Abstract
Background:
Cesarean section poses a fourfold risk for postpartum hemorrhage (PPH), necessitating accurate blood loss estimation to enable timely interventions. However, the conventional visual estimation method often leads to underestimation, resulting in undiagnosed PPH even in our setting, Uganda. Yet, the quantitative standard techniques remain underutilized.
Objective:
We compared visual and calculated blood loss among women undergoing cesarean delivery at Gulu Regional Referral Hospital in northern Uganda.
Design:
We employed a cross-sectional study design.
Methods:
We enrolled pregnant women scheduled for cesarean section and determined both calculated and visually estimated blood loss. Data analysis involved using Pearson’s moment correlation coefficient to compare the two methods and logistic regression to determine the factors associated with PPH.
Results:
We included 105 participants, most were primigravida (n = 100, 43%), aged 15–24 years (n = 100, 52%), with term gestation (n = 100, 75%). The mean visual estimated blood loss (vEBL) was 235.3 ± 123.7 ml (interquartile range (IQR) 50–600 ml), while the calculated estimated blood loss (cEBL) was 435.0 ± 1328.2 ml (IQR −11,182.1–2226.7 ml). Visual estimation underestimated blood loss in 90% of cases (n = 100), and 21% (n = 21) had undiagnosed PPH (>1000 ml blood loss). None of the respondents had PPH (>1000 ml blood loss) following vEBL. There was a small positive correlation between both methods (vEBL and cEBL; r = 0.1165; p = 0.2482). Women aged >35 years were 1.60 times more likely to experience PPH than their counterparts aged 25–34 years (adjusted odds ratio (AOR): 1.60; 95% CI: 1.11–2.30, p < 0.011). Chorioamnionitis increased the risk of PPH by 2.2 times (AOR: 2.20; 95% CI: 1.20–4.05, p < 0.012).
Conclusion:
The visual estimation technique significantly underestimated blood loss in up to 90% of cases, particularly during emergency cesarean sections. Among the 21% of cases diagnosed with PPH based on calculated blood loss, advanced maternal age and chorioamnionitis were notable contributing factors. Routine hemoglobin and hematocrit testing in obstetric care can be effectively utilized to objectively assess blood loss, aiding in the accurate diagnosis and management of PPH. Implementing these measures, even in resource-constrained settings, can significantly reduce the morbidity and mortality associated with PPH.
Trial registration:
Not applicable.
Keywords: Gulu, hemoglobin, maternal mortality, postpartum hemorrhage, Uganda, underestimation
Introduction
Maternal mortality and morbidity persist as a threat to achieving Sustainable Development Goal 3.1. 1 Globally, an estimated 287,000 women died during and after pregnancy in 2020, 2 and sub-Saharan Africa has the highest maternal mortality ratio (MMR) at 545 deaths per 100,000 live births. Though Uganda has made efforts to reduce maternal and perinatal mortality, with MMR dropping from 336 to 189 per 100,000 live births, 3 its maternal mortality and morbidity remain unacceptably high and predominantly due to obstetric hemorrhage, particularly postpartum hemorrhage (PPH).
PPH, defined as accumulative blood loss of 500 or 1000 ml following vaginal or cesarean delivery, respectively, with or without resultant hemodynamic instability after the birth of the baby up to 6 weeks postpartum has a significant impact on low- and middle-income countries, accounting for one-third of all maternal deaths.4–7 Obstetric surgeries such as cesarean sections (CS) carry a fourfold risk for PPH,8,9 presenting a notable drawback. 10
The global CS rate is projected to increase to 28.5% by the year 2030. 11 In sub-Saharan African countries, where maternal and perinatal mortality rates are high, the CS rate is lower (7.3%) than in the less and more developed countries (24.2% and 27.2%, respectively).11,12 Despite the low population CS rate in Uganda (6%), there are massive variations in the rates with facility CS rates estimated to be 36% in 2021. 13 Furthermore, unique obstacles hinder the accurate determination of the national burden and patterns of PPH. The gross underestimation of blood loss, particularly with the reliance on unreliable visual estimations, means that PPH will continue to be a significant challenge. 14
Traditionally, blood loss estimation during cesarean section and other obstetric surgeries relies on the visual technique which involves looking at items such as blood in containers, drapes, sponges, and mops which are used to determine blood loss at the end of the procedure by multiple observers including surgeons, anesthetists, assistants, and theatre nurses with inter-observer inaccuracies.15,16 In most clinical settings in Uganda, this estimate is done by the surgeon with less involvement of other operating team members. 14
However, alternative methods such as hematocrit (Hct)/hemoglobin (Hb) change, gravimetric (weighing of swabs/soiled linen), volumetric (volume of blood in canisters), and colorimetric (Triton method and graduated drapes) techniques are considered superior.17–20 For example, Briley demonstrated the efficacy of calculating blood loss by considering hematocrit change 48 h post-blood loss, multiplying pregnancy blood volume by the percentage of blood lost.21–24 Orzolek et al. noted that this calculated blood loss technique was accurate though their study noted not much difference in mean with visual estimated blood loss (vEBL) and calculated blood loss. 25 Atukunda et al. in a randomized control trial comparing oxytocin and misoprostol in the management of PPH at Mbarara Regional Referral Hospital also noted that calculated blood loss was superior to the weighted swabs technique which had poor sensitivity but high specificity. 4
Despite the widespread use of hematocrit and hemoglobin measurements in maternal care, even in resource-limited settings, their application in estimating blood loss in obstetric care remains underutilized. The common use of vEBL in clinical practice, particularly in low-income countries, leads to many cases of PPH being potentially missed, significantly contributing to maternal morbidity and mortality.
