Abstract
The Chilean papaya (Vasconcellea pubescens A.DC.) is a climacteric fruit that grows in the north and center of Chile. During its processing, residues formed mainly by mucilage and seeds are produced and mostly discarded, despite being a potential source of bioactive metabolites. This work aimed to apply untargeted metabolic analysis by HPLC-DAD-QToF to study the chemical composition of ethyl acetate and methanol extracts from Chilean papaya residues and evaluate their antioxidant and antiglycation capacities. Twenty-three metabolites were tentatively identified in papaya residues, including one carboxylic acid, one glycosylated hydroquinone, four flavan-3-ols, three proanthocyanidins, twelve glycosylated flavonols, one carbohydrate, and one alkaloid reported for the first time. The antioxidant capacity measured as the scavenging of DPPH• and ABTS•+ radicals was comparable with that of ascorbic acid. Chilean papaya extracts decreased fluorescent Advanced Glycation End (AGE) products and oxidative modifications in proteins induced by glucose.
Keywords: Chilean papaya, Mucilage, Seeds, Chemical composition, Antioxidant, Protein glycation, Protein oxidation
Highlights
-
•
Ethyl acetate and methanol extracts from Chilean papaya residues were investigated.
-
•
Twenty-three compounds were identified using a non-targeted metabolic approach.
-
•
Papaya residue extracts showed antioxidant capacity against DPPH.• and ABTS•+
-
•
Papaya residue extracts decreased fluorescent AGEs and protein oxidation markers.
-
•
Correlations were found among phenolics, antioxidant capacity, and AGEs inhibition.
1. Introduction
Chilean papaya (Vasconcellea pubescens A. DC.) is a native species to South America belonging to the Caricaceae family. It is widely distributed throughout the Andes countries, especially in Colombia, north of Ecuador, and Central Chile. Chilean papaya was introduced in Chile over 50 years ago and is cultivated in Coquimbo and Valparaiso valleys, as well as in Maule Region coast. The consumption of V. pubescens is very popular in Chile. The fruits are used to make jams, preserves, cold drinks and cocktails. Chilean papaya's chemical composition and biological activity have not been widely studied. However, there is a lot of information regarding the presence of secondary metabolites and bioactivity of other members of the Caricaceae family, such as Carica papaya L [1].
It is known that ripe Chilean papaya is a potential source of chemical compounds such as carotenoids, vitamins, proteins, and polysaccharides. Previous works on the composition of Chilean papaya have studied the volatile compounds responsible for the aroma, reporting the presence of several alcohols, aldehydes, ketones, and aliphatic esters [[2], [3], [4]]. The fatty acid profile of papaya seed oil has been also investigated, oleic (n-9) and linoleic (n-6) acids being the major acids [5]. Regarding the phenolic constituents of fruits, two quercetin glycosides (rutin and manghaslin) have been previously isolated [6]. Hydroxycinnamic acids, hydroxycinnamic acid glycosides and quercetin glycosides have been identified [6,7].
Concerning biological activities, polyphenolic enriched extracts of Chilean papaya fruits have shown antioxidant capacity [6,8]. More recently, Vega-Gálvez et al., 2019 reported the antioxidant capacity of dried papaya and its efficacy in inhibiting the α-glucosidase, a key enzyme associated with metabolic syndrome. Recently, attention has been focused on dietary polyphenols, as they inhibit the oxidation of biomolecules, thus protecting humans from the onset of various chronic diseases such as type II diabetes mellitus (T2DM). In particular, the inhibition of dietary and endogenous advanced glycation end products (AGEs), has been proposed to be significant for patients diagnosed with T2DM, as well for subjects with metabolic syndrome [[9], [10], [11]]. Numerous reports have shown that polyphenolic compounds can inhibit the generation of AGEs produced under thermal treatment of foods [12], as well as those produced under in vitro simulating physiological conditions [13,14]. However, the inhibitory effects of polyphenolic enriched extracts from Chilean papaya on AGEs have not yet been studied.
During the food industry processing of the Chilean papaya, residues formed mainly by mucilage and seeds are produced. These residues are mostly discarded because of their limited current use. Chilean papaya residues (seeds and skin) have shown antioxidant capacity and presence of polyphenolic compounds such as rutin, quercetin, and phenolic acids [5,15].
Plant mucilage constitutes a complex mixture of water-soluble polysaccharides, mainly composed of monosaccharides and uronic acids linked with glycosidic bonds, glycoproteins and bioactives. Previous studies of plant mucilage of different species have shown interesting characteristics that promote its potential use in food industry as additive, encapsulation agent of biologically active ingredients, and as edible coatings or films [16]. It has also been described that mucilages have significantly higher antioxidant activity than other hydrocolloids due to their chemical composition and presence of polyphenolic compounds [16].
The aim of the present study was to determine the chemical profile of ethyl acetate and methanol extracts from Chilean papaya residues (mucilage and seeds) and to evaluate their bioactivity in terms of their antioxidant and antiglycation capacity. To our knowledge, this is the first report of untargeted metabolic approach to study the chemical composition of waste extracts from Chilean papaya using two different solvents and the first report of their antiglycation capacity.
2. Materials and methods
2.1. Chemicals and reagents
Petroleum ether (p.a.), ethyl acetate (p.a), methanol (p.a.), formic acid (p.a.), acetonitrile (HPLC grade), 2,2-diphenyl-1-picrylhydrazyl, 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid), dimethylsulfoxide, ascorbic acid, bovine serum albumin, cryptochlorogenic acid, sodium azide, NaH2PO4, Na2HPO4, Tris base, glycine, D(+)-glucose, sodium azide, coomassie brilliant blue, and aminoguanidine hydrochloride were obtained from Sigma-Aldrich (St. Louis, MO, USA). Laemmli buffer (4 X), sodium dodecyl sulfate (SDS), and β-mercaptoethanol were purchased from Bio-Rad Laboratories, USA. Monoclonal antibody against carboxymethyllysine was obtained from R&D systems, Minneapolis, MN, USA. The enzymatic kits for total polyphenols (12815) and catechins (12834) analyses were acquired from Biosystems (Santiago, Chile).
2.2. Sample collection and pre-treatment
The Chilean papaya residues (mucilage and seeds) were obtained from the local artisan production of preserves in the town of La Pesca, Iloca, Maule Region, Chile, in March of 2022. The samples were transported to the laboratory and kept at −6 °C until processing.
Chilean papaya residues were subjected to extraction following the procedures described by Razali et al., 2012 and Gopčević et al., 2019 [17,18] with modifications. The mixture of mucilage and seeds (1000 g) was homogenized in a blender and extracted three times with petroleum ether (3x 1 L each) at room temperature in the dark for 1 day and then sonicated for 10 min using a Branson ultrasonic cleaner bath, model 1510, 115v, 1.9 L with a mechanical timer (10 min with continuous hold) and heater switch, 47 kHz to obtain the low polarity metabolites. The de-fatted residue was extracted three times with ethyl acetate, using the same extraction procedure (3 x 1 L each, at room temperature in the dark for one day and then sonicated for 10 min using a Branson ultrasonic cleaner bath). The remaining residue was re-extracted with methanol three times (3 x 1 L each, at room temperature in the dark for one day and then sonicated for 10 min using a Branson ultrasonic cleaner bath) to obtain the high polarity extract. Extracts were then filtered, combined and concentrated under reduced pressure below 40 °C in a rotary evaporator Heidolph 517-61000-00-0 (Germany), frozen at −20 °C and subsequently lyophilized in a Biobase-BK-FD10P lyophilizer (HES, Chile). The freeze-dried extracts were stored at 4 °C for further analysis.
2.3. Chemical characterization of papaya residue extracts
Total polyphenols and total catechins were measured on an Y15 automatic analyzer (Biosystems, Barcelona, Spain), using the kits COD 12815 and COD 12834 respectively, acquired from BioSystems (Santiago, Chile). Total polyphenols method (kit 12815) is based on the reaction of polyphenols in the samples with the Folin-Ciocalteu′s reagent in basic media, generating a colored complex quantified spectrophotometrically at 750 nm. Determination of total catechins by means of the kit 12834 is based on the reaction of catechins (mainly monomeric fraction of flavan-3-ols) with the chromogen p-(dimethylamine)-cinnamaldehyde (p-DAC) in ethanol/acid medium, generating a colored complex quantified spectrophotometrically at 620 nm. Measurements were performed in triplicate, and results were reported in mg/L of gallic acid, and catechin as mean ± SD.
