Abstract
To ensure food safety, food business operators must eliminate or reduce hazardous factors in manufacturing processes by implementing effective process controls. Since some beans are known to contain cyanogenic compounds, their distribution and use are permitted only as a raw bean paste material. Therefore, from the perspective of Hazard Analysis and Critical Control Points (HACCP), the purpose of this study is to demonstrate the validity of establishing CCPs and to determine the cyanogenic compounds in intermediate products for effectively managing hazardous substances in the manufacturing process. The previously reported method, post-column HPLC with fluorescence detection, was used for determine cyanogenic compounds in CCPs.
While free cyanide ions were only detected at CCP#1, cyanoglycoside analysis was crucial throughout the manufacturing process. Results indicated a decrease in cyanoglycoside concentration as manufacturing progressed, with levels below 10 ppm in the final product. Notably, cyanoglycosides decreased significantly during the shibukiri process (soaking, boiling, and discarding water). The concentration of cyanogenic compounds in raw beans were below the regulated 500 ppm, and the concentrations in the final product were below regulated 10 ppm. In conclusion, it was found that proposed method is very useful for HACCP management to monitor the decrease of cyanide compounds in the manufacturing process.
Keywords: Critical control point (CCP), Risk management, Sweetened bean paste, Post-column HPLC, Conway dish, Manufacturing process, Cyanogenic compounds
Graphical abstract
Highlights
-
•
Cyanogenic compounds in sweetened bean paste were quantified using post-column HPLC.
-
•
Cyanoglycoside concentrations decreased during manufacturing and were below the limit of quantification in the final product.
-
•
From the HACCP perspective, post-column HPLC is useful for identifying and managing hazardous factors in food production.
1. Introduction
Hazard Analysis and Critical Control Points (HACCP) is a systematic approach in which food hygiene is addressed through the analysis and control of biological, chemical, physical, and other potential hazards from raw material production, procurement and handling, to manufacturing, distribution and consumption of the final product [1]. In Japan, following partial revision of the Food Sanitation Act of Japan amended in 2021, all food business operators (FBOs) are required to implement HACCP [2]. To ensure food safety, FBOs need to understand the hazardous factors in the manufacturing processes and demonstrate the appropriateness of process controls to eliminate or reduce such hazards. Critical Control Points (CCPs) are only determined for hazards identified as significant following the results of a hazards analysis. CCPs are established at steps where control is essential and where a deviation could result in the production of a potentially unsafe food. The control measures at CCPs should result in an acceptable hazard level.
Some beans imported into Japan are known to contain cyanogenic compounds [3], and according to the Food Sanitation Act, their distribution and use are only permitted as raw bean paste materials. Therefore, in the sweetened bean paste manufacturing process, cyanogenic compounds contained in the beans as a raw material are considered to be a chemical hazard [[4], [5], [6]]. To demonstrate the efficacy of risk management measures through compliance with manufacturing standards, cyanogenic residues should be quantitatively determined at CCPs [2]. We previously reported a method for determining cyanide compounds (free cyanide ions and cyanoglycoside (linamarin)) in sweetened bean paste using post-column derivatization with HPLC-fluorescence detector [7]. However, the concentrations detected in commercially available products were lower than those validated in the previous report. Therefore, we re-validated of the method at lower concentrations to evaluate if it applicable in this study. The cyanogenic compound levels in raw beans are regulated at 500 ppm. There is no specific limitation for cyanogenic compound in sweetened bean paste, but by applying the regulatory limit of cassava flour based on the international standard set by FAO/WHO Codex Alimentarius Commission, it was considered necessary to adhere to standard of less than 10 ppm in final product. Moreover, the intermediate products at each Critical Control Point (CCP) are unregulated. Therefore, from the perspective of Hazard Analysis and Critical Control Points (HACCP), the purpose of this study is to demonstrate the validity of establishing CCPs. Furthermore, we will clarify whether it is possible to manage the hazardous substances by determining cyanogenic compounds in intermediate products using the proposed method. Thereby this study contributes to enhanced safety and quality control.
