Abstract
The Fv1 gene restricts murine leukemia virus replication via an interaction with the viral capsid protein. To study this interaction, a number of mutations, including a series of N-terminal and C-terminal deletions, internal deletions, and a number of single-amino-acid substitutions, were introduced into the n and b alleles of the Fv1 gene and the effects of these changes on virus restriction were measured. A significant fraction of the Fv1 protein was not required for restriction; however, retention of an intact major homology region as well as of domains toward the N and C termini was essential. Binding specificity appeared to be a combinatorial property of a number of residues within the C-terminal portion of Fv1.
The Fv1 gene is one of a series of mouse genes, originally described in the early 1970s, that control the susceptibility of mice to MLV (14, 15). Many of the genes were found to modify target-cell proliferation or the immune response to the virus, but Fv1 acts in a cell-autonomous manner to restrict virus replication (22). The precise mechanism for restriction is unclear. It has been shown that viral replication is blocked at a stage after virus entry into the cell but before integration of newly synthesized viral DNA into the host genome (11, 20).
There are two major alleles of Fv1: Fv1n and Fv1b. These alleles are able to block specific subclasses of MLV (8). Fv1n, found in NIH Swiss mice, is able to block replication of B-tropic MLV while allowing replication of N-tropic virus, and Fv1b, found in BALB/c mice, acts vice versa. The block to infection is not absolute in vitro, but the number of infected cells is reduced by a factor of 50 to 1,000 (8). When expressed at natural levels, neither allele shows significant restriction of NB-tropic MLV. However, recent studies involving overexpression of Fv1b indicate that its gene product can interact to a certain degree with both N- and NB-tropic virus (3). By contrast, the product of the n allele does not show such secondary effects (3).
Genetic studies have shown that the target for Fv1 restriction is the MLV CA protein (9, 21). Subsequent studies suggested that viral tropism is determined by a pair of adjacent amino acids, residues 109 and 110, in CA (6, 18). A more recent study has shown that the amino acid at position 110 appears to be the most important residue for N- and B-tropism (13). N-tropic MLVs have Arg at this position, and B-tropic MLVs have Glu. The determinants for NB-tropism have not been fully characterized.
The Fv1 gene was cloned a few years ago (2) and was found to have sequence similarity (60% identity over 1.3 kb) to families of human and murine endogenous retroviruses called HERV-L or MuERV-L, respectively (1, 2). Based on its position within the Gag gene of the element, Fv1 apparently encodes a CA-like protein. Gag proteins bind tightly to each other via interaction domains during virus assembly (16), and this suggests a possible mechanism for the way Fv1 acts on MLV CA (7). To date, however, there is no evidence for direct binding of Fv1 to CA, and the extremely low level of expression of Fv1 in vivo effectively precludes direct biochemical analysis.
One feature of CA proteins is the MHR (19, 28). This domain is characterized by three absolutely conserved residues, as well as one aromatic and two hydrophobic amino acids, with exact spacing between them (Q-X3-E-X4-Φ-O-X-R-O, where Φ is the aromatic amino acid and O indicates an aliphatic amino acid) (1). It is the only region of significant sequence homology between CA proteins of different retroviral genera (28). An MHR motif is present in all replication-competent retroviruses analyzed, except spumaviruses (25), and can also be identified in the Fv1 ORF product (1). The biological significance of the MHR is unknown, but its clear evolutionary conservation must presumably reflect an important function. Mutational analyses of the MHR of HIV-1 (17), RSV (5), and Mason-Pfizer Monkey virus (25) have shown that specific residues are required for particle assembly, maturation, and proper function of the viral core in the early stages of infection. The role of the MHR of Fv1 has not been determined.
To identify the regions of Fv1 necessary for activity and the determinants of restriction specificity, we have taken a genetic approach. We created several different types of Fv1 mutants by site-directed mutagenesis, including both N-and C-terminal deletions, internal deletions throughout the coding region, and point mutations in the putative MHR domain of Fv1 and at residues 358 and 399. Using a recently developed transient assay for Fv1 function (3), these mutants were then typed for restriction activity. This paper describes the activities of these mutants and the conclusions we can draw about the determinants involved in Fv1 restriction.
MATERIALS AND METHODS
Abbreviations.
The following abbreviations are used: CMV, cytomegalovirus; MLV, murine leukemia virus; CA, the MLV capsid protein (p30); EGFP, enhanced green fluorescent protein; EYFP, enhanced yellow fluorescent protein; IRES, internal ribosome entry site; MHR, major homology region; ORF, open reading frame; HIV-1, human immunodeficiency virus type 1; FACS, fluorescence-activated cell sorting; SDS, sodium dodecyl sulfide; PBS, phosphate-buffered saline; B-MLV, B-tropic MLV; N-MLV, N-tropic MLV; NB-MLV, NB-tropic MLV; RSV, Rous sarcoma virus.
Recombinant DNA.
All recombinant DNA work was done by established techniques (23). The structure of each plasmid prepared was verified by restriction mapping and/or sequencing prior to use. All DNA preparations were purified on Qiagen columns prior to transfection.
Synthesis of the CMV promoter-driven Fv1-EGFP expression plasmids pIRES2-EGFP/Fv1n and pIRES2-EGFP/Fv1b as well as of the plasmids encoding Fv1-EGFP delivery vectors pLFv1nIEG, pLFv1bIEG, and pLMMxIEG (mix-and-match constructs) have been described in detail previously (3).