2.4. HPLC-DAD-Q-ToF analysis
To tentatively identify phenolic compounds and other metabolites in Chilean papaya residues, the ethyl acetate and methanol extracts were analyzed using a HPLC-DAD Bruker Elute LC system coupled in tandem with a Q-ToF spectrometer Compact, Bruker (Bremen, Germany). Instrument control and data collection were carried out using Compass Data Analysis 4.4 SR1 (Bruker Daltonics GmbH). The MS conditions for compound identification were as follows: negative ionization ESI −3500 V, dry gas: 9 L/min, nebulizer: 2 Bar, T: 200 °C, end capillary 500 V, collision energy at 20–50 eV in stepping mode, Auto MS/MS mode (4 precursor/cycle), 50–1500 m/z (scan 0.2 s centroid mode), and internal calibration using sodium formate (10 % formic acid, 1 M) with a mass accuracy <3 ppm.
Chromatographic separation was performed using a C18 column (Kromasil 250 mm × 4.6 mm × 5 μm), oven temperature 32 °C. The mobile phase consisted of 0.1 % formic acid in water (A) and 0.1 % formic acid in acetonitrile (B) at a flow rate of 0.3 mL/min. The gradient of mobile phase B ranged from 15 % to 25 % for 14 min, 25 %–35 % for 11 min, 35 %–100 % for 1 min, 100 % for 9 min; 100 to 15 % for 1 min, with a stabilization period of 10 min.
2.5. Tentative identification of compounds in papaya residues
Tentative identification of the compounds present in papaya residue extracts was carried out using the Library Search tool of the Global Natural Products Social Molecular Networking (GNPS) platform [19]. The analysis was performed using the following conditions: precursor Ion Mass Tolerance: 0.025 Da; Fragment Ion Mass Tolerance: 0.02 Da; Score Threshold: 0.7. The following MS libraries were used for the analysis: MONA, MASSBANK, Sumner Spectral Library, BERKELEY-LAB, TUEBINGEN-NATURAL-PRODUCT-COLLECTION, libraries associated and included in the platform GNPS-LIBRARY (https://gnps.ucsd.edu/) [19].
Of the total compounds detected by GNPS analysis, those that met the following selection criteria were reported: compounds detected in at least two of the three replicates of the extracts analyzed, that the difference between detected mass and library mass was not greater than one, and that the elution of the compound occurs before the column cleaning process (33 min).
2.6. Antioxidant activity
The in vitro free radical scavenging capacity of papaya waste extracts was evaluated using the 2,2-diphenyl-1-picrylhydrazyl (DPPH•) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) assays.
The extracts were evaluated for DPPH• free radical scavenging capacity, according to the method previously described by Brand-Williams [20] and modified by Polo-Cuadrado [21]. 1 mL aliquot of the tested extract (10–100 μg/mL in methanol) or of the control (2 % final DMSO in methanol) were mixed with 2 mL of a methanolic DPPH• solution (0.02 mg/L). The mixture was vigorously stirred and left to stand at room temperature for 5 min in the absence of light; after this time, the absorbance of the samples was measured at 517 nm using a SYNERGY HTX multimode reader. Ascorbic acid was used as a positive control. Free radical scavenging activity was calculated as a percentage of DPPH• decoloration using the following equation: Percentage of free radical removal DPPH• = 100 × (1 − AE/AD) where AE is the absorbance of the solution after adding the extract, and AD is the absorbance of the blank DPPH• solution. All measurements were performed in triplicate. Ascorbic acid was used as the reference compound with an EC50 value of 1.5 μg/mL in methanol.
The extracts were evaluated for the radical scavenging capacity of 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS•+) according to Ref. [22] and modified by Ref. [21]. The stock solution was prepared by mixing equal volumes of a solution of 7 mM ABTS and a 2.45 mM potassium persulfate solution followed by incubation for 12 h at room temperature in the dark to produce a dark-colored solution containing ABTS•+ radicals. The working solution was prepared once it was needed in the assay to avoid its oxidation, adding 50 % ethanol to give an initial absorbance of approximately 0.70 (±0.02) at 732 nm at room temperature. Free radical scavenging capacity was evaluated by mixing 300 μL of the extract (10–100 μg/mL) with 2.7 mL of ABTS•+ working solution. The decrease in absorbance was measured exactly 30 min after mixing the solution. Ascorbic acid was used as a positive control. The scavenging activity was estimated based on the percentage of ABTS•+ radicals inhibition using the following equation: Percentage of free radical scavenging ABTS•+ = [(A0 − As)/A0] × 100.
where A0 is the absorption of the control, and As is the absorption of the extract solution. All measurements were performed in triplicate. Ascorbic acid was used as the reference compound with an EC50 value of 28 μg/mL in methanol.
2.7. Inhibition of fluorescent advanced glycation end products (AGEs) and protein oxidation markers
The inhibition of AGEs and the oxidation products arising from Tryptophane (Trp) and Tyrosine (Tyr), such as Kynurenine (Kyn) and Di-tyrosine (Di-Tyr), respectively, by means of extracts from Chilean papaya were assessed by fluorescence spectroscopy. Bovine serum albumin (BSA) was prepared at a final concentration of 10 mg/mL in phosphate buffer 100 mM, pH 7.4 and incubated with glucose (0.5 M) during 40 h at 55 °C, pH 7.4 in phosphate buffer 100 mM in the presence of PEA or PM in final concentrations of 5, 50, 100, 500 and 2500 μg/mL in phosphate buffer 100 mM. Controls incubated at the same conditions, but without glucose and containing only BSA and the extracts at concentrations of 50, 100, 250, 500 and 2500 μg/mL in phosphate buffer 100 mM, pH 7.4 were also prepared. Control of aminoguanidine (AG) was also prepared at the following concentrations: 5, 25, 50, 100 and 250 μg/mL and incubated with BSA in the presence and absence of glucose (0.5 M). Fluorescence was determined at the following λEXC/λEM for AGEs 1: 325/440; AGEs 2: 389/443; Di-Tyr: 330/415 and Kyn: 365/480 nm.
The inhibition percentage of fluorescence attributed to AGEs was calculated as:
where BSAGlu/P represents BSA incubated with glucose and extracts; BSAP corresponds to serum albumin incubated with extracts at the same concentration as BSAGlu/ and BSAGlu corresponds to serum albumin incubated with glucose.
Fluorescence quenching associated to AGEs 1, AGEs 2, Di-Tyr and Kyn was carried out using the control BSA + Glucose (3 mg/mL in phosphate buffer 100 mM, pH 7.4) and adding increasing concentration of PEA, AG and Cryptochlorogenic acid (CCGA), at 25 °C. The quenching of the fluorescence was plotted using the Stern-Volmer equation:
where F0 and F are the fluorescence intensities of BSA-glucose in the absence and presence of PEA, PM (5, 50, 100, 500 and 2500 μg/mL), AG (5, 25, 50, 100 and 250 μg/mL) or CCGA (1, 5, 50, 100 and 500 μg/mL). Ksv is the Stern-Volmer quenching constant, expressed in mL/μg and [Q] is the concentration of the different quenchers. Fluorescence analyses were performed in a Perkin Elmer LS 55 fluorescence spectrofluorometer (PerkinElmer ltd., Waltham, MA, USA).
2.8. SDS-PAGE and Western Blot analysis for carboxymethyllysine
SDS-PAGE analysis was carried out with BSA incubated in the presence and absence of glucose, as well as with extracts (from 50 to 2500 μg/mL). Controls and samples, containing 225 μg of proteins, Laemmli sample buffer and β-mercaptoethanol (676 mM, final concentration) were boiled for 5 min and loaded onto 12 % (w/v) SDS-PAGE gels. Electrophoresis was carried out at 100 V for 60–120 min. Gels were stained with Coomassie Brilliant Blue (0.1 %, w/v) and destained or electrotransferred into a nitrocellulose membrane (GE Healthcare, Germany) for Western Blot analysis. The electrotransference was performed at 100 V, 60 min and membranes were blocked overnight at room temperature under constant agitation. Monoclonal antibody against carboxymethyllysine (CML) (R&D systems, Minneapolis, MN, USA) were used diluted 1:1000 in blocking buffer and incubated 1 h with the electrotransferred membrane. After washing, membranes were incubated with a horseradish peroxidase-conjugated secondary antibody, diluted (1:10000 in phosphate buffer 100 mM, pH 7.4). Membranes were incubated with an ECL Plus chemiluminescent detection system (Amersham, GE Healthcare, Buckinghamshire, UK), and the chemiluminescence was detected using a photo-documenter Omega Lug. Gels and revealed membranes were analyzed by means of the ImageJ software (NIH, Bethesda, MD, USA). In each lane, proteins were categorized as: fragmentated (<50 kDa), monomers (between 50 and 75 kDa), dimer-trimer proteins (between 75 and 250 kDa), and high molecular weight aggregates (>250 kDa). Pixel intensities (areas under the curve) from these four categories were added and considered as 100 % and then the percentage of area corresponding to proteins in each category was calculated.