2. Materials and methods
2.1. Materials and reagents
Acetic acid, sodium acetate trihydrate, sodium perchlorate monohydrate, methanol, pyridine, citric acid monohydrate, sodium hydroxide, hydrochloric acid, sulfuric acid, linamarase, and linamarin were purchased from FUJIFILM Wako Pure Chemical Corporation (Tokyo, Japan). Sodium p-toluenesulfonchloramide trihydrate was purchased from KANTO CHEMICAL CO., INC. (Tokyo, Japan). Barbituric acid was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). A standard solution of potassium cyanide (1000 ppm as cyanide) was used (Merck, Osaka, Japan). All other chemicals were of analytical reagent grade.
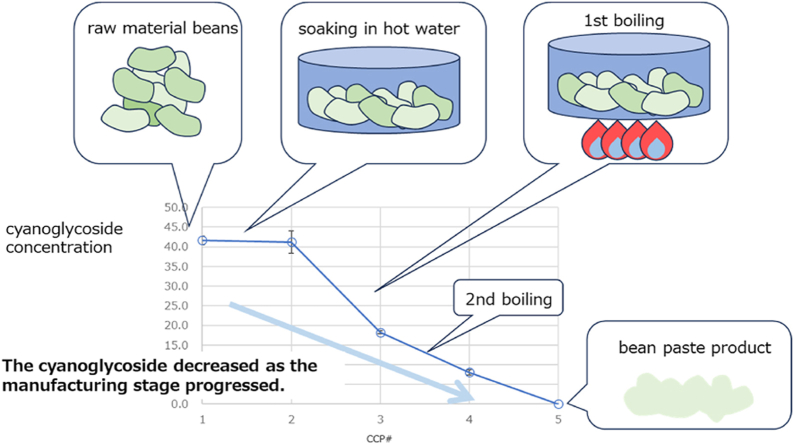
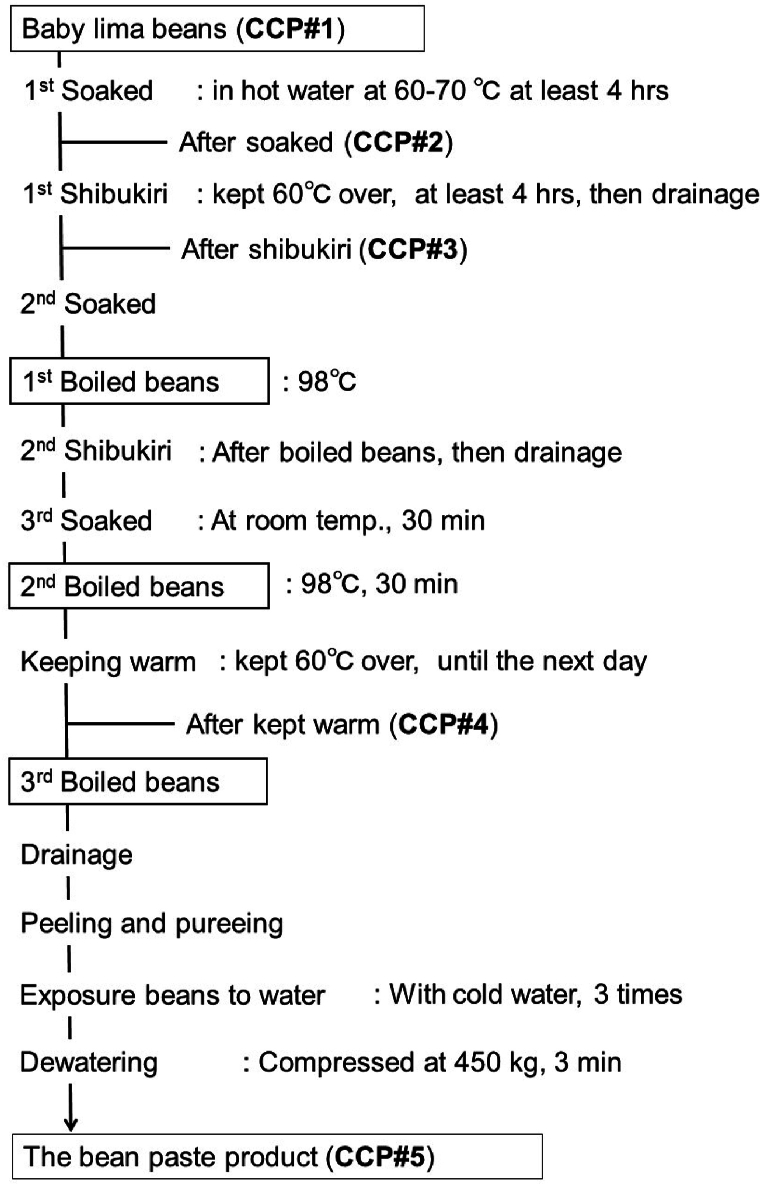
For sample analysis, five points were selected as CCPs during the manufacturing process (Fig. 1). The CCPs were as follows: #1, raw beans (baby lima beans from Myanmar; #2, intermediate product after soaking (60–70 °C, minimum of 4 h); #3, intermediate product after shibukiri, in which the water was drained after being held at > 60 °C for 4 h; #4, intermediate product after keeping warm (>60 °C, overnight); and #5, bean paste product after drying. The sampling was conducted using the same batch in the manufacturing process shown in Fig. 1, with n = 3 samples taken at each CCP.Shibukiri is a process in which beans are boiled or kept warm, and then the boiling or warm water is discarded to remove any astringent compounds, such as tannins and saponins. The raw baby lima beans and intermediate products #2 to #5 from each manufacturing stage were provided by the domestic manufacturer. The samples were kept in a frozen state (<-70 °C) until analysis.
Fig. 1.
Scheme of bean paste manufacturing process.
2.2. Instruments
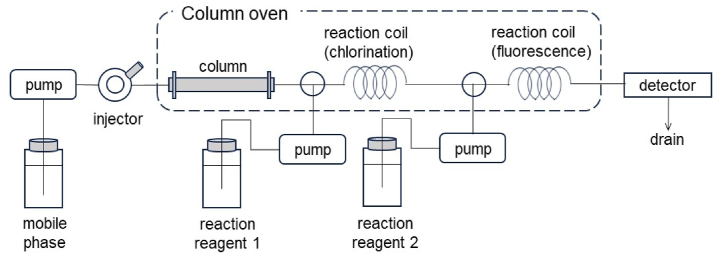