In our setting, there exists a paucity of literature exploring routine estimation of blood loss and its relevance to PPH diagnosis and management; therefore, we aimed to compare visual and calculated estimated blood loss (cEBL) in assessing blood loss among women undergoing cesarean delivery at a tertiary facility in northern Uganda, hence determining cases of PPH.
Methods
Design
We conducted a cross-sectional study design utilizing quantitative approaches to determine cEBL using maternal pregnancy volume and preoperative and postoperative Hct change. This was then compared with visual estimations to determine cases of PPH in a snapshot of time. This study conforms to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) cross-sectional studies statement. 26
Setting
Gulu regional referal hospital (GRRH) is a tertiary healthcare facility with a total annual number of deliveries of approximately 4000–4500 (from ward records). It is a public hospital that serves as a teaching hospital for Gulu University, and an internship training center for medical, nursing, midwifery, and pharmacy graduates. Recently, it has been accredited as a fellowship training center for the East Central and Southern African College of Obstetrics and Gynecology. It is a referral site for more than eight districts in northern Uganda, serving a population of approximately 2 million people. Its MMR is estimated at 122/100,000 live births, justifying its low CS rate of 14%, making it the referral hospital with the lowest CS rate in Uganda. 27
Study variables
Dependent variables
PPH (visual or calculated blood loss greater than 1000 ml).
Independent variables
Gravidity, height, weight, American College of Obstetricians and Gynecologists (ACOG) obstetric hemorrhage risk factors (previous cesarean delivery, obesity, parity, chorioamnionitis, magnesium sulfate administration, prolonged oxytocin use, platelet count, large myomas, estimated fetal weight above 4 kg and prolonged second stage), and blood loss (visual and calculated).
Participants
Selection criteria
Women undergoing CS (both emergency and elective) at GRRH and provided informed consent during the study period were included in the study.
We excluded women who had antepartum hemorrhage (APH), those who were critically ill, or received preoperative and intraoperative/postoperative blood transfusion because these conditions could alter Hct count independent of the operation’s effect. Other conditions such as anemias, pre-eclampsia, or chronic diseases that potentially affect hematocrit/hemoglobin count preoperative without affecting visual estimation were not excluded.
Sample size estimation
According to Pebalo et al., 28 the CS rate at GRRH was estimated at 14%. Also taking into account average 4500 deliveries annually, about 630 CSs per year and hence 53 per month (Hospital records). Using the Kish and Leslie (1965) formula for sample size calculation 29 :
where n0 = the desired sample size; Z = critical values of normal distribution at 95%, which corresponds to 1.96; p = the proportion of the target population estimated to have undergone cesarean delivery (0.14); q = compliment of p (1 − p) (0.86); e = estimated margin of error 5% (0.05).
Then adjusting the finite population
n = final sample size; n0 = desired sample size (185); N = monthly CS rate (53).
Hence, the sample size was 85 participants, but adjusting for design errors and non-response, the sample size was increased by 20% to 102.
Sampling technique
Simple random sampling was employed. Every mother prescribed CS chose a random number from our lottery box, and those who drew an even number were selected. This process was repeated until the desired sample size was achieved.
Data sources
Data collection tool
Adopted from various literature reviews,4,9,22,23,30,31 the study tool included sections to capture pregnancy and delivery factors (gravidity, parity, height and weight, indication for CS, surgeon’s qualification and vtn years of experience, vEBL, ACOG Obstetrics hemorrhage risk factors, and preoperative and postoperative Hct and Hb. The tool, available only in English, was designed in Kobo Toolbox to enable Android online–offline data collection and was administered by experienced research assistants who underwent training before starting data collection.
Data collection procedure
Participants who consented were recruited, and their height, weight, and risk factors for PPH were documented as per the ACOG risk factor tool. The first blood sample was then collected for CBC analysis to determine preoperative hematocrit (Hct1). Participants then underwent routine CS as per their obstetric indication, from which the surgeon’s estimated blood loss (vEBL) was identified from the operation notes later minus their knowledge. The Nihon Kohden 5-part hematology Analyzer (model name/number: MEK-7300) was used for analysis, employing three reagents (one hemolyzing reagent (cyanide-free), one diluent, and two detergents). Daily quality controls were performed to eliminate any analytical errors. Another sample was collected 48 h postoperatively to determine the second hematocrit count (Hct2). The cEBL was then determined using the perinatology.com equations that involved the use of maternal pregnancy volume (height and weight) and hematocrit change. A comparison of blood loss by visual and calculated methods was made across all other independent variables.
PPH was defined as any visual or calculated blood loss greater than 1000 ml and calculated blood loss was considered a standard estimation technique, any vEBL by the surgeon less than the calculated was considered underestimation and if higher it meant overestimation compared to calculated. All blood samples were collected by qualified staff (research assistant) through superficial venipuncture using a 5-ml syringe in the preoperative and postoperative rooms for samples 1 and 2, respectively. These were placed in purple top vacutainer tubes containing EDTA and transported to the laboratory for analysis within 12 h. For emergency cases, informed consent was sought during the second sample collection after the debrief (second postoperative day).
Formulas employed 22 :
Calculated pregnancy blood volume = (0.75 ((maternal height (m)) + (maternal weight (kg))
Percent of blood volume lost = (pre-delivery Hct − post-delivery Hct)/pre-delivery Hct.
cEBL = calculated pregnancy blood volume × percent of blood volume lost.
Bias
All the surgeons (intern doctors, medical officers, and obstetricians) were blinded from the study to reduce bias during visual blood loss estimation and their estimate was captured from their operation notes days later.