2.9. Statistical analyses
Statistical analyses were carried out using the SPSS Statistics 24 version (IBM, Armonk, NY, USA). Statistical differences among measurements were determined by one-way analysis of variance, ANOVA, and mean separations were performed using the Tukey test with a confidence level of 95 %. To establish possible correlations between the different parameters in Chilean papaya extracts, a bivariate Pearson's correlation analysis was carried out. The respective correlation coefficient (Pearson's r) was registered in each case, and their significance was denoted as ∗ or ∗∗ according to their p-values (p < 0.05 and p < 0.01, respectively).
3. Results and discussion
3.1. Total phenolics and catechins
Polyphenols are metabolites widely distributed in several plants, including food crops. The quantity and diversity of polyphenols present in plant material have been recognized as responsible for the bioactivity of many plant species [23]. The extraction yield for the PEA and PM from Chilean papaya residues, and their chemical characterization with respect to total phenolic content and total catechins are shown in Table 1. The extraction yield was 1.221 g/100 g of fresh residue for the PEA and 5.445 g/100 g of fresh residue for PM (Table 1). These yields are comparable to those obtained by Briones-Labarca [5], for 80 % aqueous methanol extracts of Chilean papaya seeds using ultrasound-assisted extraction and conventional extraction. The total phenolic content of Chilean papaya residue extracts (6.244 ± 0.342 g GAE/100 g of extract for PEA and 4.902 ± 0.702 g GAE/100 g of extract for PM) is comparable to that of aqueous extracts of Carica papaya L. seeds [24]. Total catechins were quantified in both, the PEA and PM, reaching values of 3.793 ± 0.223 and 2.796 ± 0.558 g CE/100 g of extract for PEA and PM, respectively. Total phenolics and total catechins were significantly higher in PEA, with p-values of 0.041 and 0.045, respectively.
Table 1.
Chemical characterization of ethyl acetate (PEA) and methanol extracts (PM) from Chilean papaya residues.
| Extraction yield (g/100 g of fresh residue) | Total phenolics (g GAE/100 g of extract) | Total catechins (g CE/100 g of extract) | |
|---|---|---|---|
| PEA | 1.221 | 6.244 ± 0.342a | 3.793 ± 0.223a |
| PM | 5.445 | 4.902 ± 0.702a | 2.796 ± 0.558a |
GAE: gallic acid equivalents; CE: catechin equivalents; PEA: Papaya ethyl acetate extract; PM: Papaya methanol extract. Values expressed as mean ± standard deviation. For each analysis.
indicates statistically significant differences between PEA and PM.
In this study, petroleum ether was used to degrease the plant material, separating low-polarity compounds, which would not be part of the study. Afterward, the extraction with ethyl acetate and methanol aimed to obtain medium and high polarity extracts from Papaya residues. Ethyl acetate and methanol are conventional organic solvents for extracting phenolic compounds based on solid-liquid extraction [18,25]. Previous works on Carica papaya and Chilean papaya support using methanol and ethyl acetate as extraction solvents for phenolic compounds. Phenolic compounds from Chilean papaya pulp were previously extracted using methanol as a solvent [6]. Ethyl acetate and methanol extracts of Carica papaya seeds and skins showed high polyphenolic content [26]. Phenolics, such as flavanols, phenolic acids, sinapic acid derivatives, and hydroxycinnamic acid derivatives, expected to be present in Chilean papaya residues, cannot be effectively extracted using water [27], thereby necessitating the use of organic solvents for their extraction. It has been reported that organic solvents possess some disadvantages such as toxicity and high cost, and their use in the extraction process has potential risks to human health and the environment [28]. Given the time required, cost of solvents, toxicity, and environmental impact, conventional organic solvents should be replaced with eco-friendly alternatives in future research on industrial-scale food applications. However, the work presented is preliminary and focused on identifying the potential of Chilean papaya waste as a source of bioactive compounds, requiring further studies such as optimization of the extraction process before its application on an industrial scale.
3.2. Tentative identification of metabolites in extracts from chilean papaya residue
In the last years, Global Natural Product Social (GNPS)-based molecular networking (MN) has emerged as a practical tool for metabolite identification via untargeted mass spectrometry (MS), or MS-based, providing relevant information to analyze the complexity of the matrix under study [19]. From the non-targeted analysis using HPLC-Q-ToF, a total of twenty-three metabolites were identified in Chilean papaya residues. Retention times, molecular mass of the detected ions, and tentative identification according to the GNPS analysis are shown in Table 2. Sixteen compounds were tentatively identified in both extracts of Chilean papaya residues, including one three carboxylic acid (2), one glycosylated hydroquinone (3), four flavan-3-ols (4,6,8,9), two proanthocyanidins (5,7), seven glycosylated flavonols (11, 12, 15, 16,19,20,21), and one alkaloid (10). Other four compounds were detected only in the PEA, including one proanthocyanidin (22), and three flavonols (17,18,23). Among the compounds only detected in the PM, it can be found one carbohydrate (1), and two glycosylated flavonols (13,14). According to these outcomes, the qualitative chemical profile of Chilean papaya residues is mainly composed of flavan-3-ols and glycosylated flavonols. Recently, the polyphenolic profile of Chilean papaya waste (seed and skin) was studied through LC-MS/MS analysis based on comparisons with pure standards, showing the presence of rutin, quercetin, and phenolic acids in seed and skin samples [15]. However, this is the first report of an untargeted analysis of Chilean papaya residue extracts. Our findings agree with those previously reported for the Chilean papaya fruit and those recently informed for the seeds [6,29], in which the contribution percentage of the flavonoids (rutin and quercetin) to total phenolics was higher than that of the phenolic acids. The differences in comparison to previous reports could be attributed to the origin of the samples (Northern Chile vs. Southern Chile), and the impact of biotic and abiotic factors on the qualitative and quantitative profile of secondary metabolites in plants. The degree of ripening of the fruits, which is not informed in any of the studies carried out with Chilean papaya, might also play an important role in the presence of different classes and content of phenolic compounds. Furthermore, we consider that the conservation and treatment of the samples could also be a possible cause of differences in the composition of the extracts obtained.
Table 2.
Tentative identification of secondary metabolites in ethyl acetate and methanol extracts from Chilean papaya residues by HPLC-Q-ToF.