A Conway dish (inner diameter: 60 mm, outer diameter: 77 mm) from SIBATA SCIENTIFIC TECHNOLOGY LTD. (Saitama, Japan.) was used for HCN collection. An incubator (SCL-325) from ASTEC Co., Ltd. (Fukuoka, Japan) was used. The post-column HPLC system consisted of three pumps (LC-20AD and LC-10ADvp from Shimadzu, Tokyo, Japan; UI-22 from FLOM Corporation, Tokyo, Japan), a column oven (CTO-10Avp), a fluorescence detector (RF-20A), and a system controller (SCL-10Avp) from Shimadzu. The degasser (DG661) was from GL Sciences (Tokyo, Japan). A schematic diagram of the post-column HPLC system is shown in Fig. 2.
Fig. 2.
Schematic diagram of the post-column HPLC system.
2.3. Analysis of free cyanide ions
For the analysis of free cyanide ions and cyanoglycosides, a previously reported analytical method [7] was used. The analysis of free cyanide ions is briefly described as follows. One gram of sample was extracted with 10 mL of 0.01 M NaOH aqueous solution. A NaOH aqueous solution (0.1 M; 1 mL) was added to the inner chamber of the Conway dish. Then, the lid was immediately placed on the dish after the extract (1 mL) and 2 M sulfuric acid (1 mL) were added to the outer chamber. The liquid in outer chamber was mixed and allowed to stand in an incubator at 38 °C for 2 h. After incubation, the inner chamber liquid was collected, prior to analysis by HPLC. For the analysis of cyanoglycosides, a sample (1 g) was extracted with 10 mL of 0.1 M citrate buffer (pH 5.9). After 1 mL of 0.1 M NaOH aqueous solution (1 mL) was added to the inner chamber of the Conway dish, 100 μL of the extract and 1 mL of 0.12 units/mL linamarase enzyme solution were separately added to the outer chamber, and the dish was then covered with the lid. The extract and enzyme solution were mixed in the outer chamber, and kept at 38 °C in an incubator for 4 h. Next, 1 M sulfuric acid (1 mL) was added to the outer chamber, the mixture was allowed to stand in an incubator at 38 °C for 2 h. After the incubation, the inner chamber solution was collected, prior to HPLC analysis.