Data management and analysis
The generated data were exported as Excel from the Kobo toolbox to enable cleaning, tallying, coding, and summarizing. Pregnancy volume, hematocrit change, and cEBL were computed in Excel and later exported to Stata version 14.1 for analysis. Descriptive statistics were run to determine the interaction between variables. Pearson’s product–moment correlation was employed to compare the effectiveness of surgeons at GRRH in estimating blood loss by visual estimation compared to quantitative losses. The results were presented in tables and scatter plots.
Results
Recruitment
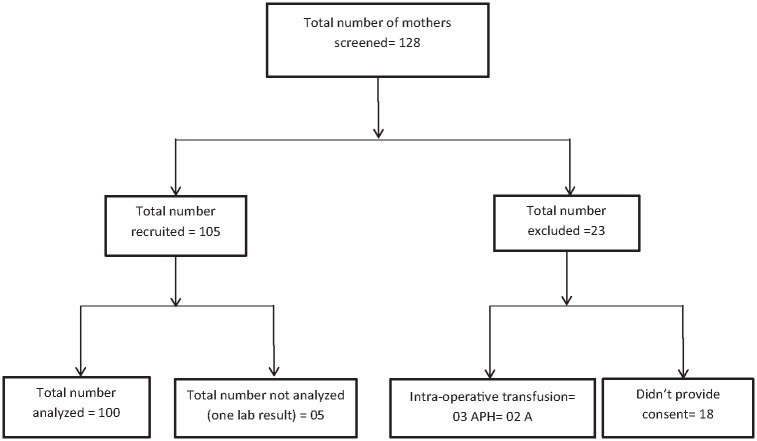
We screened a total of 128 mothers between June and August 2023, of this, 105 were recruited and an additional 23 were eliminated. Of the 105 recruited, 5 were cleaned off due to only 1 lab result. Of those screened out, 3 had received an intraoperative blood transfusion, 2 had APH, and 18 opted out. Figure 1 shows the participant recruitment procedure.
Figure 1.
Consort diagram showing recruitment of participants.
Participant characteristics
A total of 100 women were enrolled. More than half (52.0%) were in the 15–24 age group. The median age was 24, interquartile range 21.0–29.5 years. Most, 43.0% and 49.0%, were primigravida and nulliparous, respectively. The majority, 94.0%, had an emergency Caesarean section (C/S) and 75% had term pregnancies. Details are shown in Table 1.
Table 1.
Characteristics of study participants.
| Variable | PPH (calculated >1000 ml) | Total, n (%), n = 100 | |
|---|---|---|---|
| No, n (%), n = 79 | Yes, n (%), n = 21 | ||
| Age | |||
| 15–24 | 44 (55.7) | 8 (38.1) | 52 (52.0) |
| 25–34 | 30 (38) | 9 (42.9) | 39 (39.0) |
| ⩾35 | 5 (6.3) | 4 (19.0) | 9 (9.0) |
| Gravidity | |||
| Primigravida | 33 (41.8) | 10 (47.6) | 43 (43.0) |
| Multigravida (2–4) | 34 (43) | 7 (33.3) | 41 (41.0) |
| Grand multigravida (>5) | 12 (15.2) | 4 (19) | 16 (16.0) |
| Parity | |||
| Nullipara | 39 (49.4) | 10 (47.6) | 49 (49.0) |
| Primipara | 19 (24.1) | 4 (19.0) | 23 (23.0) |
| Multipara (2–4) | 17 (21.5) | 5 (23.8) | 22 (22.0) |
| Grand multipara (>5) | 4 (5.1) | 2 (9.5) | 6 (6.0) |
| Gestational age at birth | |||
| Preterm (<37 weeks) | 9 (11.4) | 4 (19.0) | 13 (13.0) |
| Term (37–41) | 62 (78.5) | 13 (61.9) | 75 (75.0) |
| Post-term (⩾42) | 8 (10.1) | 4 (19.0) | 12 (12.0) |
| Type of cesarean delivery | |||
| Elective C/S | 6 (7.6) | 0 (0.0) | 6 (6.0) |
| Emergency C/S | 73 (92.4) | 21 (100) | 94 (94.0) |
| Prior cesarean section, uterine surgery, or multiple laparotomies | |||
| No | 60 (75.9) | 15 (71.4) | 75 (75.0) |
| Yes | 19 (24.1) | 6 (28.6) | 25 (25.0) |
| Multiple gestations | |||
| No | 76 (96.2) | 20 (95.2) | 96 (96.0) |
| Yes | 3 (3.8) | 1 (4.8) | 4 (4.0) |
| Obesity (BMI above 40) | |||
| No | 76 (96.2) | 21 (100) | 97 (97.0) |
| Yes | 3 (3.8) | 0 (0.0) | 3 (3.0) |
| Chorioamnionitis | |||
| No | 79 (100) | 19 (90.5) | 98 (98) |
| Yes | 0 (0.0) | 2 (9.5) | 2 (2.0) |
| Magnesium sulfate administration | |||
| No | 76 (96.2) | 20 (95.2) | 96 (96) |
| Yes | 3 (3.8) | 1 (4.8) | 4 (4.0) |
| Prolonged second stage | |||
| No | 74 (93.7) | 19 (90.5) | 93 (93) |
| Yes | 5 (6.3) | 2 (9.5) | 7 (7.0) |
| Platelet <70,000 | |||
| No | 77 (97.5) | 20 (95.2) | 97 (97.0) |
| Yes | 2 (2.5) | 1 (4.8) | 3 (3.0) |
| Surgeon qualification | |||
| Intern doctor | 41 (51.9) | 10 (47.6) | 51 (51.0) |
| Medical officer | 35(44.3) | 10 (47.6) | 45 (45.0) |
| Consultant obstetrician | 3 (3.8) | 1 (4.8) | 4 (4.0) |
| Surgeon’s years of experience | |||
| 1–5 | 31 (39.2) | 9 (42.9) | 40 (40.0) |
| Less than 1 year | 46 (58.2) | 11 (52.4) | 57 (57.0) |
| More than 5 years | 2 (2.5) | 1 (4.8) | 3 (3.0) |
| Type of anesthesia | |||
| General | 0 (0.0) | 1 (4.8) | 1 (1.0) |
| Spinal | 79 (100.0) | 20 (95.2) | 99 (99.0) |
| Greater than four prior births | |||
| No | 67 (84.8) | 18 (85.7) | 85 (85.0) |
| Yes | 12 (15.2) | 3 (14.3) | 15 (15.0) |
| Hct <30% and other risk factor | |||
| No | 74 (93.7) | 20 (95.2) | 94 (94.0) |
| Yes | 5 (6.3) | 1 (4.8) | 6 (6.0) |
| Active bleeding | |||
| No | 79 (100.0) | 20 (95.2) | 99 (99.0) |
| Yes | 0 (0.0) | 1 (4.8) | 1 (1.0) |
| Two or more medium (admission or intrapartum) risk factors | |||
| No | 72 (91.1) | 19 (90.5) | 91 (91.0) |
| Yes | 7 (8.9) | 2 (9.5) | 9 (9.0) |
BMI, body mass index; Hct, hematocrit; PPH, postpartum hemorrhage.