| No | Rt (min) | Tentative identification | Adduct detected | Detected mass (m/z) | Library mass (m/z) | Mass difference (m/z) | MQ Score | Extract |
|---|---|---|---|---|---|---|---|---|
| 1 | 8.22 | 6:3+6O fatty acyl hexoside | [M − H]- | 353.074 | 353.072 | 0.002 | 0.88 | M |
| 2 | 9.11 | Citric acid | [M − H]- | 191.017 | 191.019 | 0.002 | 0.94 | EA; M |
| 3 | 9.48 | Arbutin | [M + HCOO]- | 317.083 | 317.088 | 0.005 | 0.84 | EA; M |
| 4 | 10.38 | Epigallocatechin | [M − H]- | 305.064 | 305.067 | 0.003 | 0.96 | EA; M |
| 5 | 10.41 | Procyanidin B1 | [M − H]- | 577.13 | 577.135 | 0.005 | 0.90 | EA; M |
| 6 | 15.05 | Gallocatechin | [M − H]- | 305.063 | 305.066 | 0.003 | 0.84 | EA; M |
| 7 | 15.85 | Procyanidin B2 | [M − H]- | 577.127 | 577.135 | 0.008 | 0.81 | EA; M |
| 8 | 19.01 | Catechin | [M − H]- | 289.068 | 289.072 | 0.004 | 0.98 | EA; M |
| 9 | 19.07–19.33 | Epicatechin | [M − H]- | 289.068 | 289.072 | 0.004 | 0.98 | EA; M |
| 10 | 19.8 | Tetrahydroharman-3-carboxylic acid | [M − H]- | 229.1 | 229.098 | 0.002 | 0.82 | EA; M |
| 11 | 22.27 | Quercetin 3-(2-glucosylrhamnoside) | [M − H]- | 609.143 | 609.146 | 0.003 | 0.93 | EA; M |
| 12 | 22.33 | Rutin | [M − H]- | 609.141 | 609.146 | 0.005 | 0.98 | EA; M |
| 13 | 25.21 | 6''-O-p-Coumaroyltrifolin | [M − H]- | 593.155 | 593.130 | 0.025 | 0.73 | M |
| 14 | 25.23 | 3''-O-L-Rhamnopyranosylastragalin | [M − H]- | 593.155 | 593.151 | 0.004 | 0.84 | M |
| 15 | 25.5 | Tiliroside | [M − H]- | 593.151 | 593.100 | 0.051 | 0.92 | EA; M |
| 16 | 25.54 | Nicotiflorin | [M − H]- | 593.152 | 593.200 | 0.048 | 0.90 | EA; M |
| 17 | 25.64 | Kaempferol-3-O-glucoside-3''-rhamnoside | [M − H]- | 593.134 | 593.151 | 0.017 | 0.73 | EA |
| 18 | 25.88 | 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-[[(2S,3S,4R,5S)-3,4,5-trihydroxy-6-[(2S,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxyoxan-2-yl] methoxy]chromen-4-one | [M − H]- | 623.162 | 623.162 | 0.000 | 0.87 | EA |
| 19 | 25.91 | Isorhamnetin-3-O-robinobioside | [M − H]- | 623.162 | 623.162 | 0.000 | 0.89 | EA; M |
| 20 | 25.93 | Isorhamnetin-3-O-rutinoside | [M − H]- | 623.163 | 623.161 | 0.002 | 0.84 | EA; M |
| 21 | 25.95 | 5,7-dihydroxy-2-(4-hydroxy-3-methoxyphenyl)-3-[3,4,5-trihydroxy-6-[[(2R,3R,4R,5R,6S)-3,4,5-trihydroxy-6-methyloxan-2-yl]oxymethyl]oxan-2-yl]oxychromen-4-one | [M − H]- | 623.162 | 623.162 | 0.000 | 0.87 | EA; M |
| 22 | 31.66 | 2,2′-bis(3,4-dihydroxyphenyl)-3′,4,5′,6-tetrahydroxyspiro[2H-1-benzofuran-3,9′-3,4-dihydro-2H-furo[2,3-h]chromene]-8′-one | [M − H]- | 573.108 | 573.104 | 0.004 | 0.70 | EA |
| 23 | 32.66 | Myricetin | [M − H]- | 317.032 | 317.000 | 0.032 | 0.76 | EA |
M: Papaya methanol extract; EA; Papaya ethyl acetate extract.
It is interesting to point out that this is the first time reporting the presence of alkaloids in Chilean papaya residue. The alkaloid tetrahydroharman-3-carboxylic acid was detected in the PEA and PM extracts. The presence of alkaloids in Carica papaya seeds has been previously reported, including the β-carboline alkaloid 1-carbomethoxy-β-carboline [[30], [31], [32], [33]]. More recently, the presence of alkaloids in the V. pubescens fruit pulp was shown by infrared spectroscopic analysis, but they remain unidentified [34]. Tetrahydroharman-3-carboxylic acid is an aromatic β-carboline alkaloid naturally occurring in fruits such as oranges, grapes, and traditional medicinal plants. It has shown antimitotic, anti-neuroinflammatory, and antioxidant activities [[35], [36], [37]].
3.3. Antioxidant capacity and inhibition of protein glycation and oxidation
3.3.1. Antioxidant capacity
The antioxidant capacity of PEA was assessed using different assays, including DPPH• and ABTS•+, and expressed as the amount of sample that can scavenge the radical by 50 % (EC50 in μg/mL) (Table 3). The free radical scavenging capacity by DPPH• assay was 55.99 ± 3.55 μg/mL for the PEA and 94.80 ± 2.69 μg/mL for the PM. According to these values, the ethyl acetate extract was the most active sample (p = 1.13E-4) scavenging the DPPH• radical. A similar trend was observed for the extracts through ABTS•+ assay (p = 3.6E-5), with EC50 of 29.77 ± 1.25 μg/mL for the PEA and 56.74 ± 1.96 μg/mL for the PM. In both assays, the antioxidant capacity is concentration-dependent and could be attributable to the polyphenolic components present in each extract. EC50 values showed a significant inverse correlation with TPC (Pearson's r = −0.814 for DPPH•; Pearson's r = −0.849 for ABTS•+) (Table 4), showing that when TPC increases, antioxidant capacity increases. The differences in the capacities of scavenging DPPH• and ABTS•+ radicals between the PEA and PM could be because of the concentration of polyphenolic compounds in the PEA. For both extracts, the free radical scavenging capacity was higher when measured through ABTS•+ assay. The present outcomes agree with Floegel et al., 2011 [38] when evaluating the comparability of antioxidant capacity measurements obtained by ABTS•+ and DPPH• assay applied to food samples. They observed that the antioxidant capacity detected by the ABTS•+ assay was significantly higher for fruits, vegetables, and beverages when compared to that obtained by the DPPH• assay. The scavenging efficiency of the flavonols quercetin, kaempferol, and isorhamnetin and their glycosylated derivatives was found to be higher for the ABTS•+ radical when compared to that for the DPPH• radical [39]. Likewise, Chen and Yu [40], evaluated the antioxidant activity of flavan-3-ols and their derivatives and observed that all the tested compounds displayed higher scavenging activities against the ABTS•+ radical, with lower IC50 values than against DPPH•.
Table 3.
IC50 or EC50 values of Chilean papaya residue extracts in forming fluorescent AGEs, protein oxidation products Di-Tyr and Kyn, and scavenging DPPH• and ABTS•+ radicals.
| EC50 (μg/mL) | IC50 (μg/mL) | |||||
|---|---|---|---|---|---|---|
| DPPH• | ABTS•+ | AGEs 1 | AGEs 2 | Di-Tyr | Kyn | |
| PEA | 55.99 ± 3.55b | 29.77 ± 1.25b | 420.41 ± 34.15a,c | 307.82 ± 35.48a,c | 480.18 ± 39.36c | 274.50 ± 41.04c |
| PM | 94.80 ± 2.69b | 56.74 ± 1.96b | 962.47 ± 67.96a,c | 714.11 ± 14.85a,c | 1283.47 ± 50,86c | 887.40 ± 79.31c |
| AG | – | – | 39.70 ± 1.12 | 82.35 ± 2.47 | 43.28 ± 0.31 | 75.97 ± 2.18 |
PEA: Papaya ethyl acetate extract; PM: Papaya methanol extract; AG: aminoguanidine; AGEs 1: λ exc 325/λ em 440; AGEs 2: λ exc 389/λ em 443; Di-Tyr: λ exc 330/λ em 415; Kyn; λ exc 365/λ em 480.
Indicates statistically significant differences when compared AGEs 1 with AGEs 2 for the same extract.
Indicates statistically significant differences when compared EC50 of different extracts for the same analysis.
Indicates statistically significant differences when compared IC50 of different extracts at the same λEXC/λEM pair.
Table 4.
Correlation matrix (Pearson's r) in relation to the analyzed parameters for papaya extracts.
| TPC | DPPH• | ABTS•+ | AGES 2 | AGES 1 | Di-Tyr | Kyn | Catechins | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1 | −0.814a | −0.849a | −0.870a | −0.742 | −0.844a | −0.873a | 0.467 |
| DPPH• | 1 | 0.982b | 0.950b | 0.973b | 0.983b | 0.963b | −0.846a | |
| ABTS•+ | 1 | 0.986b | 0.974b | 0.999b | 0.992b | −0.776 | ||
| AGES 2 | 1 | 0.927b | 0.985b | 0.981b | −0.673 | |||
| AGES 1 | 1 | 0.977b | 0.965b | −0.859a | ||||
| Di-Tyr | 1 | 0.993b | −0.773 | |||||
| Kyn | 1 | −0.720 | ||||||
| Catechins | 1 |
TPC: Total phenolic content; DPPH•: 2,2-diphenyl-1-picrylhydrazyl radical; ABTS•+: 3-ethylbenzothiazoline-6-sulfonic acid; AGES 1: Advanced glycation end products (λEXC = 325/λEM = 440 nm); AGEs 2: Advanced glycation end products (λEXC = 389/λEM = 443 nm); Di-Tyr: Di-tyrosine; Kyn: Kynurenine.
Correlation is significant at the 0.05 level (two-tailed).
Correlation is significant at the 0.01 level (two tailed).