Fv1 terminal deletion mutants were created by PCR from the pIRES2-EGFP/Fv1n and pIRES2-EGFP/Fv1b plasmids. N-terminal PCR primers were designed with an identical 16-nucleotide sequence containing a BglII site 5′ of a start codon, followed by 18 nucleotides of an Fv1 ORF encoding amino acids 2 to 7 (5′-CGCGAGATCTAAGATGAATTTCCCACGTGCGCTT-3′), 33 to 38 (5′-CGCGAGATCTAAGATGACTGTTAACCCATGGCGT-3′), 51 to 56 (5′-CGCGAGATCTAAGATGGATTCATCCTTTTCGAGC-3′), 62 to 67 (5′-CGCGAGATCTAAGATGGACTCTGTGTACCATACT-3′), or 93 to 98 (5′-CGCGAGATCTAAGATGAAGGAAAGGGACCAATTC-3′). C-terminal PCR primers were designed to introduce a stop codon followed by a SaII site at positions following Fv1 amino acids 410 (5′-ACCGCGGTCGACTCATCAGAGGAGGCTAAATACAAA-3′), 437 (5′-AACGCGGTCGACTCATCAAGCTGCTGTTGGCTTTAA-3′), and 440 for both n and b alleles (5′-ATCGCGGTCGACTCATCAGAGTTTTGTAGCTGC-3′ [Fv1n] and 5′-AATACGGTCGACTCATCAAGTCAAGCCAGCTGCTGT-3′ [Fv1b]), as well as amino acid 459 for Fv1b (5′-GCCGCGGTCGACTTATTAACTGTTGCTTTGATG-3′). To synthesize each Fv1 mutant, 50 ng of the template and 50 pmol of each primer of the appropriate primer pair were added to 5 U of Pfu polymerase (Stratagene) and 35 cycles of PCR, with a 1-min annealing step at 58°C and a 3-min elongation step at 72°C, were performed. The PCR products were digested with BglII and SalI and ligated into the large fragment of the BglII/SalI-digested pLFv1bIEG.
The internal deletion, MHR, and 358 or 399 Ala mutants of Fv1 were synthesized using the QuikChange site-directed mutagenesis kit (Stratagene). Primers (33 to 39 nucleotides long) with a melting temperature greater than 78°C were designed. The desired point mutation (in the case of the MHR and Ala mutants) was situated in the middle of the primer, with 16 to 19 bases of correct sequence on either side. For the internal deletion mutants, primers consisting of 18 bases on either side of the region to be deleted were synthesized as one oligomer. Primer sequences are available upon request. A total of 15 ng of pLFv1nIEG, pLFv1bIEG, or each mix-and-match mutant was used as the DNA template in a reaction with 12 cycles of an 18-min extension time at 68°C.
Cells and viruses.
Cells were cultivated in Dulbecco's modified Eagle medium containing 10% fetal calf serum and antibiotics. Viruses were generated by simultaneous CaPO4-mediated transient transfections of 293T cells with three plasmids providing vector, gag-pol and env functions (3, 24). CMV promoter-driven expression was stimulated by incubation in Dulbecco's modified Eagle medium containing 10 mM sodium butyrate for 8 to 10 h. Virus-containing supernatant was harvested, passed through a 0.45-μm-pore-size filter (Millipore), and stored at −70°C.
Fv1 transduction assay.
Fv1 assays were carried out as previously described (3). Briefly, 2 × 104 to 3 × 104 Mus dunni cells per well were seeded on a 12-well plate. Sixteen hours later, cells were transduced with an NB-tropic delivery virus carrying a wild-type or mutant allele of Fv1 in the Fv1-IRES-EGFP cassette. Approximately 56 h later, cells were split 1:12, and after a further 16 h, they were transduced with an aliquot of N-, B-, or NB-tropic tester virus encoding EYFP which yielded 35% yellow cells in control (Fv1-null) cells. Forty-eight hours after the second transduction, cells were harvested, fixed in PBS–3.5% formaldehyde, and examined for EGFP and EYFP expression by FACS analysis with a FACS Vantage apparatus (Becton Dickinson). The XF500/T filter set (Glen Spectra) was used to separate the EGFP from the EYFP fluorescence signal. The data were analyzed with the FCSPress 1.1 package (Ray Hicks, Cambridge, United Kingdom).
In every assay, Fv1n and Fv1b were tested as controls. The extremely high degree of reproducibility allowed the use of symbols to report comparisons of the number of infected cells in Fv1− and Fv1+ populations. (see Fig. 3 and 4 for examples).
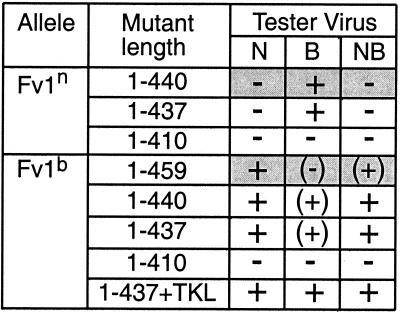
FIG. 3.
