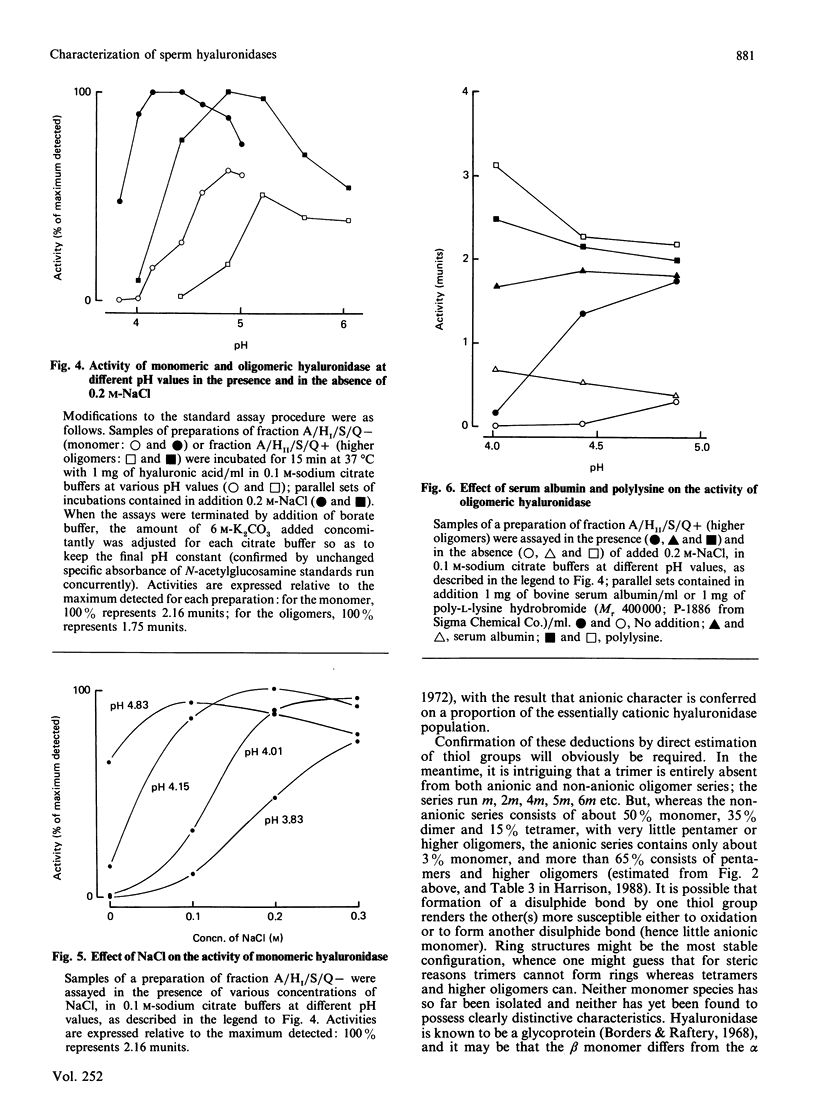
Abstract
An investigation was made of the inter-relationships and characteristics of various hyaluronidase forms isolated from ram spermatozoa. They were shown to be members of an oligomeric series, apparently formed by intermolecular disulphide cross-linking. Two monomer species were detected, alpha (Mr 89,600) and beta (Mr 81,200). Although the alpha species predominated, the two were evenly distributed throughout the oligomer population, and they shared antigenic determinants; the beta species did not arise from the alpha species as a result of catabolism following cell disruption. The oligomeric series was of the form [Hyal]n, where n = 1, 2, 4, 5, 6, 7 etc.; no trimer was detectable. Though essentially cationic, part of the hyaluronidase population also had anionic characteristics, probably due to oxidation of free thiol groups. In the anionic subpopulation tetramers and higher oligomers predominated, whereas the non-anionic subpopulation was composed of monomers, dimers and tetramers. The pH optimum of the monomer was 4.3 in 0.2 