2.4. HPLC analysis
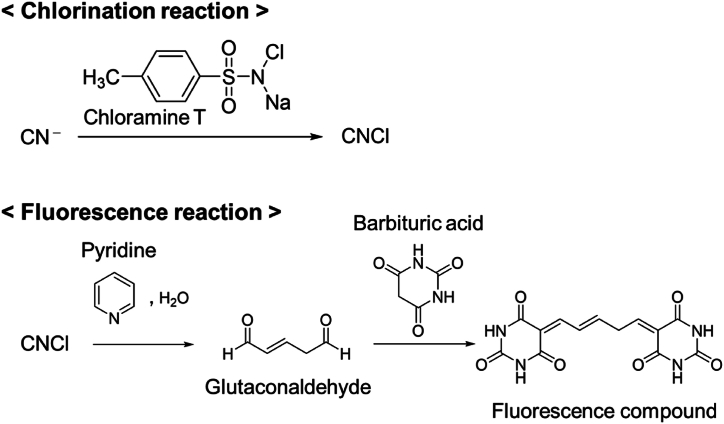
As shown in Fig. 1, the post column-HPLC method involves two reactions [8], chlorination and a fluorescence reaction. The two reaction mechanisms are shown in Fig. 3. The mobile phase consisted of (A) 0.1 M sodium acetate buffer (pH 5.0) containing 12.5 mM sodium perchlorate, and (B) methanol at a ratio of A:B = 90:10 (v/v). The flow rate was 0.5 mL/min. The cyanide compounds were separated on a Scherzo SS-C18 column (3 μm, 4.6 mm i.d. × 250 mm; Imtact Corporation, Kyoto, Japan) at an oven temperature of 40 °C. For reaction reagent 1, 0.1 w/v% chloramine T aqueous solution was used at a flow rate of 0.1 mL/min. The reaction coil for chlorination was TEFZEL™ tubing (0.2 mm i.d. × 5 m; (FLOM Corporation, Tokyo, Japan)). For reaction reagent 2, a pyridine-barbituric acid solution (mixture of 1.5 w/v% barbituric acid, 15 v/v% pyridine, and 3 v/v% hydrochloric acid) was used at a flow rate of 0.1 mL/min. The reaction coil for fluorescence was PEEK tubing (0.25 i.d. × 15 m; (FLOM Corporation, Tokyo, Japan)). The excitation and emission wavelengths of the detector were set at 583 and 607 nm, respectively.
Fig. 3.
Reaction mechanisms of chlorination and fluorescence.
2.5. Method validation
The authors previously reported the method validation of cyanide compounds (free cyanide ions and cyanoglycoside (linamarin)) [7]. However, to determine trace levels of cyanoglycosides, the method was validated again at a lower concentration. In the recovery test of cyanoglycosides, the spiked samples were prepared at a final concentration of 1 ppm as hydrogen cyanide in the bean paste. The spiked recovery was calculated to assess trueness, and the standard deviation of the recovery (n = 5) was calculated to evaluate intra-day precision. The recovery and intra-day precision were calculated using in Microsoft Excel for Microsoft 365. Statistical analysis for multiple comparison was performed using the free software WinSTAT, a general-purpose software downloaded from the Vector software library.