Summary of restriction by Fv1 C-terminal deletion mutants. The activity of each mutant against N-, B-, or NB-tropic tester virus is shown. The mutant length indicates at which residue the mutant terminates. The data for wild-type alleles are shaded. The extent of restriction is described by symbols, as follows: +, full restriction (equivalent to a reduction from 35% infection of Fv1− cells to 2% infection of Fv1+ cells, as shown by Fv1n restriction of B-MLV and Fv1b restriction of N-tropic MLV); (+), partial restriction; (−), slight restriction; −, no restriction.
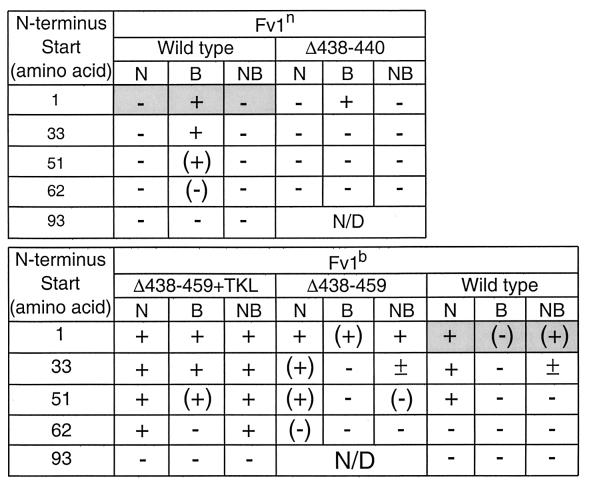
FIG. 4.
Restriction by Fv1 N-terminal deletion mutants. The activity of each mutant against N-, B-, or NB-tropic tester virus is shown. The data for wild-type alleles are shaded. The extent of restriction is indicated by symbols, as described in the legend to Fig. 3. ±, 50% restriction (half the percentage of Fv1+ cells are infected composed to the percentage infected in the Fv1− population).
Western blot analysis.
M. dunni cells were transduced with delivery virus carrying one wild-type or mutant allele of Fv1 in the Fv1-IRES-EGFP cassette, as described for the transduction assay (3), under conditions where 50 to 80% of the cells were infected, as judged by the number of green fluorescent cells 2 days after infection. Approximately 2 × 106 cells were lysed in 200 μl of lysis buffer (50 mM Tris [pH 8.0], 150 mM NaCl, 0.02% sodium azide, 0.1% SDS, 1% Nonidet P-40, 0.5% sodium deoxycholate) containing complete mini-protease inhibitor cocktail (Roche). Insoluble material was removed from the samples by centrifugation. A 100-μg portion of total protein was diluted in SDS loading buffer containing 10 mM dithiothreitol and boiled for 3 min. Samples were subjected to electrophoresis on a 12% polyacrylamide gel and transferred to an Immobilon-P membrane (Millipore) with a semidry electrotransfer apparatus (Ancos). Filters were blocked with 5% nonfat dry milk in PBS–0.1% Tween 20 and incubated for 1 h at room temperature with anti-Fv1 diluted (3:1,000) in 5% milk–PBS–0.1% Tween 20. Rabbit anti-Fv1 antiserum was prepared following repeated immunization with the gel-purified product of Fv1 expressed in Escherichia coli. After three washes in 0.5% milk–0.1% Tween 20–PBS, the filters were incubated for 1 h at room temperature in 1:1,000 solution of horseradish peroxidase-protein A (Bio-Rad) in 5% milk–PBS–0.1% Tween 20. After three washes, bound peroxidase activity was revealed with the ECL kit (Amersham Life Science) and exposed on Kodak MXB Film.
RESULTS
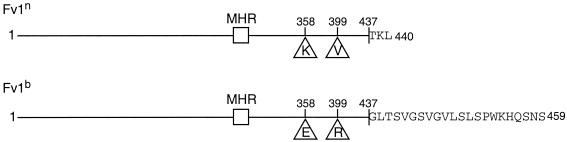
Fv1 restriction shows unique specificity for the incoming virus (3), a specificity that must result from the three differences between the predicted products of the n and b alleles of Fv1, at amino acids 358 and 399 and at the C-terminus (Fig. 1). To investigate which regions of Fv1 are necessary for restriction and what factors determine the specificity of the interaction, we have carried out a detailed genetic analysis of a series of Fv1 derivatives, measuring their ability to restrict incoming MLV.
FIG. 1.
The differences between Fv1n and Fv1b proteins. The lines are to scale and represent the Fv1 gene product. The three positions that differ between Fv1n and Fv1b, residues 358 and 399 and the C terminus, are highlighted, with the amino acids in each case indicated by their single-letter codes. Sequences for Fv1n and Fv1b can be found under GenBank accession no. X97720 and no. X97719, respectively.
C-terminal deletions.