M-NaCl/0.1 M-sodium citrate, whereas that of the anionic oligomers was 4.9. Both serum albumin and polylysine stimulated enzyme activity at pH 4.0 in the absence of NaCl; polylysine was particularly effective. NaCl diminished the stimulatory effects, and essentially suppressed them above the pH optimum. The specific activities of different oligomer populations were the same as that of the monomer, and conversion of oligomers into monomer by reduction had likewise no effect upon the specific activity. Low concentrations of poly(vinyl alcohol), poly(ethylene glycol) or polyvinylpyrrolidone stabilized soluble hyaluronidase activity by preventing the enzyme's binding to surfaces; solutions of anionic oligomers were further stabilized by NaCl. Enzyme preparations were stable for several months frozen in the presence of poly(vinyl alcohol) and salt.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borders C. L., Jr, Raftery M. A. Purification and partial characterization of testicular hyaluronidase. J Biol Chem. 1968 Jul 10;243(13):3756–3762. [PubMed] [Google Scholar]
- Doak G. A., Zahler W. L. Stimulation of bull sperm hyaluronidase by polycations. Biochim Biophys Acta. 1979 Oct 11;570(2):303–310. doi: 10.1016/0005-2744(79)90150-5. [DOI] [PubMed] [Google Scholar]
- Gacesa P., Savitsky M. J., Dodgson K. S., Olavesen A. H. A recommended procedure for the estimation of bovine testicular hyaluronidase in the presence of human serum. Anal Biochem. 1981 Nov 15;118(1):76–84. doi: 10.1016/0003-2697(81)90159-7. [DOI] [PubMed] [Google Scholar]
- Harrison R. A., Gaunt S. J. Multiple forms of ram and bull sperm hyaluronidase revealed by using monoclonal antibodies. J Reprod Fertil. 1988 Mar;82(2):777–785. doi: 10.1530/jrf.0.0820777. [DOI] [PubMed] [Google Scholar]
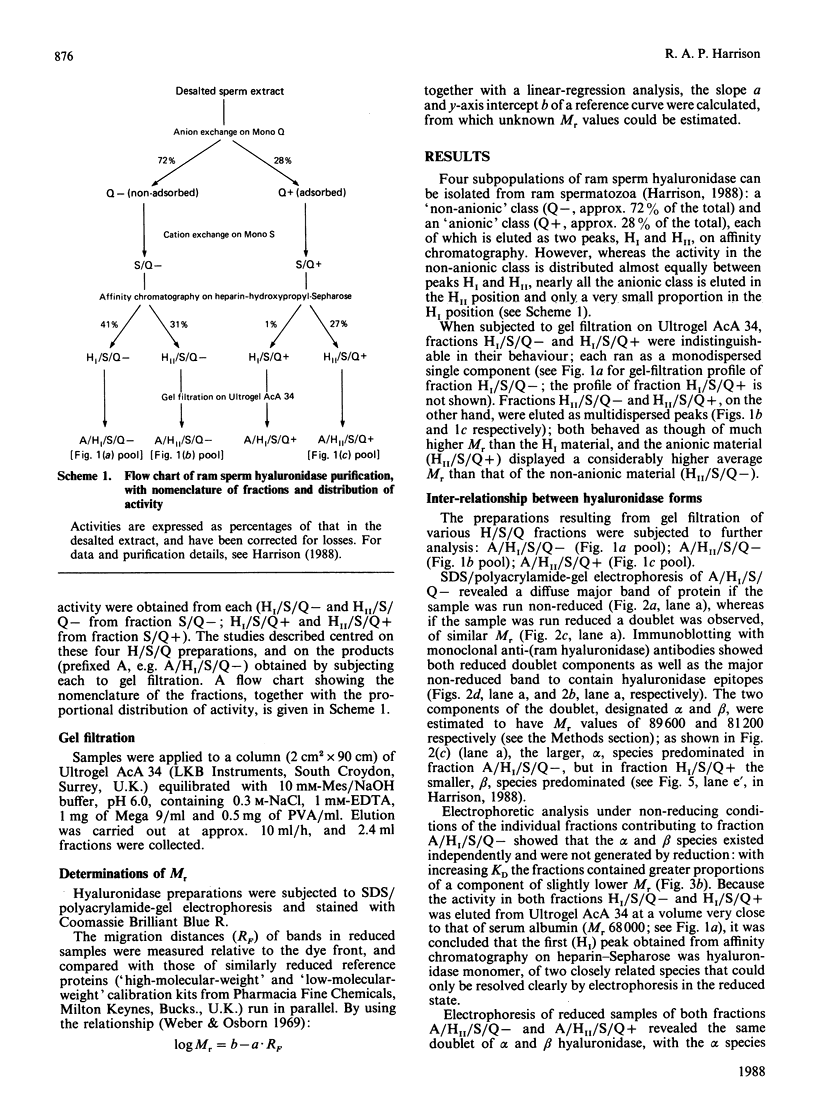
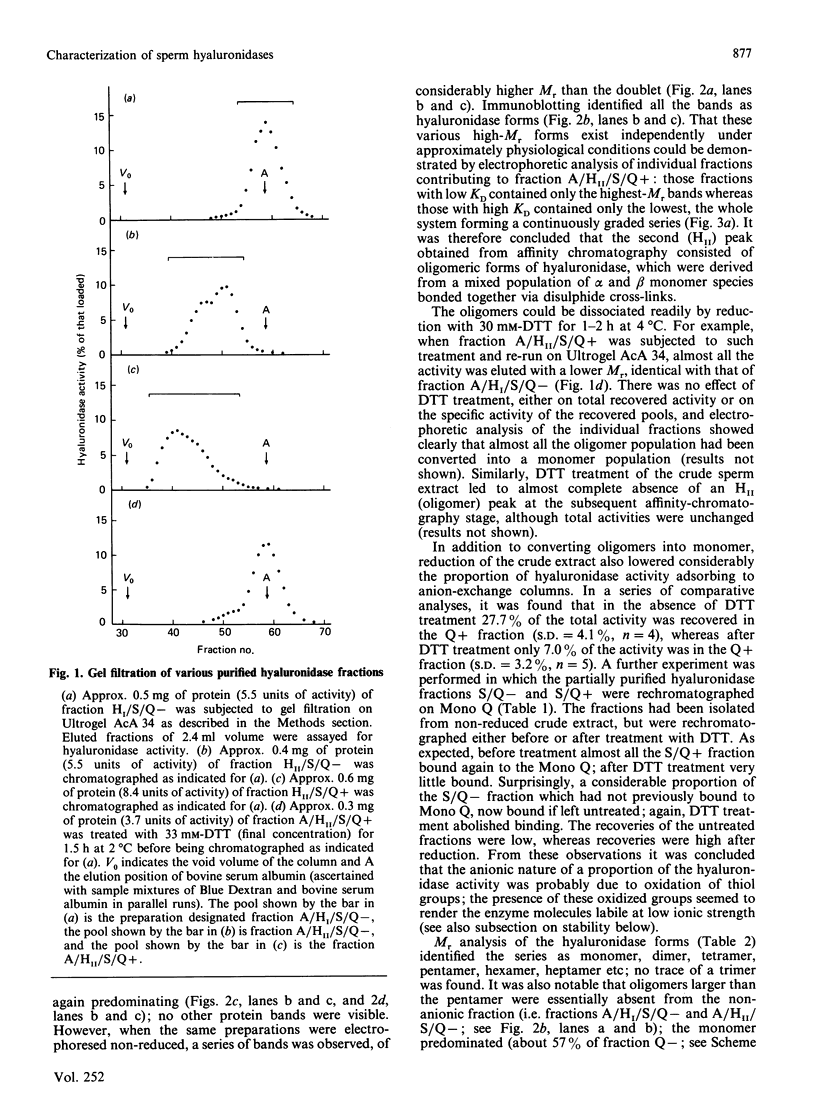
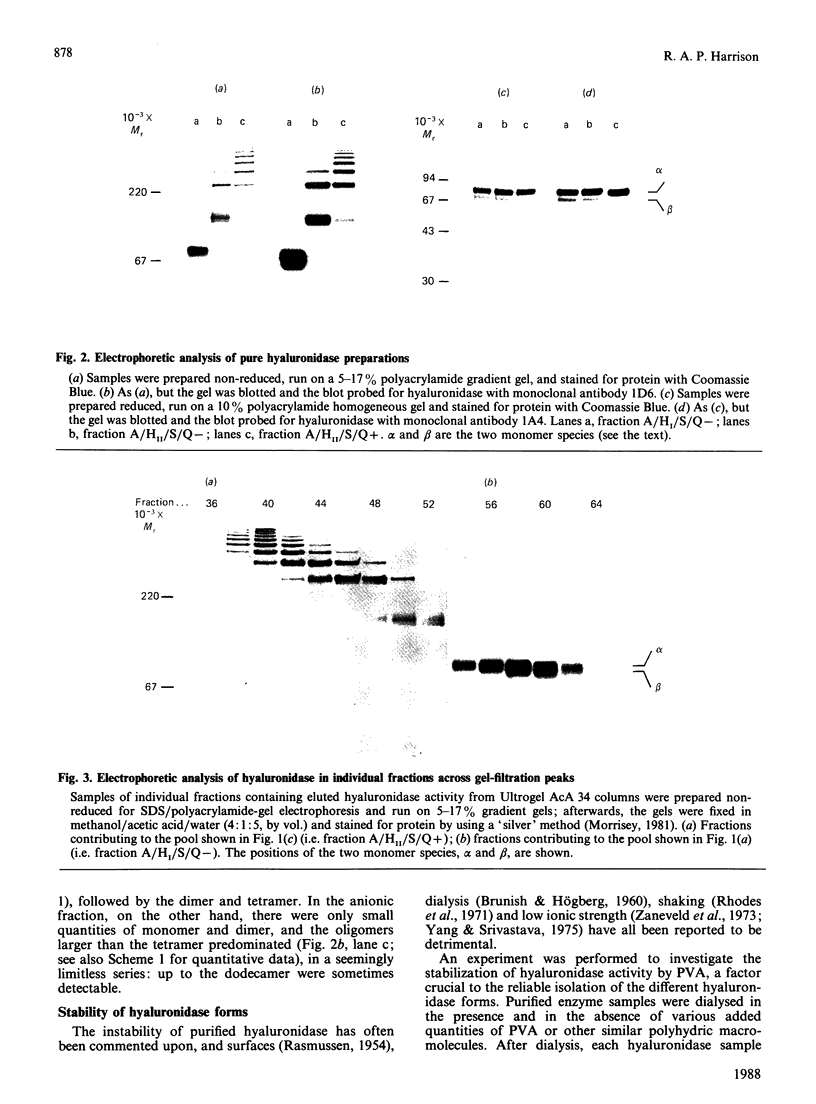
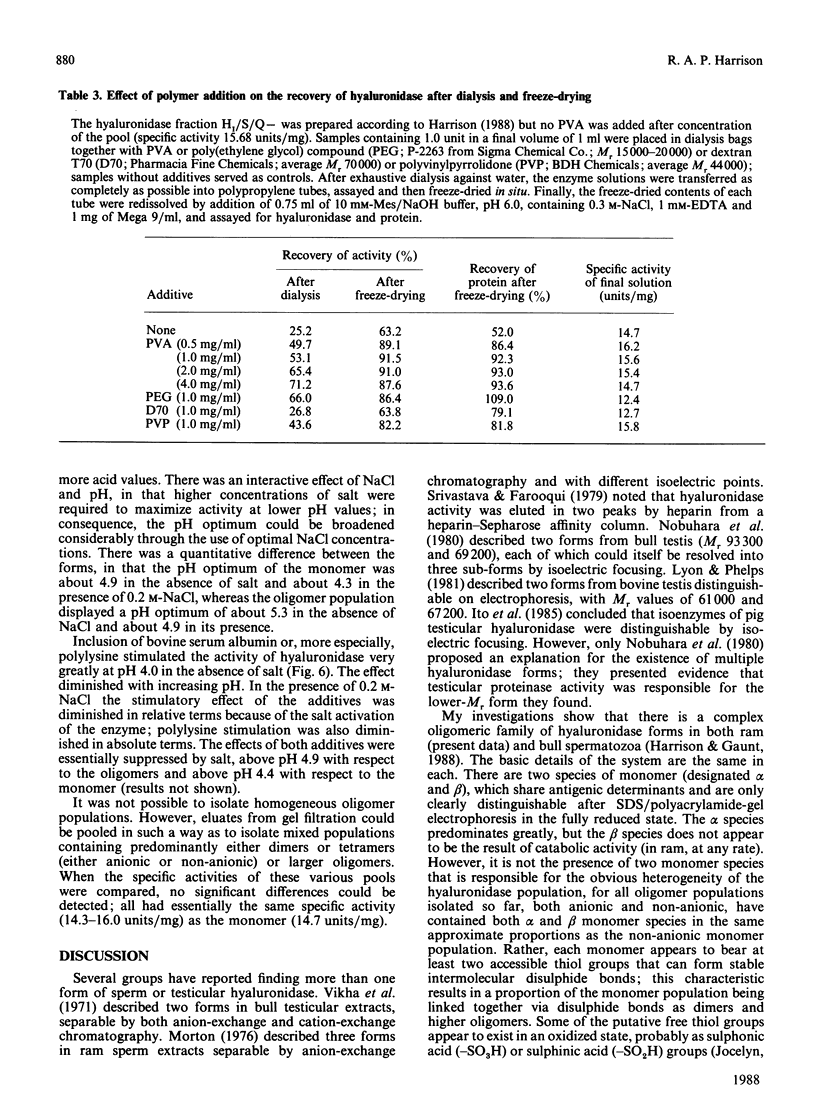
- Harrison R. A. Hyaluronidase in ram semen. Quantitative determination, and isolation of multiple forms. Biochem J. 1988 Jun 15;252(3):865–874. doi: 10.1042/bj2520865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito A., Tamaki J., Ishihara Y., Nonaka M., Mori Y. [Partial purification and characterization of hyaluronidase from pig testis]. Yakugaku Zasshi. 