3. Results and discussion
3.1. HPLC analysis and method validation
We re-evaluated the validity of the analytical method previously reported by us for application to this study. The correlation coefficient (r) of the calibration curve was r > 0.99 in the concentration range of 0.02–20 μM. When calculating the hydrogen cyanide concentration, the cyanide ion concentration in the sample was multiplied by 1.04 (=HCN/CN- = 27.025/26.017). The method quantification limit (MQL) of free cyanide ions in sweetened bean paste was 0.1 ppm as hydrogen cyanide, whereas the MQL of cyanoglycosides in sweetened bean paste was 1 ppm as hydrogen cyanide. As shown in Table 1, the trueness and intra-day precision of cyanoglycoside analysis were 88.4 % and 1.5 %, respectively (n = 5). The recovery met the target values in the validation guidelines for residual pesticides in Japan [9]. Therefore, we considered that the proposed method was valid for the verification of HACCP.
Table 1.
The validation data of analytical method.
| Spiked level (ppm) | Trueness (recovery%) | Intra-day precision (%) | |
|---|---|---|---|
| Cyanoglycoside | 1 | 88.4 | 1.5 |
(n = 5).
3.2. Determination of free cyanide ions and cyanoglycosides at each CCP
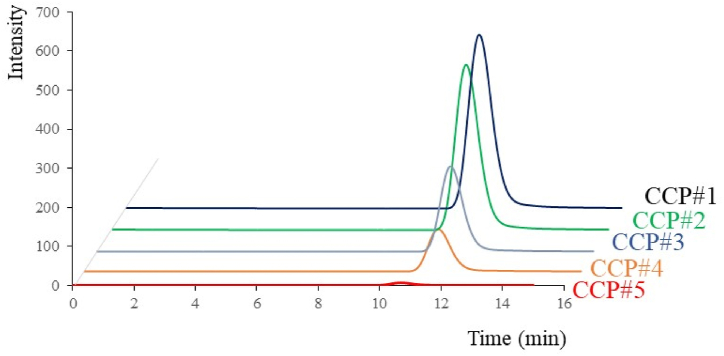
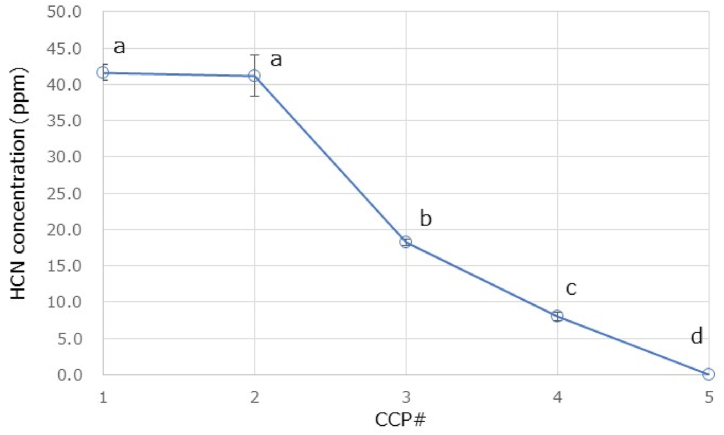
We applied the validated method to determine free cyanide ions at each CCP. Free cyanide ions were determined at the concentration of 30.4 ± 3.2 ppm in CCP#1, whereas they were not determined for CCP#2 to #5. In addition, we determined cyanoglycosides at each CCP. These chromatograms were shown in Fig. 4. As shown in Fig. 5, the concentrations of cyanoglycosides were determined to be 41.7 ± 1.1 ppm in CCP#1, 41.2 ± 2.8 ppm in CCP#2, 18.2 ± 0.4 ppm in CCP#3, 8.0 ± 0.6 ppm in CCP#4, and below the MQL in CCP#5. The cyanoglycosides concentration decreased as the manufacturing stage progressed; in the final product (CCP#5), cyanoglycosides concentration was below the MQL level. The amount of cyanoglycosides decreased significantly from CCP#2 to #3 and from CCP#3 to #4. An initial shibukiri process was employed during CCP#2 and #3, and a second shibukiri process was also performed before CCP#4, suggesting that the shibukiri process was greatly involved in the decrease of cyanoglycosides. The results suggest that the cyanoglycosides were removed along with the astringent compounds by dissolving in water. It was also observed that boiling decrease the cyanide compounds concentration, which was consistent with the report by Kawamura et al. [10].
Fig. 4.
HPLC chromatograms of cyanoglycosides at each CCP.
The cyanoglycosides were determined in each CCPs during the manufacturing process.