The most striking difference between the n and b alleles of Fv1 lies at the predicted C terminus. Fv1b encodes a protein 459 amino acids long, 19 amino acids longer than the Fv1n gene product. To test the effect of these extra residues, we first deleted nucleotides in Fv1b encoding amino acids 441 to 459, thereby truncating Fv1b to the length of Fv1n. This mutant showed a marked increase in activity in the transduction assay against both B- and NB-tropic MLV, compared to full-length Fv1b (Fig. 2, compare panels C and B). This implies that the long C terminus of Fv1b is a negative factor for restriction. Next, Fv1b and Fv1n were both deleted to produce proteins terminating at residue 437. This is the last amino acid before the C terminus that is identical in both alleles. Thus, these constructs differed only at positions 358 and 399. Fv1n terminating at residue 437 had activity identical to that of wild-type Fv1n, i.e., showing full restriction of B-tropic MLV and no restriction of N- or NB-tropic MLV (Fig. 3). Fv1b truncated to residue 437 had the same activity as Fv1b terminating at residue 440, fully restricting N- and NB-MLV and showing slightly less restriction of B-MLV. Interestingly, when the three amino acids that form the C terminus of the n allele (TKL) were added to the Fv1b mutant protein that was truncated to residue 437, the previously slightly reduced restriction of B-MLV was increased to full restriction. From this it seems that restriction of B-tropic MLV is more sensitive to the specific amino acids found at the C terminus. Deleting more residues from the C terminus of Fv1, creating proteins 410 amino acids long, completely abolished restriction activity in both alleles. The differences in the restriction patterns for Fv1n and Fv1b truncated to residue 437 indicate that the amino acids at residues 358 and 399 are important and sufficient to determine specificity and that full activity can be maintained when the C terminus is deleted.
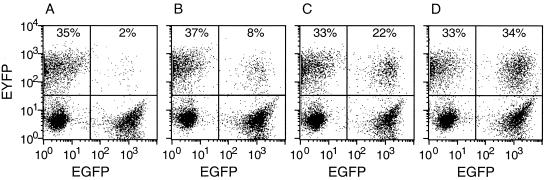
FIG. 2.
Fv1 activity of C-terminal deletion mutants. FACS profiles illustrating the effect of the C terminus on restriction of B-tropic tester virus are shown. EGFP expression is shown on the x axis; EYFP expression is shown on the y axis. The percentages given each indicate the proportion of EYFP-positive, i.e., B-MLV-infected, cells in the EGFP-negative (Fv1−) and the EGFP-positive (Fv1+) subpopulation. Wild-type Fv1n (A), mutant Fv1bΔ441–459 (B), wild-type Fv1b (C), and mutant Fv1bΔ411–459 (D) were introduced. The amount of restriction decreased from the full restriction shown in panel A to the partial restriction shown in panel B the slight restriction shown in panel C, and the lack of restriction shown in panel D. The percentages given are typical of the values for each level of restriction.
N-terminal deletions.
A series of N-terminal deletions were made to obtain five different proteins with variations in the C-terminal end: full-length Fv1n (wild-type Fv1n), Fv1n truncated to residue 437 (Fv1nΔ438-440), full-length Fv1b (wild-type Fv1b), Fv1b truncated to residue 437 (Fv1bΔ438-459), and Fv1b terminating at residue 437 with the TKL sequence then added (Fv1bΔ438-459+TKL). Using PCR, constructs were synthesized that encoded proteins that began progressively further from the wild-type N terminus, at residues 33, 51, 62, or 93. The activities of these mutant proteins in the transduction assay are summarized in Fig. 4.
The general trend for all C-terminal variants was that restriction decreased as the N terminus was deleted until complete inactivation occurred, always with removal of 92 residues, and by deletion of 61 residues for wild-type Fv1b. Inactivation occurred as soon as the first 32 residues were removed from Fv1nΔ438-440, while Fv1bΔ438-459 had some activity until 92 residues had been removed. Mutants with N-terminal deletions of more than 92 residues were all inactive (data not shown). It is clear that Fv1b is more active than Fv1n with the same ends and that Fv1b derivatives all lose activity first against B-MLV, followed by NB-MLV and finally N-MLV as the N terminus is deleted.
Restriction of B-MLV was lost as soon as the first residues of the protein were removed unless the mutant had the TKL sequence at the C terminus. In these cases, restriction was normal until 50 residues had been deleted, when the degree of restriction fell slightly. Some inhibition of B-MLV was seen when 61 residues had been removed from wild-type Fv1n, but at that point Fv1bΔ438-459+TKL did not restrict B-MLV. It seems either that the TKL sequence at the C terminus or the first 32 amino acids of the N terminus are required for restriction of B-MLV. Fv1nΔ438-440 showed the same degree of restriction of B-MLV as wild-type Fv1n, which was greater than the restriction by Fv1bΔ438-459. This confirms one or both of the two amino acid differences between the n and b gene products are important in B-MLV restriction.
Neither derivative of Fv1n showed any restriction of N- or NB-MLV, as expected. However, all variants of Fv1b restricted both these viruses, to a greater or lesser degree, with wild-type Fv1b showing slightly less restriction of NB-MLV than Fv1bΔ438-459 or Fv1bΔ438-459+TKL. Fv1bΔ438-459+TKL fully restricted NB-MLV until 92 residues were removed. Both other Fv1b allele variants showed only 50% restriction of this virus as soon as 32 amino acids were deleted. Although Fv1bΔ438-459 showed slight inhibition with a deletion of 50 residues, wild-type Fv1b with the same deletion had no activity against NB-MLV. Thus, the presence of the C terminus from the b allele seems slightly detrimental to restriction, while addition of the TKL motif greatly enhances the restriction capability of Fv1b without enabling Fv1n to restrict NB-MLV.