Comparison of vEBL and cEBL
The mean vEBL for CS was 235.3 ± 123.7 ml with a range of 50–600 ml, while the mean cEBL was 435.0 ± 1328.2 ml with a range of −11,182.1, 2226.7 ml. Visual estimation was less than calculated (standard) EBL (underestimation) in 90% (90/100) of the participants. Details are shown in Table 2.
Table 2.
Comparison of vEBL and cEBL.
| Variable | Overestimation of blood loss by the surgeon, no, n (%), n = 10 | Underestimation of blood loss by surgeon, yes, n (%), n = 90 | Total, n (%), n = 100 | p |
|---|---|---|---|---|
| Type of cesarean delivery | ||||
| Elective C/S | 1 (10.0) | 5 (5.6) | 6 (6.0) | 0.575 |
| Emergency C/S | 9 (90.0) | 85 (94.6) | 94 (94.0) | |
| Surgeon qualification | ||||
| Intern doctor | 5 (50.0) | 46 (30.0) | 51 (51.0) | 0.587 |
| Medical officer | 4 (40.0) | 41 (45.6) | 45 (45.0) | |
| Consultant obstetrician | 1 (10.0) | 3 (3.3) | 4 (4.0) | |
| Surgeon’s years of experience | ||||
| 1–5 | 6 (60.0) | 34 (37.8) | 40 (40.0) | 0.366 |
| Less than 1 year | 4 (40.0) | 53 (58.9) | 57 (57.0) | |
| More than 5 years | 0 (0.0) | 3 (3.3) | 3 (3.0) | |
| Type of anesthesia | ||||
| General | 0 (0.0) | 1 (1.1) | 1 (1.0) | 0.738 |
| Spinal | 79 (100.0) | 89 (98.9) | 99 (99.0) | |
cEBL, calculated estimated blood loss; vEBL, visual estimated blood loss.
A Pearson product–moment correlation coefficient was computed to assess the relationship between vEBL and cEBL. There was a small positive correlation between both methods (r = 0.1165; p = 0.2482) as shown in Figure 2.
Figure 2.
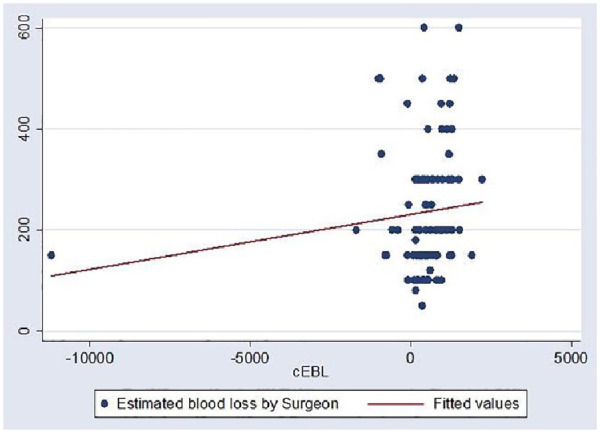
Relationship of vEBL and cEBL.
cEBL, calculated estimated blood loss; vEBL, visual estimated blood loss.
PPH cases from calculated blood loss among women undergoing C/S at GRRH
A total of 21 out of 100 women (21.0%) had PPH (>1000 ml) by cEBL.
Risk factors of PPH across calculated blood loss estimation techniques among women undergoing C/S at GRRH.
Women in the age bracket above 35 years were 1.60 times more likely to have PPH compared to their counterparts between 25 and 34 years (adjusted odds ratio (AOR): 1.60; 95% CI: 1.11–2.30).
Women who had chorioamnionitis were 2.21 times more likely to have PPH as compared to those who did not have chorioamnionitis (AOR: 2.21; 95% CI: 1.20–4.05). Details are shown in Table 3.
Table 3.