The antioxidant activity of PEA and PM extracts was moderate compared to the standard used, according the DPPH• results, however, according to ABTS•+ assay results, the EC50 value of PEA is similar to ascorbic acid. The EC50 values are two orders of magnitude lower than those of the methanolic extracts of Carica papaya seeds that showed an EC50 of 1000 ± 0.08 μg/mL in the DPPH• assay [41]. Briones-Labarca et al., 2015 [5] investigated the antioxidant capacity of the 80 % aqueous methanol extract from the seeds of Chilean papaya, but not from the mucilage nor or the ethyl acetate extracts from papaya residues. They reported high antioxidant capacity for the Chilean papaya seeds compared to other fruit crops such as melon, pear, tomato, apple, banana, white and pink grapes, pink grapefruit, orange, kiwi, plum, and strawberry.
3.3.2. Inhibition of protein glycation and oxidation
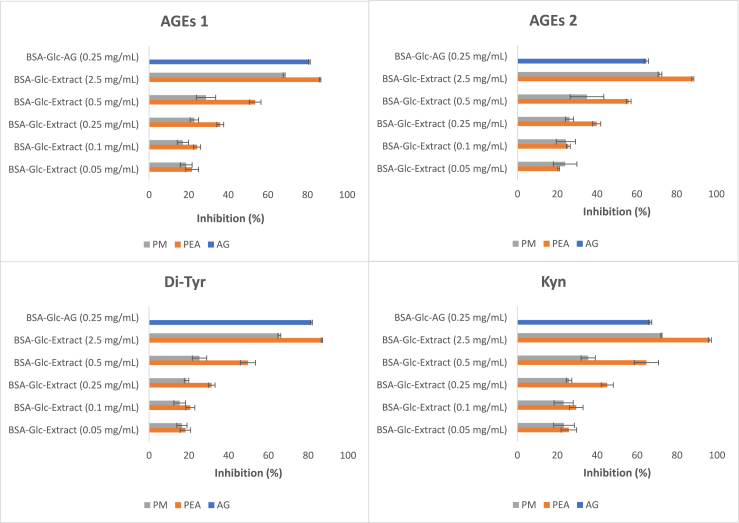
The effect of PEA and PM from Chilean papaya residues on protein glycation and oxidation was assessed by inhibition of fluorescence attributed to AGEs, Di-Tyr, and Kyn. Table 3 shows that IC50 for AGEs 1 (λEXC = 325/λEM = 440 nm) and for AGEs 2 (λEXC = 389/λEM = 443 nm) presented statistically significant differences (p = 0.017 for PEA and p = 0.008 for PM). This fact is consistent with previous reports in which the time-resolved fluorescence properties of fluorophores (λEXC = 316/λEM = 440 nm and λEXC = 375/λEM = 443 nm), resulting from the incubation of BSA with glucose, showed a slightly different lifetime and contribution factor [13]. Our present results support that both pairs of excitation/emission wavelengths assigned for AGEs 1 and AGEs 2 correspond to different AGEs. In this study, the IC50 for AGEs 2 (compatible with fluorescence properties of vesperlysine and crossline) was lower (p < 0.05) than the IC50 value of AGEs 1 (compatible with fluorescence properties of argpyrimidine and pentosidine), suggesting that different mechanisms can inhibit a different group of fluorophores assigned as AGEs. On the other hand, the inhibition of the fluorescence associated with protein oxidation markers such as Di-Tyr and Kyn was also assessed. Fig. 1 shows an increase in the fluorescence inhibition attributed to AGEs 1, AGEs 2, Di-Tyr, and Kyn, which is dose-dependent for PM and PEA extracts. The generation of oxidative processes during the Maillard reaction has been previously reported, showing the generation of protein carbonyls and oxidated amino acids, including Di-Tyr, N-formyl Kyn, and Kyn, among others [13,42]. In this work, PEA showed a lower IC50 value than PM for the fluorescence associated with AGEs, as well as Kyn and Di-Tyr. Concomitantly, the PEA showed a content of total polyphenols and total catechins 27 and 36 % higher than PM, respectively. The catechin content in the PEA was 1.3 times higher than its content in PM, which could partly explain the higher inhibition (2.3 times) of protein glycation and oxidation observed for PEA. The rate constants of methylglyoxal scavenging mediated by epicatechin have shown synergic, antagonist, and additive effects, depending on the interaction with other compounds, including flavonoids, phenolic acids, and amino acids [43]. The fact that the total phenolic content in the PEA was 21.5 % higher than that of the PM could also explain the higher antiglycation activity found in the PEA. On the other hand, myricetin, which was detected by HPLC-Q-ToF only in the PEA, has shown IC50 values of 0.86 μg/mL for the inhibition of fluorescent AGEs in the model BSA-glucose, which was higher when compared with other flavonoids such as rutin, catechin, procyanidin B1 and procyanidin B2 (ranging from 1.41 to 4.14 μg/mL) [42].
Fig. 1.
Fluorescent AGEs, Di-Tyr, and Kyn forming inhibition percentage as induced by Chilean papaya residue extracts.
Free radical scavenging capacity and total phenolic content have been shown to correlate with antiglycation activity [13,42]. We observed significant negative correlations among TPC, EC50, and IC50 values for protein glycation and protein oxidation markers (Table 4), reinforcing that phenolic compounds are associated with AGEs inhibition and protein oxidation occurring in complex food matrices.
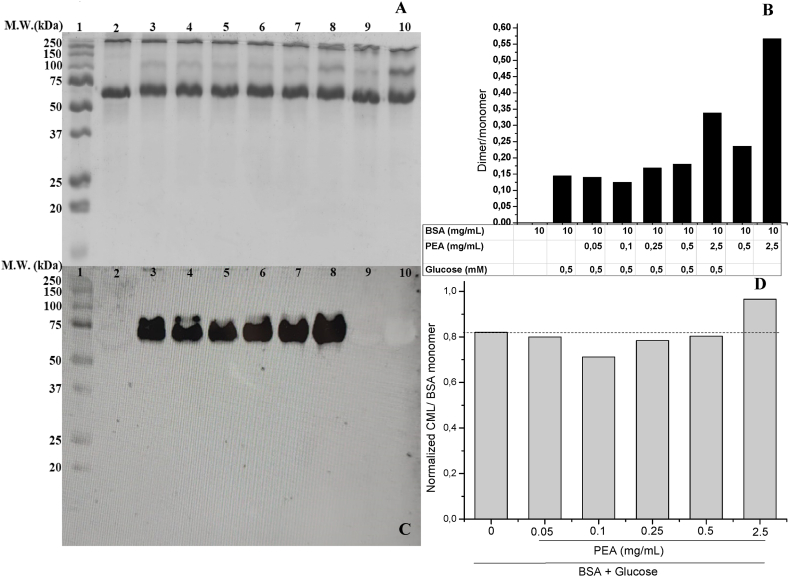
Different mechanisms, including dynamic and static quenching, can attenuate fluorescence. This fact could contribute to overestimating the inhibitory capacity of some compounds on the fluorescence of AGEs and oxidation products. To evaluate whether these effects could contribute to a decrease in the fluorescence of modified proteins, the quenching by steady-state fluorescence for PM and PEA extracts was assessed at the four pairs of excitation/emission wavelengths attributed to AGEs 1, AGEs 2, Di-Tyr, and Kyn. Table 5 shows the Stern-Volmer constants for AGEs inhibition. As an external reference, to compare with literature values, the Stern-Volmer constant (KSV) for cryptochlorogenic acid was determined, and it was found to be similar to the isomer chlorogenic acid, (6.81 ± 0.59 x 104 L/mol) [44]. When comparing KSV between PEA and AG for AGEs 1, the constant of AG was three times higher than PEA (Table 5). On the contrary, KSV for PEA was approximately 1.4, 3.4, and 2 times higher than KSV of AG for AGEs 2, Di-Tyr, and Kyn, respectively (Table 5). The fact that the quenching of the fluorescence for AGEs 1 was higher in the AG and that KSV of PEA for AGEs 2 was similar to KSV of AG suggests that the antiglycation activity of PEA towards AGEs 1 occurs by inhibition of the generation of AGEs and not as a result of fluorescence quenching. Therefore, we carried out additional experiments determining by immunochemistry the inhibition of CML mediated by PEA. Fig. 2 Panel A shows a representative SDS-PAGE analysis of BSA, BSA in the presence of glucose (BSA-Glu), BSA with different concentrations of PEA (BSA-Glu-PEA), and BSA incubated only with PEA (BSA-PEA). Fig. 2 panel B shows that the ratio dimer/monomer increases for samples BSA-Glu when compared with BSA. It was observed that when increasing PEA concentrations, the ratio of dimer/monomer tends to increase at the highest concentrations, which is consistent with the high ratio of dimer/monomer at 2.5 mg/mL for the sample BSA-PEA (Fig. 2, panel B). An increase in protein crosslinking was observed when sarcoplasmic proteins from chicken, beef, salmon, or turkey were incubated with high concentrations (1–5 mg/mL) of polyphenolic-enriched extracts from Ribes cucullatum Hook. & Arn. [Grossulariaceae] [45]. The fluorescence percentage associated with Kyn, increased for the sample BSA-PEA (Fig. S1) compared with BSA, also supporting the occurrence of oxidative processes. This increment is related to a decrease in the Trp fluorescence particularly observed at 2.5 mg/mL (Fig. S1). The dimerization of BSA in the absence of glucose could be attributed to pro-oxidant effects of the PEA at the highest concentration, which could promote free radical reactions involving Trp or Tyr residues, generating protein cross-linking [46]. Fig. 2, panel C shows the immunochemical detection of CML residues. It can be observed that no CML detection was observed for BSA or BSA-PEA samples (lanes 2, 9, and 10), indicating the absence of non-specific interactions between BSA and the anti-CML-antibody. Fig. 2 panel C shows that CML detection was present in molecular masses that can be attributed to the monomer of BSA (66 kDa). Densitometry analysis of CML levels showed that BSA-Glu-PEA samples at 0.5 mg/mL decreased the CML levels compared to the positive control (BSA-Glu). An increase in the CML levels of 18 % compared with the BSA-Glu sample was observed for the BSA-Glu-PEA sample at 2.5 mg/mL. This fact agrees with the oxidative fragmentation of the Amadori product [47], which results in CML generation (glycoxidative pathway), which agrees with the pro-oxidant effect of PEA at the highest concentration discussed above.