Interestingly, Fv1bΔ438-459 showed less restriction of N-MLV than the Fv1b wild type with 32 or 50 amino acids missing from the N terminus. This is contrary to restriction of the other two classes of virus, where the presence of the C terminus from the b allele caused the mutant protein to be less active. However, with 61 amino acids removed, wild-type Fv1b had no activity against N-MLV, whereas Fv1bΔ438-459 showed slight inhibition of the virus. The same mutation in Fv1bΔ438-459+TKL did not decrease its activity. From this it seems that Fv1b can restrict N-MLV when up to 50 N-terminal residues have been removed and retain activity when a further 11 residues are removed if the n end is present. Overall, 50 amino acids can be removed from the N terminus of Fv1 without the primary activity of either allele (restriction of B-MLV by Fv1n and N-MLV by Fv1b) being lost.
Internal deletions.
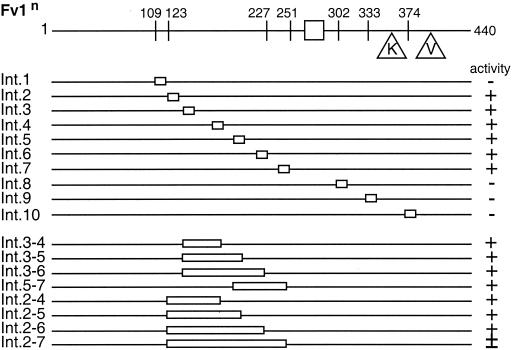
Even though only a relatively short region could be removed from either terminus of Fv1 without losing activity, we decided to create some internal deletion mutants to examine the role of the rest of the protein. For simplicity, only Fv1n mutants were synthesized. Constructs were created that encoded proteins with stretches of 10 amino acids deleted. Ten mutants were synthesized with deletions that resulted in the removal of amino acids between residues 109 and 384 (Fig. 5). Surprisingly, 6 out of the 10 mutants were fully active. The inactive mutants included those with deletions C-terminal of the MHR and the deletion of residues 109 to 118. Removing residues 123 to 132, only 5 amino acids along the protein, had no effect on restriction. In fact, none of the deletions of regions encoding amino acids between residues 123 and 250 caused loss of activity. Following these results, we decided to create a series of mutants with larger deletions in this region. In each case the construct synthesized resulted in the deletion of two sections previously deleted and the region in between them. All but the largest deletion (removal of 128 amino acids) had no effect on activity. This mutant, Int.2–7, showed only 50% restriction. However, as the regions deleted in separate mutants overlapped and covered the whole of the region encoding residues 123 to 250, it is clear that the specific residues found in this section of the protein are dispensable for activity. Further, taken together with the data from the preceding section, these results suggest the presence of two functional domains in Fv1.
FIG. 5.
Restriction of Fv1n internal deletions. The diagram at the top shows the major features of Fv1n, with the lines below representing mutants. The stretch of sequence deleted is indicated (open boxes). The name of each mutant is shown to the left of each line, and its activity against B-tropic MLV is shown to the right. Symbols indicate the extent of restriction, as follows: +, complete; ±, 50% restriction −, none.
MHR mutants.
Capsid proteins from all known replication competent viruses except spumaviruses contain an MHR (25). The function of this region is unclear, but mutation of the conserved residues causes several defects in virus replication, including blocking of assembly and release of viral particles, and production of noninfectious virions (5, 17, 25). Despite not requiring budding, maturation, or infection functions for activity, sequence comparisons with capsid have identified an MHR in Fv1 (1). This may be a remnant of Fv1's evolutionary past and have no function, or it may serve some purpose in restriction. To determine the importance of this motif for Fv1 activity, each of the invariant residues in the MHR was mutated by site-directed mutagenesis to amino acids that had caused an effect in HIV (17) or RSV (5) capsid proteins, and the activity of each mutant was measured in the transduction assay. The results are shown in Fig. 6.
FIG. 6.
Restriction of Fv1 MHR mutants. The diagram shows the MHR sequence of wild-type Fv1 with the single-amino-acid changes made shown below. The consensus sequence is written at the bottom (Φ and O represent aromatic and aliphatic amino acids, respectively). The extent of restriction of Fv1n and Fv1b with each mutation is shown on the right. +, restriction; −, no restriction; n/d, not determined.
Even the conservative changes of Gln to Asn at position 269 and Glu to Asp at position 273 resulted in loss of activity. The residue adjacent to the invariant Arg (position 282 in Fv1) is always aliphatic. Changing the Val to Leu did not reduce restriction of either Fv1n or Fv1b, except that the slight restriction of B-MLV by Fv1b was lost (data not shown). However, changing this position to Glu completely abolished all activity. Mutations in nonconserved regions of the MHR, such as positions 271 and 276, did not affect activity. Taken together, these results imply that the same structural constraints underlying MHR function in CA are also applicable to Fv1 restriction.
Protein expression.
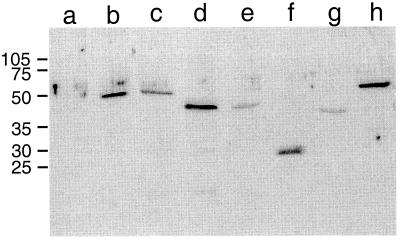
To ensure that the loss of activity seen in certain mutants was due to the effects of the specific mutation and not to a defect in protein expression or stability, Western blot analysis was carried out on cell lysates of M. dunni cells expressing each mutant. Figure 7 shows representative examples of each of the four types of mutants referred to above. For every mutant analyzed, including all the C-terminal deletion mutants, all the N-terminal deletions of Fv1n and Fv1nΔ438–440, several N-terminal deletions of Fv1b derivatives, all the inactive internal deletion mutants, A271P and V282E MHR mutants, and all the alanine specificity mutants, a protein of the correct size was identified by anti-Fv1 antibodies (Fig. 7 and data not shown). This indicates that each mutant was synthesized in M. dunni cells. We have been unable to detect wild-type Fv1 in N-3T3 or B-3T3 cells by Western blot analysis (data not shown), confirming the reported low-level expression in these cell lines (2). Thus, Fv1 is expressed at higher levels from our constructs than natural Fv1. However, even with these elevated expression levels, some of these mutant proteins were unable to restrict incoming MLV, implying that the region mutated is necessary for activity.