1985 Jan;105(1):53–58. doi: 10.1248/yakushi1947.105.1_53. [DOI] [PubMed] [Google Scholar]
- Lyon M., Phelps C. F. A rapid purification of bovine testicular hyaluronidase by chromatography on dermatan sulphate-substituted 1,6-diaminohexane--sepharose 4B. Biochem J. 1981 Nov 1;199(2):419–426. doi: 10.1042/bj1990419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrissey J. H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981 Nov 1;117(2):307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- Morton D. B. Lysosomal enzymes in mammalian spermatozoa. Front Biol. 1976;45:203–255. [PubMed] [Google Scholar]
- Nobuhara M., Sakamaki M., Takemura M., Onishi H., Suzuki Y. [The purification and the characteristics of testicular hyaluronidase (author's transl)]. Yakugaku Zasshi. 1980 Aug;100(8):832–838. doi: 10.1248/yakushi1947.100.8_832. [DOI] [PubMed] [Google Scholar]
- Pope C. E., 2nd One for the gripper? Dig Dis Sci. 1984 Jul;29(7):657–658. doi: 10.1007/BF01347299. [DOI] [PubMed] [Google Scholar]
- Rhodes C., Dodgson K. S., Olavesen A. H., Högberg B. Unsuitability of indoxyl acetate as a substrate for the assay of testicular hyaluronidase. Biochem J. 1971 May;122(4):575–582. doi: 10.1042/bj1220575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava P. N., Farooqui A. A. Heparin-sepharose affinity chromatography for purification of bull seminal-plasma hyaluronidase. Biochem J. 1979 Dec 1;183(3):531–537. doi: 10.1042/bj1830531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikha I. V., Fedurkina N. V., Khorlin A. Ia. Razdelenie testikuliarnykh gialuronidaz. Biokhimiia. 1971 Jan-Feb;36(1):216–218. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Yang C. H., Srivastava P. N. Purification and properties of hyaluronidase from bull sperm. J Biol Chem. 1975 Jan 10;250(1):79–83. [PubMed] [Google Scholar]
- Zaneveld L. J., Polakoski K. L., Schumacher G. F. Properties of acrosomal hyaluronidase from bull spermatozoa. Evidence for its similarity to testicular hyaluronidase. J Biol Chem. 1973 Jan 25;248(2):564–570. [PubMed] [Google Scholar]