Table 5.
Fluorescence quenching of ethyl acetate extract of Chilean papaya residues and antiglycant reference compound aminoguanidine.
| Ksv (mL/μg) | ||||
|---|---|---|---|---|
| AGEs 1 | AGEs 2 | Di-Tyr | Kyn | |
| PEA | 1.1 x 10−3 ± 1.1 x 10−4 | 5.3 x 10−4 ± 1.1 x 10−4 | 1.0 x 10−3 ± 1.1 x 10−4 | 5.3 x 10−4 ± 1.1 x 10−4 |
| AG | 3.3 x 10−3 ± 2.4 x 10−3 | 3.7 x 10−4 ± 5.8 x 10−5 | 3.0 x 10−4 ± 1.0 x 10−4 | 2.7 x 10−4 ± 5.8 x 10−5 |
| CCGAa | 7.7 x 104 ± 5.9 x 103 | 2.9 x 103 ± 2.2 x 102 | 7.4 x 104 ± 3.3 x 103 | 9.1 x 103 ± 3.7 x 102 |
PEA: Papaya ethyl acetate extract; PM: Papaya methanol extract; AG: aminoguanidine; CCGA: Crytpochlorogenic acid.
For CCGA the KSV are expressed as L/mol.
Fig. 2.
SDS-PAGE electrophoresis and Western blot analysis of PEA.
Lane 1: Molecular weight marker; lane 2: BSA; lane 3: BSA + Glucose; lane 4: BSA + Glu + PEA 2.5 mg/mL; lane 5: BSA + Glu + PEA 0.5 mg/mL; lane 6: BSA + Glu + PEA 0.25 mg/mL; lane 7: BSA + Glu + PEA 0.1 mg/mL; lane 8: BSA + Glu + PEA 0.05 mg/mL; lane 9: BSA + PEA 0.5 mg/mL; lane 10: BSA + PEA 2.5 mg/mL. Panel A shows representative SDS-PAGE analysis under reducing conditions. Panel B shows a densitometry analysis of SDS-PAGE quantifying the ratio of the pixels corresponding to dimer (approx. 100 kDa) and monomer (50–75 kDa). Panel C shows representative western Blot analysis detecting carboxymethyl lysine (CML) modifications using a monoclonal anti-CML antibody. Panel D shows a densitometry analysis of CML levels normalized by pixels quantified from SDS-PAGE analysis considering the band attributed to the BSA monomer (50–75 kDa). The dashed line indicates the comparison with BSA + glucose controls.
According to the results, the analyzed residues of Chilean papaya formed by seed and mucilage are interesting sources of polyphenolic compounds, the content of which is crucial for bioactivity properties. Understanding their health-promoting potential and incorporating nutraceuticals into daily consumption could enhance nutritional strategies in preventing lifestyle diseases and give added value to waste from the artisanal canning industry. Subsequent studies using waste from different geographical areas could complement and compare the information obtained, establishing a possible effect of the origin of the sample, the climate, and the geographical distribution in terms of the chemical composition and bioactivity shown. Likewise, the study of seeds and mucilage separately can contribute to expanding the information obtained in this first study.
In recent years, the use of the principles of green chemistry in extraction methods has led to the research of new strategies, including new solvents to replace conventional organic solvents, as the latter are more harmful to human and animal health, and the environment and characterized by their high volatility, flammability, and toxicity [28]. As a consequence, developing green solvents for the extraction of bioactive compounds with food applications has acquired significant attention in response to the growing demand for sustainable and environmentally friendly processes [48,49]. Considering that this is the first report on the bioactivity and chemical composition of Chilean papaya residues, it is necessary to highlight that the use of solvents focused on green chemistry, including amphiphilic solvents, ionic liquids, and deep eutectic solvents, maybe a new methodological strategy as an extraction technique for minimize the toxicity generated by organic solvents in the preparation of extracts as well as reduce the environmental impact generated by the use of organic solvents.
4. Conclusions
This study utilized untargeted metabolic profiling for the first time to analyze the chemical composition of waste extracts from Chilean papaya, which were found to be mainly composed of flavan-3-ols and glycosylated flavonols. The alkaloid tetrahydroharman-3-carboxylic acid was reported for the first time in this species. This study showed for the first time that papaya residue extracts decreased the fluorescent intensity of AGEs and protein oxidation markers. The antioxidant capacity of Chilean papaya residue extracts was comparable with ascorbic acid. EC50 values from ABTS•+ and DPPH• assays strongly correlate with total phenolic content, which means that phenolic compounds contribute to the antioxidant properties of extracts. Significant correlations were found among TPC, EC50, and IC50 values for protein glycation and protein oxidation markers, supporting the inhibitory effects of phenolic composition on the generation of AGEs and protein oxidation markers.
CRediT authorship contribution statement
Liudis L. Pino-Ramos: Writing – original draft, Methodology, Investigation. Dafne Reyes Farias: Investigation. Lia Olivares-Caro: Formal analysis, Data curation. Christina Mitsi: Formal analysis, Data curation. Claudia Mardones: Supervision, Methodology. Javier Echeverria: Supervision, Data curation. Felipe Avila: Writing – review & editing, Methodology, Investigation. Margarita Gutierrez: Writing – review & editing, Supervision, Project administration, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This research was financially supported by the Chilean National Agency of Research and Development (ANID) through the project ANILLO ACT210025 (M. Gutiérrez, L.L. Pino-Ramos, and D. Reyes), FONDECYT grant 1221280 (F. Ávila), and FONDEQUIP EQM 170023 (C. Mardones and L. Olivares).
The authors also knowledge the artisans from La Pesca, Iloca for providing the papaya residues and technical personal of Laboratorio de Nutrición y Bromatología, Universidad de Talca. Christina Mitsi was supported by the Scholarship Program of the Agencia Nacional de Investigación y Desarrollo de Chile (ANID Doctorado Nacional 2022/21220376).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e38837.
Contributor Information
Felipe Avila, Email: favilac@utalca.cl.