FIG. 7.
Detection of mutant Fv1 proteins. Shown are the results of Western blot analysis of M. dunni cells transduced with Fv1 derivatives Lanes: a, negative control; b, wild-type Fv1n; c, wild-type Fv1b; d, C-terminal deletion mutant Fv1nΔ1–410; e, C-terminal deletion mutant Fv1bΔ1–410; f, N-terminal deletion mutant Fv1nΔ1–201; g, internal deletion mutant Int.3–6; h, MHR mutant Fv1nE273D. Virus input was adjusted to result in 50 to 80% transduction levels as measured by EGFP expression. Total protein (100μg) was loaded in each lane, and Fv1 was detected with anti-Fv1 polyclonal antibody. Wild-type Fv1 and mutant Int. 3–6 are active; the remaining mutants are inactive.
Specificity mutants.
To examine the relative roles of the three differences between Fv1n and Fv1b, we had already constructed a series of mix-and-match derivatives (3). Results from these mutants as well as the Fv1n and Fv1b proteins, with and without their normal C termini, are summarized in Fig. 8A. Herein, Fv1 derivatives are described in three-letter codes depending on whether they have n or b sequences at positions 358, 399, and the C terminus (thus, e.g., the derivative nbn has the n allele sequence at 359 (Lys), the b allele Arg at 399 and the TKL of n origin at the C terminus) (Fig. 8).
FIG. 8.
Restriction of specificity mutants. Mutants are named with three letters; the first refers to position 358, the second to position 399, and the third to the C-terminus of the allele. n indicates the mutant is Fv1n-like at that position, b indicates it is Fv1b like at that position, A means that the residue has been mutated to alanine, and a dash in the third position indicates that the allele terminates at residue 437. Symbols are used to indicate the extent of restriction, as follows: +, full restriction; (+), partial restriction; (−), slight restriction; −, no restriction.
Inspection of the data shown in Fig. 8A reveals no perfect correlation between specific amino acids and Fv1 tropism. However position 358 seems to have the strongest influence on Fv1 restriction. This residue is positively charged in Fv1n (Lys) and negatively charged in Fv1b (Glu) (Fig. 1), which is opposite to the charge at residue 110 on the CA protein of the MLV primarily restricted by each protein (13). If a reciprocal charge interaction is needed for restriction, mutating residue 358 to alanine might be expected to abolish activity of both Fv1n and Fv1b. Figure 8B shows this is not the case. Mutating this residue to Ala allowed Fv1 derivatives previously unable to restrict N-MLV (all mutants with a Fv1n-derived Lys at 358) to block this virus. Changing the Fv1b-derived Glu to Ala had no effect on N-MLV restriction. It seems that a Lys at this position prevents restriction of N-MLV. All mutants with a Lys at 358 were able to restrict B-MLV. However, mutants with Glu or Ala at this position could also restrict B-MLV providing they did not terminate with the C terminus of Fv1b. Derivatives with this C terminus and Ala at 358 had a greater degree of restriction of B-MLV than the same derivative with a Glu at 358. It seems the absence of a positive charge at residue 358 is the important factor for N-MLV restriction, and the absence of the C terminus of Fv1b is important for restriction of B-MLV, unless residue 358 is Lys.
The results of an analysis of the Fv1 derivatives shown in Fig. 8A and B reveal that all variants capable of restricting NB-MLV had an Fv1b-derived Arg at position 399. Restriction was strongest when this was combined with the C terminus from Fv1n. Therefore, residue 399 was mutated to alanine for all the mutants shown in Fig. 8A and B. Figure 8C shows that only one mutant was still able to restrict NB-MLV. This mutant, bAn, showed slightly less restriction than bbn, which was able to fully restrict all three subclasses of MLV. It seems that a Glu at 399 and the TKL sequence are both necessary for full restriction of NB-MLV but that other combinations can cause partial restriction of this virus.
The third position that differs between the n and b alleles of Fv1 is the C terminus (Fig. 1). It has been shown above that the C terminus of Fv1b is a negative factor for activity against B-MLV and that full restriction can occur even when the C-terminus is deleted, but mutation of both residue 358 and residue 399 to Ala makes the effect of the C terminus even more obvious. Figure 8 shows that all three mutants were able to restrict both N- and B-MLV and unable to restrict NB-MLV. The C terminus of Fv1b seemed to prevent full restriction of B-MLV, and the C terminus of Fv1n allowed full restriction of N-MLV. Taken together, these data indicate that the ability of Fv1 to interact with CA is determined in a complex combinatorial fashion.