CCP#1: raw beans, CCP#2: intermediate product, CCP#3: intermediate product, CCP#4: intermediate product, and CCP#5: the bean paste product.
Fig. 5.
Monitoring of cyanoglycosides behavior at CPPs.
Data are mean ± standard deviation (n = 3). A multiple comparison was carried out using one-way ANOVA and post hoc Bonferroni test (p < 0.01). Different letters above the markers indicate significant difference between the five CCPs.
The variability of the determinations was also discussed; the validation guideline [9] sets a target RSD <10 % for concentrations greater than 0.1 ppm. The RSD at each CCP was CCP #1: 2.6 %, #2: 6.8 %, #3: 2.2 %, and #4: 7.5 %, and these data were within the target variability. This indicates that sufficient determination was achieved. On the other hand, for CCP#2 and CCP#4, the RSD was within the target variability but greater than the repeatability (%RSD) of this method. Therefore, there was concern about the cyanoglycoside content and variation in the removal process, but no variation was observed in CCP#5 and stable cyanoglycoside removal was confirmed.
As a result, the cyanoglycoside concentration in the sweetened bean paste was below the limit of quantification, indicating that cyanoglycosides were properly removed throughout the manufacturing process, and the CCP settings were judged to be appropriate.
3.3. Differential determination of free cyanide ions and cyanoglycoside-derived cyanide ions
In the free cyanide ion analysis, the extraction was performed with 0.1 M NaOH, a strong alkali, which was outside the optimum pH for linamarase (pH 5.9); thus, we considered that the enzyme (linamarase) would be largely inactive. On the other hand, in the analysis of cyanoglycosides, the extraction was performed using citrate buffer (pH 5.9), and we estimated that free cyanide ions were also extracted with cyanoglycosides. Therefore, we determined the level of free cyanide ions that could be extracted under the conditions of cyanoglycosides extraction. An identical procedure as the extraction method for cyanoglycosides was performed without the addition of the enzyme (linamarase). Consequently, the concentration of free cyanide ions in CCP#1 was 50.2 ± 8.5 ppm, whereas it was not detected in CCP#2 to #5. Moreover, this result and the result of free cyanide ion analysis in CCP#2 to #5 revealed that both levels were below the MQL. Therefore, the results suggest that differential determination of free cyanide ions and cyanoglycoside-derived cyanide ions was achieved in CCP#2 to #5. In the determination of CCP#1, differential determination of free cyanide ions and cyanoglycosides was not achieved. In CCP#1, the RSD of the cyanide ion determination value met the target values in the guidelines [9], however, it was larger than the repeatability (%RSD) of this method. In other words, the RSD of the quantification value of cyanide ion was not due to the variation derived from the analytical method. We attributed this result to digestion by endogenous enzymes contained in the beans. In fact, in CCP#1, the free cyanide ion concentration was not significantly different from that obtained by applying the cyanoglycoside extraction procedure without the addition of linamarase (t-test, p = 0.29), suggesting that cyanide ions were derived from cyanoglycosides by the action of endogenous enzymes.
4. Conclusions
The previously reported method [7] was re-validated to confirm its applicability to the present study. As the method was found to be applicable, it was employed to determine the cyanide compounds in each manufacturing stage (CCP#1–5). Since free cyanide ions are found at a much lower concentration than cyanoglycosides, the cyanoglycoside analysis is critical in the study of cyanide compounds in sweetened bean paste. Results of an investigation of the decrease of cyanoglycosides showed that the concentration of cyanoglycosides significantly decreased during the shibukiri process. In this study, the concentration of cyanide compounds was below the regulated concentration of 500 ppm in raw material beans, and below the MQL in the final product. The method used here exhibited higher sensitivity than the official method (LOQ: 10 ppm). In conclusion, not only is the validated method more sensitive than the conventional method, our study suggests that the method can be applied to determine cyanogenic compounds in the manufacturing process of sweet bean paste and to confirm the decrease of cyanogenic compounds at each CCP, thereby contributing to food safety.
Funding
This study was supported by grants from the Ministry of Health, Labour and Welfare of Japan (20KA1005, 21KA1009 and 23KA1007).
