biophys Acta. 1985 Jun 27;816(2):358–364. doi: 10.1016/0005-2736(85)90503-6. [DOI] [PubMed] [Google Scholar]
- Kempf C., Klausner R. D., Weinstein J. N., Van Renswoude J., Pincus M., Blumenthal R. Voltage-dependent trans-bilayer orientation of melittin. J Biol Chem. 1982 Mar 10;257(5):2469–2476. [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Roise D., Horvath S. J., Tomich J. M., Richards J. H., Schatz G. A chemically synthesized pre-sequence of an imported mitochondrial protein can form an amphiphilic helix and perturb natural and artificial phospholipid bilayers. EMBO J. 1986 Jun;5(6):1327–1334. doi: 10.1002/j.1460-2075.1986.tb04363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sessa G., Freer J. H., Colacicco G., Weissmann G. Interaction of alytic polypeptide, melittin, with lipid membrane systems. J Biol Chem. 1969 Jul 10;244(13):3575–3582. [PubMed] [Google Scholar]
- Terwilliger T. C., Weissman L., Eisenberg D. The structure of melittin in the form I crystals and its implication for melittin's lytic and surface activities. Biophys J. 1982 Jan;37(1):353–361. doi: 10.1016/S0006-3495(82)84683-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosteson M. T., Holmes S. J., Razin M., Tosteson D. C. Melittin lysis of red cells. J Membr Biol. 1985;87(1):35–44. doi: 10.1007/BF01870697. [DOI] [PubMed] [Google Scholar]
- Weiner R. N., Schneider E., Haest C. W., Deuticke B., Benz R., Frimmer M. Properties of the leak permeability induced by a cytotoxic protein from Pseudomonas aeruginosa (PACT) in rat erythrocytes and black lipid membranes. Biochim Biophys Acta. 1985 Nov 7;820(2):173–182. doi: 10.1016/0005-2736(85)90110-5. [DOI] [PubMed] [Google Scholar]
- Young J. D., Peterson C. G., Venge P., Cohn Z. A. Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature. 1986 Jun 5;321(6070):613–616. doi: 10.1038/321613a0. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986 Jun;5(6):1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