DISCUSSION
The mechanism of Fv1 restriction is unknown but is thought to involve a specific interaction between the MLV CA protein and the Fv1 gene product (3, 7). To probe the regions of Fv1 that are important for activity, we prepared a series of Fv1 mutants and examined their ability to restrict virus replication. These studies permit a number of conclusions. First, sequences toward the N and C termini of Fv1 are necessary for function, but perhaps surprisingly, one-third of the specific internal sequences are dispensible (amino acids 123 to 250). Second, specific sequences within the MHR are essential for function. Third, restriction specificity appears to be a property of a combination of amino acids located within the C-terminal portion of Fv1. Finally, the fact that such a wide range of mutations affect Fv1 function seems to rule out the possibility that Fv1 is acting as RNA (29); instead, they provide evidence for a direct interaction between CA and the Fv1 gene product.
If the C-terminal third of the protein is responsible for the specificity of Fv1 binding, what role does the N-terminal region play? One possibility is that the N- and C-terminal domains interact to form one binding pocket. This is consistent with the observation that the nature of the C terminus affects the function of N-terminal deletions (Fig. 4). One of the amino acids deleted in the inactive mutant Int.1 is a Cys which might form a disulphide bond with a C-terminal Cys (370 or 411). However, this is unlikely in a cytoplasmic protein, and site-directed mutagenesis of the Cys to an Ala does not affect restriction (data not shown). Alternatively, the N-terminal domain might play some other role essential for Fv1 function, such as providing a cellular localization signal.
The MHR region is highly conserved within retroviruses (1) but its functional significance remains to be fully elaborated (26). Fv1 also contains the same motif, with identical mutations preventing virus assembly or maturation and inhibiting Fv1 function, apparently lending further weight to the idea that the viral origin of Fv1 is important for function (7). However, attempts to align Fv1 and CA, using a sophisticated threading program (27), have not revealed any further similarities between the two proteins (W. Taylor, personal communication). Structural studies of a variety of CA proteins (4, 10, 12) indicate that much of the MHR forms a linker region joining separate domains of CA; our finding of two functional domains in Fv1 is consistent with a similar role for the MHR in Fv1. Resolution of this issue must await structural studies on Fv1.
When the sequences of the n and b alleles of Fv1 were first determined (2), a striking inverse relationship between the charge of amino acid 358 on Fv1 and that of amino acid 110 on restricted virus was noted, suggesting the possibility that a salt bridge might be important for restriction. This appears not to be the case. Our results with the mix-and-match mutants and the Ala substitution at 358, as well as the observation that MLV with a Trp at CA position 110 is N-tropic (13), are inconsistent with this notion. Rather, the introduction of a specific charged residue in the viral capsid will prevent restriction. This suggests a complex evolutionary interplay between virus and Fv1, with viral changes to allow replication selecting different Fv1 variants capable of restricting virus. These changes to CA can occur in multiple positions; we have preliminary evidence that alterations in at least 6 amino acids of CA can influence restriction by Fv1 (A. Stevens and M. Bock, unpublished data) and that minor alleles of Fv1 have other changes (S. Ellis and J. P. Stoye, unpublished data). It appears likely that the Fv1-CA interaction is complex, with multiple amino acids on both sides playing important roles. Again, structural information appears essential for understanding the interaction in detail.
ACKNOWLEDGMENTS
We thank Willie Taylor for helpful discussions about protein alignments, Chris Atkins for facilitating the FACS analyses, Steve Smerdon for advice about protein expression, and John Skehel and Melvyn Yap for comments on the manuscript.
This work was supported by the United Kingdom Medical Research Council.
REFERENCES
- 1.Bénit L, de Parseval N, Casella J-F, Callebaut I, Cordonnier A, Heidmann T. Cloning of a new murine endogenous retrovirus, MuERV-L, with strong similarity to the human HERV-L element and a gag coding sequence closely related to the Fv1 restriction gene. J Virol. 1997;71:5652–5657. doi: 10.1128/jvi.71.7.5652-5657.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Best S, Le Tissier P, Towers G, Stoye J P. Positional cloning of the mouse retrovirus restriction gene Fv1. Nature. 1996;382:826–829. doi: 10.1038/382826a0. [DOI] [PubMed] [Google Scholar]
- 3.Bock M, Bishop K N, Towers G, Stoye J P. Use of a transient assay for studying the genetic determinants of Fv1 restriction. J Virol. 2000;74:7422–7430. doi: 10.1128/jvi.74.16.7422-7430.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campos-Olivas R, Newman J L, Summers M F. Solution structure and dynamics of the Rous Sarcoma Virus capsid protein and comparison with capsid proteins of other retroviruses. J Mol Biol. 2000;296:633–649. doi: 10.1006/jmbi.1999.3475. [DOI] [PubMed] [Google Scholar]