Margarita Gutierrez, Email: mgutierrez@utalca.cl.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Dotto J.M., Abihudi S.A. Nutraceutical value of Carica papaya: a review. Sci Afr. 2021;13 doi: 10.1016/j.sciaf.2021.e00933. [DOI] [Google Scholar]
- 2.Balbontín C., Gaete-Eastman C., Vergara M., Herrera R., Moya-León M.A. Treatment with 1-MCP and the role of ethylene in aroma development of mountain papaya fruit. Postharvest Biol. Technol. 2007;43:67–77. doi: 10.1016/j.postharvbio.2006.08.005. [DOI] [Google Scholar]
- 3.Withopf B., Richling E., Roscher R., Schwab W., Schreier P. Sensitive and selective screening for 6‘- O -malonylated glucoconjugates in plants. J. Agric. Food Chem. 1997;45:907–911. doi: 10.1021/jf960578l. [DOI] [Google Scholar]
- 4.Idstein H., Keller T., Schreier P. Volatile constituents of mountain papaya (Carica candamarcensis, syn. C. pubescens Lenne et Koch) fruit. J. Agric. Food Chem. 1985;33:663–666. [Google Scholar]
- 5.Briones-Labarca V., Plaza-Morales M., Giovagnoli-Vicuña C., Jamett F. High hydrostatic pressure and ultrasound extractions of antioxidant compounds, sulforaphane and fatty acids from Chilean papaya (Vasconcellea pubescens) seeds: effects of extraction conditions and methods. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2015;60:525–534. doi: 10.1016/j.lwt.2014.07.057. [DOI] [Google Scholar]
- 6.Simirgiotis M.J., Caligari P.D.S., Schmeda-Hirschmann G. Identification of phenolic compounds from the fruits of the mountain papaya Vasconcellea pubescens A. DC. grown in Chile by liquid chromatography–UV detection–mass spectrometry. Food Chem. 2009;115:775–784. doi: 10.1016/j.foodchem.2008.12.071. [DOI] [Google Scholar]
- 7.Vega-Gálvez A., Poblete J., Quispe-Fuentes I., Uribe E., Bilbao-Sainz C., Pastén A. Chemical and bioactive characterization of papaya (Vasconcellea pubescens) under different drying technologies: evaluation of antioxidant and antidiabetic potential. J. Food Meas. Char. 2019;13:1980–1990. doi: 10.1007/s11694-019-00117-4. [DOI] [Google Scholar]
- 8.Uribe E., Delgadillo A., Giovagnoli-Vicuña C., Quispe-Fuentes I., Zura-Bravo L. Extraction techniques for bioactive compounds and antioxidant capacity determination of Chilean papaya (Vasconcellea pubescens) fruit. J. Chem. 2015:1–8. doi: 10.1155/2015/347532. (2015) [DOI] [Google Scholar]
- 9.Vlassara H., Cai W., Tripp E., Pyzik R., Yee K., Goldberg L., Tansman L., Chen X., Mani V., Fayad Z.A., Nadkarni G.N., Striker G.E., He J.C., Uribarri J. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: a randomised controlled trial. Diabetologia. 2016;59:2181–2192. doi: 10.1007/s00125-016-4053-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anis M.A., Sreerama Y.N. Inhibition of protein glycoxidation and advanced glycation end-product formation by barnyard millet (Echinochloa frumentacea) phenolics. Food Chem. 2020;315 doi: 10.1016/j.foodchem.2020.126265. [DOI] [PubMed] [Google Scholar]
- 11.Baye E., Kiriakova V., Uribarri J., Moran L.J., de Courten B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: meta-analysis of randomised controlled trials. Sci. Rep. 2017;7:2266. doi: 10.1038/s41598-017-02268-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ávila F., Ravello N., Manriquez C., Jiménez-Aspee F., Schmeda-Hirschmann G., Theoduloz C. Antiglycating effect of phenolics from the Chilean currant Ribes cucullatum under thermal treatment. Antioxidants. 2021;10:665. doi: 10.3390/antiox10050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ávila F., Cruz N., Alarcon-Espósito J., Nina N., Paillan H., Márquez K., Fuentealba D., Burgos-Edwards A., Theoduloz C., Vejar-Vivar C., Schmeda-Hirschmann G. Inhibition of advanced glycation end products and protein oxidation by leaf extracts and phenolics from Chilean bean landraces. J. Funct.Foods. 2022;98 doi: 10.1016/j.jff.2022.105270. [DOI] [Google Scholar]
- 14.Anwar S., Khan S., Almatroudi A., Khan A.A., Alsahli M.A., Almatroodi S.A., Rahmani A.H. A review on mechanism of inhibition of advanced glycation end products formation by plant derived polyphenolic compounds. Mol. Biol. Rep. 2021;48:787–805. doi: 10.1007/s11033-020-06084-0. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes Y., Giovagnoli-Vicuña C., Faúndez M., Giordano A. Microencapsulation of Chilean papaya waste extract and its impact on physicochemical and bioactive properties. Antioxidants. 2023;12:1900. doi: 10.3390/antiox12101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cakmak H., Ilyasoglu-Buyukkestelli H., Sogut E., Ozyurt V.H., Gumus-Bonacina C.E., Simsek S. A review on recent advances of plant mucilages and their applications in food industry: extraction, functional properties and health benefits. Food Hydrocolloids for Health. 2023;3 doi: 10.1016/j.fhfh.2023.100131. [DOI] [Google Scholar]
- 17.Gopčević K., Grujić S., Arsenijević J., Karadžić I., Izrael-Živković L., Maksimović Z. Phytochemical properties of satureja kitaibelii, potential natural antioxidants: a new insight. Plant Foods Hum. Nutr. 2019;74:179–184. doi: 10.1007/s11130-019-0716-3. [DOI] [PubMed] [Google Scholar]
- 18.Razali N., Mat-Junit S., Abdul-Muthalib A.F., Subramaniam S., Abdul-Aziz A. Effects of various solvents on the extraction of antioxidant phenolics from the leaves, seeds, veins and skins of Tamarindus indica L. Food Chem. 2012;131:441–448. doi: 10.1016/j.foodchem.2011.09.001. [DOI] [Google Scholar]
- 19.Wang M., Carver J.J., V Phelan V., Sanchez L.M., Garg N., Peng Y., Nguyen D.D., Watrous J., Kapono C.A., Luzzatto-Knaan T., Porto C., Bouslimani A., V Melnik A., Meehan M.J., Liu W.-T., Crüsemann M., Boudreau P.D., Esquenazi E., Sandoval-Calderón M., Kersten R.D., Pace L.A., Quinn R.A., Duncan K.R., Hsu C.-C., Floros D.J., Gavilan R.G., Kleigrewe K., Northen T., Dutton R.J., Parrot D., Carlson E.E., Aigle B., Michelsen C.F., Jelsbak L., Sohlenkamp C., Pevzner P., Edlund A., McLean J., Piel J., Murphy B.T., Gerwick L., Liaw C.-C., Yang Y.-L., Humpf H.-U., Maansson M., Keyzers R.A., Sims A.C., Johnson A.R., Sidebottom A.M., Sedio B.E., Klitgaard A., Larson C.B., Boya P C.A., Torres-Mendoza D., Gonzalez D.J., Silva D.B., Marques L.M., Demarque D.P., Pociute E., O'Neill E.C., Briand E., Helfrich E.J.N., Granatosky E.A., Glukhov E., Ryffel F., Houson H., Mohimani H., Kharbush J.J., Zeng Y., Vorholt J.A., Kurita K.L., Charusanti P., McPhail K.L., Nielsen K.F., Vuong L., Elfeki M., Traxler M.F., Engene N., Koyama N., Vining O.B., Baric R., Silva R.R., Mascuch S.J., Tomasi S., Jenkins S., Macherla V., Hoffman T., Agarwal V., Williams P.G., Dai J., Neupane R., Gurr J., Rodríguez A.M.C., Lamsa A., Zhang C., Dorrestein K., Duggan B.M., Almaliti J., Allard P.-M., Phapale P., Nothias L.-F., Alexandrov T., Litaudon M., Wolfender J.-L., Kyle J.E., Metz T.O., Peryea T., Nguyen D.-T., VanLeer D., Shinn P., Jadhav A., Müller R., Waters K.M., Shi W., Liu X., Zhang L., Knight R., Jensen P.R., Palsson B.Ø., Pogliano K., Linington R.G., Gutiérrez M., Lopes N.P., Gerwick W.H., Moore B.S., Dorrestein P.C., Bandeira N. Sharing and community curation of mass spectrometry data with global natural products social molecular networking. Nat. Biotechnol. 2016;34:828–837. doi: 10.1038/nbt.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 1995;28:25–30. doi: 10.1016/S0023-6438(95)80008-5. [DOI] [Google Scholar]
- 21.Polo-Cuadrado E., Rojas-Peña C., Acosta-Quiroga K., Camargo-Ayala L., Brito I., Cisterna J., Moncada F., Trilleras J., Rodríguez-Núñez Y.A., Gutierrez M. Design, synthesis, theoretical study, antioxidant, and anticholinesterase activities of new pyrazolo-fused phenanthrolines. RSC Adv. 2022;12:33032–33048. doi: 10.1039/D2RA05532E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Re R., Pellegrini N., Proteggente A., Pannala A., Yang M., Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Fraga C.G., Croft K.D., Kennedy D.O., Tomás-Barberán F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019;10:514–528. doi: 10.1039/C8FO01997E. [DOI] [PubMed] [Google Scholar]