Association between PPH and ACOG obstetric hemorrhage risk factors.
| Variable | COR | 95% CI | p | AOR | 95% CI | p |
|---|---|---|---|---|---|---|
| Age | ||||||
| 15–24 | 1 | 1 | ||||
| 25–34 | 1.08 | 0.91–1.28 | 0.37 | 1.15 | 0.96–1.37 | 0.126 |
| ⩾35 | 1.34 | 1.00–1.78 | 0.047 | 1.60 | 1.11–2.30 | 0.012* |
| Gestational age at birth | ||||||
| Preterm | 1 | 1 | ||||
| Term | 0.87 | 0.69–1.11 | 0.274 | 0.92 | 0.72–1.19 | 0.524 |
| Post-term | 1.03 | 0.74–1.41 | 0.875 | 1.05 | 0.75–1.46 | 0.788 |
| Chorioamnionitis | ||||||
| No | 1 | 1 | ||||
| Yes | 2.24 | 1.29–3.89 | 0.004 | 2.21 | 1.20–4.05 | 0.011* |
| Magnesium sulfate administration | ||||||
| No | 1 | 1 | ||||
| Yes | 1.04 | 0.69–1.57 | 0.843 | 1.31 | 0.78–2.20 | 0.306 |
| Platelet <70,000 | ||||||
| No | 1 | 1 | ||||
| Yes | 1.14 | 0.71–1.82 | 0.598 | 1.15 | 0.71–1.85 | 0.577 |
| Prolonged second stage | ||||||
| No | 1 | 1 | ||||
| Yes | 1.08 | 0.79–1.49 | 0.613 | 1.16 | 0.84–1.60 | 0.374 |
| Type of delivery | ||||||
| Elective C/S | 1 | 1 | ||||
| Emergency C/S | 1.25 | 0.89–1.75 | 0.193 | 1.22 | 0.85–1.75 | 0.284 |
| Surgeon qualification | ||||||
| Intern doctor | 1 | 1 | . | |||
| Medical officer | 1.03 | 0.87–1.21 | 0.757 | 1.00 | 0.83–1.20 | 1.000 |
| Consultant obstetrician | 1.06 | 0.69–1.61 | 0.802 | 1.04 | 0.68–1.60 | 0.842 |
| Prior cesarean section, uterine surgery, or multiple laparotomies | ||||||
| No | 1 | 1 | ||||
| Yes | 1.04 | 0.86–1.25 | 0.673 | 1.08 | 0.88–1.33 | 0.443 |
| Multiple gestations | ||||||
| No | 1 | 1 | . | |||
| Yes | 1.04 | 0.69–1.57 | 0.843 | 1.14 | 0.74–1.74 | 0.553 |
| Greater than four prior births | ||||||
| No | 1 | 1 | ||||
| Yes | 0.99 | 0.79–1.24 | 0.919 | 0.87 | 0.64–1.19 | 0.388 |
| Obesity (BMI above 40) | ||||||
| No | 1 | 1 | ||||
| Yes | 0.81 | 0.50–1.29 | 0.367 | 0.74 | 0.43–1.27 | 0.272 |
| Hct <30% and other risk factor | ||||||
| No | 1 | 1 | ||||
| Yes | 0.95 | 0.68–1.34 | 0.79 | 0.98 | 0.65–1.46 | 0.913 |
| Two or more medium (admission or intrapartum) risk factors | ||||||
| No | 1 | 1 | ||||
| Yes | 1.01 | 0.76–1.34 | 0.926 | 0.82 | 0.54–1.23 | 0.334 |
ACOG, American College of Obstetricians and Gynecologists; AOR, adjusted odds ratio; BMI, body mass index; COR, crude odds ratio; Hct, hematocrit; PPH, postpartum hemorrhage.
Discussion
Our study compared visual and calculated blood loss among women undergoing cesarean delivery at a tertiary facility in northern Uganda. Most of our participants were primigravida aged 15–24 years, the majority of whom had done an emergency CS. We noted a significant underestimation of blood loss and undiagnosed PPH with only a small correlation between visual and calculated blood loss.
Despite such revealing results, the calculated blood loss utilizing the Hct change formula has potential limitations. Factors influencing hematocrit, such as dehydration, perioperative blood transfusion, and burns, could affect the accuracy of quantitative (calculated) blood loss. Elevated white blood cell and reticulocyte count might result in falsely high hematocrit values. However, we used other full blood count parameters to mitigate false elevation or decrease in Hct (Hb change). Postoperative Hct measured at least 48 h apart allowed for the redistribution of fluids and demonstrated a drop in Hct. Patients with burns and those who received blood transfusions were excluded. In addition, potential bias from the surgeon’s visual estimation in the event of research was mitigated by blinding them. To address this further, combining various quantitative estimation approaches, such as weighing swabs, graduated drapes, and the triton technique, as suggested by Hancock et al., may enhance accuracy.17,18,32 But this technique presents a feasible approach worth popularizing since a complete blood count and specifically hemoglobin estimation remains one of the most done investigations even in primary care providing maternal and child health services.