Data availability
Data will be made available on request.
CRediT authorship contribution statement
Rie Ito: Writing – review & editing, Writing – original draft, Visualization, Validation, Investigation, Formal analysis, Data curation, Conceptualization. Ayaka Kikuchi: Validation, Investigation, Formal analysis, Data curation, Conceptualization. Airi Ishibashi: Validation, Investigation, Formal analysis. Tsuyoshi Kai: Validation, Investigation, Formal analysis. Akira Terashima: Writing – review & editing. Yusuke Iwasaki: Writing – review & editing, Conceptualization. Takaaki Taguchi: Writing – review & editing, Conceptualization. Tomohide Fukiwake: Writing – review & editing, Conceptualization. Tomoaki Tsutsumi: Writing – review & editing, Conceptualization. Tomoaki Imamura: Writing – review & editing, Project administration, Funding acquisition. Hiroshi Akiyama: Writing – review & editing, Writing – original draft, Visualization, Supervision, Resources, Project administration, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
The authors thank Hiroshi Asakura for advice.
References
- 1.FAO and WHO, General principles of food hygiene CXC 1-1969, 2022 edition, https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXC%2B1-1969%252FCXC_001e.pdf (access data: 27/September/2023).
- 2.Ministry of Health, Labour and Welfare of Japan Shokuhintou jigyousyadantai ni yoru eiseikanri keikaku tebikisho no tameno gaidansu dai 4 han. https://www.mhlw.go.jp/content/11130500/000796211.pdf (written in Japanese) (access data.
- 3.Tsutsumi T., Ishii R., Takatsuki S., Matsuda R. Evaluation of an analytical method for cyanogenic compounds in beans and surveillance of cyanogenic compounds in beans. Shokuhin Eiseigaku Zasshi. Food Hyg. Saf. Sci.) 2011;52:370–375. doi: 10.3358/shokueishi.52.370. [DOI] [PubMed] [Google Scholar]
- 4.Vetter J. Plant cyanogenic glycosides. Toxicon. 2000;38(1):11–36. doi: 10.1016/s0041-0101(99)00128-2. [DOI] [PubMed] [Google Scholar]
- 5.Cressey P., Saunders D., Goodman J. Cyanogenic glycosides in plant-based foods available in New Zealand. Food Addit. Contam. Part A. 2013;30(11):1946–1953. doi: 10.1080/19440049.2013.825819. [DOI] [PubMed] [Google Scholar]
- 6.Zhong Y., Xu T., Chen Q., Li K., Zhang Z., Song H., Wang M., Wu X., Lu B. Development and validation of eight cyanogenic glucosides via ultra-high-performance liquid chromatography-tandem mass spectrometry in agri-food. Food Chem. 2020;331 doi: 10.1016/j.foodchem.2020.127305. [DOI] [PubMed] [Google Scholar]
- 7.Akiyama H., Ishibashi A., Kai T., Kikuchi A., Taguchi T., Fukiwake T., Tsutsumi T., Asakura H., Ito R. Determination of cyanide and cyanoglycosides in sweetened bean paste by HPLC with fluorescence detection. Biol. Pharm. Bull. 2023;46:1024–1026. doi: 10.1248/bpb.b23-00118. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki R., Higashi K., Okamoto Y., Abe H., Iwase H., Toida T. Detection of selenocyanate in biological samples by HPLC with fluorescence detection using König reaction. Chem. Pharm. Bull. 2019;67:884–887. doi: 10.1248/cpb.c19-00277. [DOI] [PubMed] [Google Scholar]
- 9.Ministry of Health, Labour and Welfare of Japan . 2006. Guideline for the Validation of Analytical Methods for Testing Agricultural Chemical Residues in Food.https://www.mhlw.go.jp/english/topics/foodsafety/positivelist060228/dl/181130_21.pdf (access data. [Google Scholar]
- 10.Kawamura Y., Hikidi S., Maruyama K., Uchiyama S., Saito Y. Fate of cyanogenic compounds in beans during the manufacturing process of bean paste. J. Food Hyg. Soc. Jpn. 1992;34:80–83. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.