- 24.Gonçalves Rodrigues L.G., Mazzutti S., Vitali L., Micke G.A., Ferreira S.R.S. Recovery of bioactive phenolic compounds from papaya seeds agroindustrial residue using subcritical water extraction. Biocatal. Agric. Biotechnol. 2019;22 doi: 10.1016/j.bcab.2019.101367. [DOI] [Google Scholar]
- 25.Irakli M., Chatzopoulou P., Ekateriniadou L. Optimization of ultrasound-assisted extraction of phenolic compounds: oleuropein, phenolic acids, phenolic alcohols and flavonoids from olive leaves and evaluation of its antioxidant activities. Ind. Crops Prod. 2018;124:382–388. doi: 10.1016/j.indcrop.2018.07.070. [DOI] [Google Scholar]
- 26.Asghar N., Naqvi S.A.R., Hussain Z., Rasool N., Khan Z.A., Shahzad S.A., Sherazi T.A., Janjua M.R.S.A., Nagra S.A., Zia-Ul-Haq M., Jaafar H.Z. Compositional difference in antioxidant and antibacterial activity of all parts of the Carica papaya using different solvents. Chem. Cent. J. 2016;10:5. doi: 10.1186/s13065-016-0149-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignat I., Volf I., Popa V.I. A critical review of methods for characterisation of polyphenolic compounds in fruits and vegetables. Food Chem. 2011;126:1821–1835. doi: 10.1016/j.foodchem.2010.12.026. [DOI] [PubMed] [Google Scholar]
- 28.Cañadas R., González-Miquel M., González E.J., Díaz I., Rodríguez M. Overview of neoteric solvents as extractants in food industry: a focus on phenolic compounds separation from liquid streams. Food Res. Int. 2020;136 doi: 10.1016/j.foodres.2020.109558. [DOI] [PubMed] [Google Scholar]
- 29.Vega-Gálvez A., Miranda M., Vergara J., Uribe E., Puente L., Martínez E.A. Nutrition facts and functional potential of quinoa (Chenopodium quinoa willd.), an ancient Andean grain: a review. J. Sci. Food Agric. 2010;90 doi: 10.1002/jsfa.4158. [DOI] [PubMed] [Google Scholar]
- 30.Agada R., Usman W.A., Shehu S., Thagariki D. In vitro and in vivo inhibitory effects of Carica papaya seed on α-amylase and α-glucosidase enzymes. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alfarabi M., Siagian F.E., Cing J.M., Suryowati T., Turhadi T., Suyono M.S., Febriyanti M.S., Naibaho F.B. Bioactivity and metabolite profile of papaya (Carica papaya) seed extract. Biodiversitas. 2022;23 doi: 10.13057/biodiv/d230926. [DOI] [Google Scholar]
- 32.Madinah N., Nozmo M., Ezekiel I. The protective effects of aqueous extract of Carica papaya seeds in paracetamol induced nephrotoxicity in male wistar rats. Afr. Health Sci. 2015;15:598. doi: 10.4314/ahs.v15i2.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sharma A., Bachheti A., Sharma P., Bachheti R.K., Husen A. Phytochemistry, pharmacological activities, nanoparticle fabrication, commercial products and waste utilization of Carica papaya L.: a comprehensive review. Curr Res Biotechnol. 2020;2:145–160. doi: 10.1016/j.crbiot.2020.11.001. [DOI] [Google Scholar]
- 34.Uribe E., Vega-Gálvez A., Pasten A., Cantuarias C., Stucken K., García V., Rodríguez A., Valenzuela-Barra G., Delporte C. Effect of high- and low-temperature drying methods on fatty acid profile and antimicrobial and anti-inflammatory traits of papaya (Vasconcellea pubescens) ACS Food Science & Technology. 2023;3:77–84. doi: 10.1021/acsfoodscitech.2c00288. [DOI] [Google Scholar]
- 35.Gaikwad S., Kamble D., Lokhande P. Iodine-catalyzed chemoselective dehydrogenation and aromatization of tetrahydro-β-carbolines: a short synthesis of kumujian-C, eudistomin-U, norharmane, harmane harmalan and isoeudistomine-M. Tetrahedron Lett. 2018;59:2387–2392. doi: 10.1016/j.tetlet.2018.04.043. [DOI] [Google Scholar]
- 36.Kim D.-C., Quang T.H., Yoon C.-S., Ngan N.T.T., Lim S.-I., Lee S.-Y., Kim Y.-C., Oh H. Anti-neuroinflammatory activities of indole alkaloids from kanjang (Korean fermented soy source) in lipopolysaccharide-induced BV2 microglial cells. Food Chem. 2016;213:69–75. doi: 10.1016/j.foodchem.2016.06.068. [DOI] [PubMed] [Google Scholar]
- 37.Herraiz T., Galisteo J. Tetrahydro-β-carboline alkaloids occur in fruits and fruit juices. Activity as antioxidants and radical scavengers. J. Agric. Food Chem. 2003;51:7156–7161. doi: 10.1021/jf030324h. [DOI] [PubMed] [Google Scholar]
- 38.Floegel A., Kim D.-O., Chung S.-J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24:1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- 39.Xiao Z., He L., Hou X., Wei J., Ma X., Gao Z., Yuan Y., Xiao J., Li P., Yue T. Relationships between structure and antioxidant capacity and activity of glycosylated flavonols. Foods. 2021;10:849. doi: 10.3390/foods10040849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen M., Yu S. Characterization of lipophilized monomeric and oligomeric grape seed flavan-3-ol derivatives. J. Agric. Food Chem. 2017;65:8875–8883. doi: 10.1021/acs.jafc.7b03530. [DOI] [PubMed] [Google Scholar]
- 41.Maisarah A.M., Amira N.B., Asmah R., Fauziah O. Antioxidant analysis of different parts of Carica papaya. Int. Food Res. J. 2013;20:1043–1048. https://www.proquest.com/scholarly-journals/antioxidant-analysis-different-parts-carica/docview/1426249736/se-2?accountid=14675 [Google Scholar]
- 42.Harris C., Beaulieu L.-P., Fraser M.-H., McIntyre K., Owen P., Martineau L., Cuerrier A., Johns T., Haddad P., Bennett S., Arnason J. Inhibition of advanced glycation end product formation by medicinal plant extracts correlates with phenolic metabolites and antioxidant activity. Planta Med. 2011;77:196–204. doi: 10.1055/s-0030-1250161. [DOI] [PubMed] [Google Scholar]
- 43.Cömert E.D., Gökmen V. Interactions of epicatechin and cysteine with certain other dicarbonyl scavengers during their reaction with methylglyoxal under simulated physiological conditions. Food Chem. 2022;369 doi: 10.1016/j.foodchem.2021.130884. [DOI] [PubMed] [Google Scholar]
- 44.Yu L., Wang J., Zhang N., Yang Y., Guo C., Li M. Inhibition of fluorescent advanced glycation end-products by ferulic acid and chlorogenic acid: a fluorescence spectroscopy and molecular docking study. Food Biosci. 2024;58 doi: 10.1016/j.fbio.2024.103790. [DOI] [Google Scholar]
- 45.Ávila F., Ravello N., Manriquez C., Jiménez-Aspee F., Schmeda-Hirschmann G., Theoduloz C. Antiglycating effect of phenolics from the Chilean currant Ribes cucullatum under thermal treatment. Antioxidants. 2021;10:665. doi: 10.3390/antiox10050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carroll L., Pattison D.I., Davies J.B., Anderson R.F., Lopez-Alarcon C., Davies M.J. Formation and detection of oxidant-generated tryptophan dimers in peptides and proteins. Free Radic. Biol. Med. 2017;113:132–142. doi: 10.1016/j.freeradbiomed.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 47.Lyons T.J., Jenkins A.J. Glycation, oxidation, and lipoxidation in the development of the complications of diabetes: a carbonyl stress hypothesis. Diabetes Rev. 1997;5:365. [PMC free article] [PubMed] [Google Scholar]
- 48.Chen J., Li Y., Wang X., Liu W. Application of deep eutectic solvents in food analysis: a review. Molecules. 2019;24:4594. doi: 10.3390/molecules24244594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nayak N., Bhujle R.R., Nanje-Gowda N.A., Chakraborty S., Siliveru K., Subbiah J., Brennan C. Advances in the novel and green-assisted techniques for extraction of bioactive compounds from millets: a comprehensive review. Heliyon. 2024;10 doi: 10.1016/j.heliyon.2024.e30921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.