We observed a significant underestimation of blood loss by visual technique (in 90% of cases) compared to the calculated method, more than that in the prospective study by Sharashchandra and Shivaraj, where they reported a 50% underestimation in their setting. 23 However, they utilized both weighing of swabs and hematocrit change, and also both the surgeon and anesthetist agreed on the visual blood loss at the end of the procedure compared to ours where only the surgeon documented blood loss. In addition, in our context, emergency CS showed a higher rate of underestimation compared to the elective category, where overestimation was noted. 30 Contrary to a retrospective cohort study by Blosser et al., which indicated that age, experience, or expertise did not enhance clinicians’ ability to estimate blood loss, our study found that the higher the surgeon’s qualification and years of experience, the better their visual estimation, highlighting the potential influence of confounding variables. 31
Atukunda et al. documented 22.6% PPH from calculated blood loss hemoglobin drop of >10% among 1148 women enrolled in a randomized control trial comparing the effectiveness of misoprostol and oxytocin in the management of PPH, this showed a comparable result to our finding however their larger sample size and also the inclusion of vaginal deliveries compared to ours would predict high rates of PPH in our setting. It further aligns with findings from a systematic review of prognostic models for predicting PPH by Carr et al. 33 Notably, none of these participants received interventions to actively manage PPH, emphasizing the clinical dilemma of establishing a threshold for PPH management.17,18,34–37
Regarding the medium- or high-risk factors assessed by the Obstetric Hemorrhage Risk Factor Tool, both chorioamnionitis and advanced maternal age showed a statistical association with PPH at bivariable and multivariable analyses. These findings are consistent with retrospective cohort studies by Pubu et al., 38 which indicated that advanced maternal age is a surrogate risk factor for PPH due to associated increased risk factors and obstetric complications. In addition, chorioamnionitis has been associated with decreased myometrial contractility during CS, leading to PPH, as observed in studies by Zackler et al. and Schwartz and Gaudet.39,40
Limitations of the study
This study is limited by its quantitative design; however, given the low CS rate at Gulu Regional Referral Hospital, the sample size was optimal allowing for the generalization of findings within our setting. Also, conditions that cause hematocrit change independent of operation such as APH and intraoperative or postoperative blood transfusion, potentially affect the calculated blood loss; however, we excluded participants with such medical conditions from the study.
Conclusion
The visual estimation technique significantly underestimated blood loss in up to 90% of cases, particularly during emergency cesarean sections. Among the 21% of cases diagnosed with PPH based on calculated blood loss, advanced maternal age and chorioamnionitis were notable contributing factors. Routine hemoglobin and hematocrit testing in obstetric care can be effectively utilized to objectively assess blood loss, aiding in the accurate diagnosis and management of PPH. Implementing these measures, even in resource-constrained settings, can significantly reduce the morbidity and mortality associated with PPH.
We recommend further studies to enhance the reliability of quantitative methods, and flexible care protocols may improve measurement, diagnosis, and timely management of PPH. At the facility level, quality improvement projects can expedite better estimation of blood loss during cesarean delivery utilizing common methods like hematocrit change.
Supplemental Material
Supplemental material, sj-docx-1-reh-10.1177_26334941241289552 for Comparing visual estimation and hematocrit change in the assessment of blood loss among women undergoing cesarean delivery in a tertiary facility in northern Uganda by Robert Edilu, Aaron Sanvu, James Ecuut, Alban Odong, Felix Bongomin, Ritah Nantale, Jackline Ayikoru, Baifa Arwinyo, Sande Ojara and Pebalo Francis Pebolo in Therapeutic Advances in Reproductive Health
Acknowledgments
We acknowledge the support from the PPH committee, Laboratory of Gulu Regional Referral Hospital, and Gulu University Research and Ethics Committee. We acknowledge the contribution of the following people: (1) Mrs. Lamaro Harriet—Gulu Regional Referral Hospital; (2) Mr. Okumu Thomas—Gulu Regional Referral Hospital; (3) Mr. Tito Okello Lutwa—Gulu Regional Referral Hospital; (4) Mrs. Acayo Irene—Gulu Regional Referral Hospital; and (5) Mr. Kiduma Robert—Gulu University.
Footnotes
ORCID iDs: Robert Edilu  https://orcid.org/0009-0006-2887-4596
https://orcid.org/0009-0006-2887-4596
Felix Bongomin  https://orcid.org/0000-0003-4515-8517
https://orcid.org/0000-0003-4515-8517
Ritah Nantale  https://orcid.org/0000-0001-8433-7054
https://orcid.org/0000-0001-8433-7054
Pebalo Francis Pebolo  https://orcid.org/0000-0002-1205-1150
https://orcid.org/0000-0002-1205-1150
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Robert Edilu, Department of Reproductive Health, Faculty of Medicine, Gulu University, Pece-Laroo, Gulu City, Gulu, Uganda.
Aaron Sanvu, Department of Reproductive Health, Faculty of Medicine, Gulu University, Gulu City, Uganda.
James Ecuut, Department of Reproductive Health, Faculty of Medicine, Gulu University, Gulu City, Uganda.
Alban Odong, Department of Reproductive Health, Faculty of Medicine, Gulu University, Gulu City, Uganda.
Felix Bongomin, Department of Medical Microbiology & Immunology, Faculty of Medicine, Gulu University, Gulu, Uganda; Department of Internal Medicine, Gulu Regional Referral Hospital, Gulu, Uganda.
Ritah Nantale, Department of Community and Public Health, Faculty of Health Sciences Mbale, Busitema University, Mbale, Uganda.
Jackline Ayikoru, Department of Reproductive Health, Faculty of Medicine, Gulu University, Gulu City, Uganda.
Baifa Arwinyo, Department of Obstetrics and Gynecology, Gulu Regional Referral Hospital, Gulu, Uganda.
Sande Ojara, Department of Obstetrics and Gynecology, St. Mary’s Hospital Lacor, Gulu, Uganda.
Pebalo Francis Pebolo, Department of Reproductive Health, Faculty of Medicine, Gulu University, Gulu City, Uganda.