- 5.Craven R C, Leure-duPree A E, Weldon J R A, Wills J W. Genetic analysis of the major homology region of the Rous sarcoma virus Gag protein. J Virol. 1995;69:4213–4227. doi: 10.1128/jvi.69.7.4213-4227.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DesGroseillers L, Jolicoeur P. Physical mapping of the Fv-1 tropism host range determinant of BALB/c murine leukemia viruses. J Virol. 1983;48:685–696. doi: 10.1128/jvi.48.3.685-696.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goff S P. Operating under a gag order: a block against incoming virus by the Fv1 gene. Cell. 1996;86:691–693. doi: 10.1016/s0092-8674(00)80141-5. [DOI] [PubMed] [Google Scholar]
- 8.Hartley J W, Rowe W P, Huebner R J. Host-range restrictions of murine leukemia viruses in mouse embryo cell cultures. J Virol. 1970;5:221–225. doi: 10.1128/jvi.5.2.221-225.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins N, Schindler J, Hynes R. Six NB-tropic leukemia viruses derived from a B-tropic virus of BALB/c have altered p30. J Virol. 1977;21:309–318. doi: 10.1128/jvi.21.1.309-318.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Z, Jin L, Peterson D L, Lawson C L. Model for lentiviral capsid core assembly based on crystal dimers of EIAV p26. J Mol Biol. 1999;286:83–93. doi: 10.1006/jmbi.1998.2443. [DOI] [PubMed] [Google Scholar]
- 11.Jolicoeur P. The Fv-1 gene of the mouse and its control of murine leukemia virus replication. Curr Top Microbiol Immunol. 1979;86:67–122. doi: 10.1007/978-3-642-67341-2_3. [DOI] [PubMed] [Google Scholar]
- 12.Khorasanizadeh S, Campos-Olivas R, Summers M F. Solution structure of the capsid protein from the human T-cell leukemia virus type-1. J Mol Biol. 1999;291:491–505. doi: 10.1006/jmbi.1999.2986. [DOI] [PubMed] [Google Scholar]
- 13.Kozak C A, Chakraborti A. Single amino acid changes in the murine leukemia virus capsid protein gene define the target for Fv1 resistance. Virology. 1996;225:300–306. doi: 10.1006/viro.1996.0604. [DOI] [PubMed] [Google Scholar]
- 14.Lilly F. Fv-2: identification and location of a second gene governing the spleen focus response to Friend leukemia virus in mice. J Natl Cancer Inst. 1970;45:163–169. [PubMed] [Google Scholar]
- 15.Lilly F, Pincus T. Genetic control of murine viral leukemogenesis. Adv Cancer Res. 1973;17:231–277. [Google Scholar]
- 16.Luban J, Alin K B, Bossolt K L, Humaran T, Goff S P. Genetic assay for multimerization of retroviral gag polyproteins. J Virol. 1992;66:5157–5160. doi: 10.1128/jvi.66.8.5157-5160.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mammano F, Öhagen A, Höglund S, Göttlinger H G. Role of the major homology region of human immunodeficiency virus type 1 in virion morphogenesis. J Virol. 1994;68:4927–4936. doi: 10.1128/jvi.68.8.4927-4936.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou C-Y, Boone L R, Koh C-K, Tennant R W, Yang W K. Nucleotide sequences of gag-pol regions that determine the Fv-1 host range property of BALB/c N-tropic and B-tropic murine leukemia viruses. J Virol. 1983;48:779–784. doi: 10.1128/jvi.48.3.779-784.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patarca R, Haseltine W A. A major retroviral core protein related to EPA and TIMP. Nature. 1985;318:390. doi: 10.1038/318390a0. [DOI] [PubMed] [Google Scholar]
- 20.Pryciak P M, Varmus H E. Fv-1 restriction and its effects on murine leukemia virus integration in vivo and in vitro. J Virol. 1992;66:5959–5966. doi: 10.1128/jvi.66.10.5959-5966.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rommelaere J, Donis-Keller H, Hopkins N. RNA sequencing provides evidence for allelism of determinants of the N-, B- or NB-tropism of murine leukemia viruses. Cell. 1979;16:43–50. doi: 10.1016/0092-8674(79)90186-7. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberg N, Jolicoeur P. Retroviral pathogenesis, P. 475–585. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. [PubMed] [Google Scholar]
- 23.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1989. [Google Scholar]
- 24.Soneoka Y, Cannon P M, Ramsdale E E, Griffiths J C, Romano G, Kingsman S M, Kingsman A J. A transient three-plasmid expression system for the production of high titer retroviral vectors. Nucleic Acids Res. 1995;23:628–633. doi: 10.1093/nar/23.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Strambio-de-Castillia C, Hunter E. Mutational analysis of the major homology region of Mason-Pfizer monkey virus by use of saturation mutagenesis. J Virol. 1992;66:7021–7032. doi: 10.1128/jvi.66.12.7021-7032.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swanstrom R, Wills J W. Synthesis, assembly, and processing of viral proteins. In: Coffin J M, Hughes S H, Varmus H E, editors. Retroviruses. Cold Spring Harbor, N.Y: Cold Spring Harbor Press; 1997. pp. 263–334. [PubMed] [Google Scholar]
- 27.Taylor W R. Dynamic sequence databank searching with templates and multiple alignments. J Mol Biol. 1998;280:375–406. doi: 10.1006/jmbi.1998.1853. [DOI] [PubMed] [Google Scholar]
- 28.Wills J W, Craven R C. Form, function, and use of retroviral gag proteins. AIDS. 1991;5:639–654. doi: 10.1097/00002030-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 29.Yang W K, Tennant R W, Rascati R J, Otten J A, Schluter B, Kiggains J O, Myer F E, Brown A. Transfer of Fv-1 locus-specific resistance to murine N-tropic and B-tropic retroviruses by cytoplasmic RNA. J Virol. 1978;27:288–299. doi: 10.1128/jvi.27.2.288-299.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]