Declarations
Ethics approval and consent to participate: The study was conducted in line with the declaration of Helsinki (2008). Ethical and institutional approvals were obtained from Gulu University Research and Ethics Committee (GUREC, Protocol number: GUREC-2023-529) and Gulu Regional Referral Hospital (Correspondence-ADM/2023/05/02), respectively. All participants provided informed consent (verbal and written), and those unable to write provided a thumbprint on the consent form. Participants below 18 years provided informed consent for emancipated minors in line with GUREC guidelines. Mothers undergoing emergency C/S consented on the second postoperative day after being debriefed before the second sample for Hct determination was obtained. Research assistants were trained on the study protocol to ensure all principles of research ethics were adhered to during the course of the study. To ensure the privacy and confidentiality of participants and their information, all consent forms were kept under lock and key in a designated cupboard in the researcher’s rooms. Participant identifiable information such as names were not collected.
Consent for publication: Not applicable.
Author contributions: Robert Edilu: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Aaron Sanvu: Conceptualization; Investigation; Methodology; Project administration; Supervision; Writing – original draft.
James Ecuut: Conceptualization; Data curation; Investigation; Methodology; Project administration; Supervision; Writing – original draft; Writing – review & editing.
Alban Odong: Conceptualization; Methodology; Project administration; Supervision; Writing – original draft.
Felix Bongomin: Formal analysis; Methodology; Supervision; Writing – review & editing.
Ritah Nantale: Conceptualization; Data curation; Formal analysis; Writing – original draft.
Jackline Ayikoru: Conceptualization; Data curation; Supervision; Writing – review & editing.
Baifa Arwinyo: Conceptualization; Investigation; Supervision.
Sande Ojara: Conceptualization; Writing – original draft; Writing – review & editing.
Pebalo Francis Pebolo: Conceptualization; Methodology; Project administration; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: We consent to the provision and publication of primary statistical data sets accessible within the journal and through contacting the first author.
References
- 1. WHO. Maternal mortality 19, World Health Organization, pp.1–5, https://www.who.int/news-room/fact-sheets/detail/maternal-mortality (2019, accessed 17 September 2023).
- 2. WHO. A woman dies every two minutes due to pregnancy or childbirth: UN agencies, WHO,. https://www.who.int/news/item/23-02-2023-a-woman-dies-every-two-minutes-due-to-pregnancy-or-childbirth–un-agencies (2023, accessed 15 June 2024). [PMC free article] [PubMed]
- 3. UBOS. The Republic of Uganda Uganda Demographic and Health Survey (UDHS) 2022 extension, https://www.ubos.org/wp-content/uploads/publications/05_20232022_Statistical_Abstract.pdf (2023, accessed 21 October 2023).
- 4. Atukunda EC, Mugyenyi GR, Obua C, et al. Measuring post-partum haemorrhage in low-resource settings: the diagnostic validity of weighed blood loss versus quantitative changes in hemoglobin. PLoS One 2016; 11(4): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cameron MJ. Definitions, vital statistics and risk factors: an overview, pp.133–146, https://www.semanticscholar.org/paper/Definitions-%2C-Vital-Statistics-and-Risk-Factors-%3A-Cameron/727292a60f381a4abbbfb926a0a637a167146559 (2012, accessed 24 May 2023).
- 6. Faso B, Day E. Reducing the global burden: mother’s day campaign make your mother’s day, every day, WHO, (4), http://www.who.int/goodwill_ambassa-dors/liya_kebede/en/index.html (2007).
- 7. Lancaster L, Richard BS, Correia MPHM, et al. Maternal death and postpartum hemorrhage in sub-Saharan Africa – a pilot study in metropolitan Mozambique. Res Pract Thromb Haemost 2020; 4(3): 402–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Belfort M. Symptoms related to blood loss with postpartum hemorrhage systolic blood pressure, mmHg. UpToDate, p. 56885, https://www.uptodate.com/contents/overview-of-postpartum-hemorrhage?search=postpartumhemorrhagesource=search_resultselectedTitle=…1/37 (2023, accessed 24 May 2024).
- 9. Borders AE. Quantitative blood loss in obstetric hemorrhage. ACOG 2019; 134(794): 150–156. [Google Scholar]
- 10. Jane E. Cesarean section – a brief history. NIH, pp.5–7, https://www.nlm.nih.gov/exhibition/cesarean/part1.html (1993, accessed 27 May 2023).
- 11. Betran AP, Ye J, Moller B, et al. Trends and projections of caesarean section rates : global and regional estimates. BMJ Glob Health 2021; 6(6): e005671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison MS, Goldenberg RL. Cesarean section in sub-Saharan Africa. Matern Health Neonatol Perinatol 2016; 2: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Atuheire EB, Opio DN, Kadobera D, et al. Spatial and temporal trends of cesarean deliveries in Uganda: 2012-2016. BMC Pregnancy Childbirth 2019; 19(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ononge S, Mirembe F, Wandabwa J, et al. Incidence and risk factors for postpartum hemorrhage in Uganda. Reprod Health 2016; 13(1): 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ladella S, Nguyen L, O’Byrne H, et al. Quantitative blood loss is a more accurate measure of blood loss compared to estimated blood loss. Obstet Gynecol 2018; 3: 1–7. [Google Scholar]
- 16. Lagrew D, McNulty J, Sakowski C, et al. Improving health care response to obstetric hemorrhage, V3.0: a California Maternal Quality Care Collaborative toolkit. CMQCC, pp.1–279, https://www.cmqcc.org/sites/default/files/HEMToolkit_03252022 Errata 7.2022 %282%29.pdf (2022, accessed 8 October 2024). [Google Scholar]
- 17. Patel A, Goudar SS, Geller SE, et al. Drape estimation vs. visual assessment for estimating postpartum hemorrhage. Int J Gynecol Obstet 2006; 93(3): 220–224. [DOI] [PubMed] [Google Scholar]
- 18. Com TJ, Torre D. Quantification of blood loss: AWHONN practice brief number 1. J Obstet Gynecol Neonatal Nurs 2015; 44(1): 158–160. [DOI] [PubMed] [Google Scholar]
- 19. Kodkany BS, Derman RJ, Sloan NL. Pitfalls in assessing blood loss and decision to transfer. In: A comprehensive textbook of postpartum hemorrhage – an essential clinical reference for effective management. 2018, pp.81–88. https://www.glowm.com/pdf/PPH_2nd_edn_Chap-11.pdf
- 20. Ruiz MT, Azevedo NF, de Resende CV, et al. Quantification of blood loss for the diagnosis of postpartum hemorrhage: a systematic review and meta-analysis. Rev Bras Enferm 2023; 76(6): 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Briley S. Maternal hemorrhage. Indiana State Department of Health. IPQIC, 2019. https://www.in.gov/health/mch/files/ipqic/maternal-hemorrhage-tool-kit-august-2019.pdf [Google Scholar]
- 22. Perinatology.com. Calculated blood loss (cEBL) calculator – BETA, Vol. 1, 2015, p. 2015, https://perinatology.com/calculators/CalculatedBloodLossCalculatorO.htm [Google Scholar]
- 23. Sharashchandra KV, Shivaraj SP. Intra operative allowable blood loss : estimation made easy. MedPulse Int J Anesthesiol 2020; 14: 27–31. https://medpulse.in/Anesthsiology/Article/Volume14Issue1/Anes_14_1_7.pdf [Google Scholar]
- 24. Thorson CM, Ryan ML, Van Haren RM, et al. Change in hematocrit during trauma assessment predicts bleeding even with ongoing fluid resuscitation. Am Surg 2013; 79(4): 398–406. [PubMed] [Google Scholar]
- 25. Orzolek C, Durie D, Flicker A, et al. Quantitative blood loss in cesarean delivery is more accurate than visual estimation [ID: 1376884]. Obstet Gynecol 2023; 141(5S): 27S. [Google Scholar]
- 26. von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol 2008; 61(4): 344–349. [DOI] [PubMed] [Google Scholar]
- 27. GRRH. Monthly HMIS report. 2022. [Google Scholar]
- 28. Pebalo FP, Steven B, Grace AA. Is the 14% cesarean section rate in Gulu Regional Referral Hospital justifiable? PAMJ Clin Med 2021; 5: 74. https://www.clinical-medicine.panafrican-med-journal.com/content/article/5/74/full/ [Google Scholar]
- 29. Oakland GB. Determining sample size. Can Entomol 1953; 85(3): 108–113. [Google Scholar]
- 30. Jaramillo S, Montane-Muntane M, Gambus PL, et al. Perioperative blood loss: estimation of blood volume loss or haemoglobin mass loss? Blood Transfus 2020; 1: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blosser C, Smith A, Poole AT. Quantification of blood loss improves detection of postpartum hemorrhage and accuracy of postpartum hemorrhage rates : a retrospective cohort study. Cureus 2021; 13(2): 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hancock A, Weeks AD, Lavender DT. Is accurate and reliable blood loss estimation the ‘crucial step’ in early detection of postpartum haemorrhage: an integrative review of the literature. BMC Pregnancy Childbirth 2015; 15(1): 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carr BL, Jahangirifar M, Nicholson AE, et al. Predicting postpartum haemorrhage : a systematic review of prognostic models study selection. Aust N Z J Obs Gynaecol 2022; 62(6): 813–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sivahikyako SA, Owaraganise A, Tibaijuka L, et al. Prevalence and factors associated with severe anaemia post-caesarean section at a tertiary hospital in Southwestern Uganda. BMC Pregnancy Childbirth 2021; 21(1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mremi A, Rwenyagila D, Mlay J. Prevalence of post-partum anemia and associated factors among women attending public primary health care facilities: an institutional based cross-sectional study. PLoS One 2022; 17: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hassen AE, Agegnehu AF, Admass BA, et al. Preoperative anemia and associated factors in women undergoing cesarean section at a comprehensive specialized referral hospital in Ethiopia. Front Med 2023; 10: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Glonnegger H, Glenzer MM, Lancaster L, et al. Prepartum anemia and risk of postpartum hemorrhage: a meta-analysis and brief review. Clin Appl Thromb Hemost 2023; 29(2): 10760296231214536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pubu ZM, Bianba ZM, Yang G, et al. Factors affecting the risk of postpartum hemorrhage in pregnant women in tibet health facilities. Med Sci Monit 2021; 27: 1–9. doi: 10.12659/MSM.928568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schwartz C, Gaudet C. Chorioamnionitis versus maternal sepsis with postpartum hemorrhage. J Obstet Gynecol Neonatal Nurs 2020; 49(6): S30–S31. [Google Scholar]
- 40. Zackler A, Flood P, Dajao R, et al. Suspected chorioamnionitis and myometrial contractility: mechanisms for increased risk of cesarean delivery and postpartum hemorrhage. Reprod Sci 2019; 26(2): 178–183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-reh-10.1177_26334941241289552 for Comparing visual estimation and hematocrit change in the assessment of blood loss among women undergoing cesarean delivery in a tertiary facility in northern Uganda by Robert Edilu, Aaron Sanvu, James Ecuut, Alban Odong, Felix Bongomin, Ritah Nantale, Jackline Ayikoru, Baifa Arwinyo, Sande Ojara and Pebalo Francis Pebolo in Therapeutic Advances in Reproductive Health