Abstract
Purpose
This study examines how MRI distortions affect frame‐based SRS treatments and assesses the need for clinical distortion corrections.
Methods
The study included 18 patients with 80 total brain targets treated using frame‐based radiosurgery. Distortion within patients' MRIs were corrected using Cranial Distortion Correction (CDC) software, which utilizes the patient's CT to alter planning MRIs to reduce inherent intra‐cranial distortion. Distortion was evaluated by comparing the original planning target volumes (PTVORIG) to targets contoured on corrected MRIs (PTVCORR). To provide an internal control, targets were also re‐contoured on uncorrected (PTVRECON) MRIs. Additional analysis was done to assess if 1 mm expansions to PTVORIG targets would compensate for patient‐specific distortions. Changes in target volumes, DICE and JACCARD similarity coefficients, minimum PTV dose (D min), dose to 95% of the PTV (D95%), and normal tissue receiving 12 Gy (V 12Gy), 10 Gy (V 10Gy), and 5 Gy (V 5Gy) were calculated and evaluated. Student's t‐tests were used to determine if changes in PTVCORR were significantly different than intra‐contouring variability quantified by PTVRECON.
Results
PTVRECON and PTVCORR relative changes in volume were 6.19% ± 10.95% and 1.48% ± 32.92%. PTVRECON and PTVCORR similarity coefficients were 0.90 ± 0.08 and 0.73 ± 0.16 for DICE and 0.82 ± 0.12 and 0.60 ± 0.18 for JACCARD. PTVRECON and PTVCORR changes in D min were –0.88% ± 8.77% and −12.9 ± 17.3%. PTVRECON and PTVCORR changes in D95% were −0.34% ± 5.89 and −8.68% ± 13.21%. The 1 mm expanded PTVORIG targets did not entirely cover 14 of the 80 PTVCORR targets. Normal tissue changes (V 12Gy, V 10Gy, V 5Gy) calculated with PTVRECON were (−0.09% ± 7.39%, −0.38% ± 5.67%, −0.08% ± 2.04%) and PTVCORR were (−2.14% ± 7.34%, −1.42% ± 5.45%, −0.61% ± 1.93%). Except for V 10Gy, all PTVCORR changes were significantly different (p < 0.05) than PTVRECON.
Conclusion
MRIs used for SRS target delineation exhibit notable geometric distortions that may compromise optimal dosimetric accuracy. A uniform 1 mm expansion may result in geometric misses; however, the CDC algorithm provides a feasible solution for rectifying distortions, thereby enhancing treatment precision.
Keywords: distortions, MRI, SRS
1. INTRODUCTION
Stereotactic radiosurgery (SRS) employs magnetic resonance imaging (MRI) for target delineation in the treatment of brain metastases with or without computed tomography (CT). 1 While MRIs offer excellent soft tissue contrast with the capacity to evaluate imaging in multiple planes, they are also inherently susceptible to geometric distortion that may affect spatial accuracy. 2 , 3 , 4 This distortion arises in part due to inhomogeneities of the magnetic field and magnetic susceptibility of the object being imaged. The magnetic field's characterization can be incorrect, leading to unknown perturbations that can shift the targets in the MRI. 5 Some common examples include the “potato‐chip” and “bowtie” effects. Planning on an MRI without CT for dose calculation could potentially exacerbate dosimetric treatment inaccuracies. 6 Despite these challenges, MRIs remain crucial for detecting and contouring soft tissue cranial SRS targets and distortion‐induced errors persist in routine clinical practice.
Alzahrani et al. conducted an audit of 11 MR scanners used for target contouring in radiotherapy in the UK and found that the mean geometric distortions for head scanning protocols were <2 mm, but the maximum distortion was observed to be >10 mm. 7 Previous studies using polymer gel‐filled phantoms have measured the magnitudes of scanner‐specific MRI distortions to be <1 mm. 8 Distortions due to the magnetic susceptibility of the head in a 3T MRI have been generally found to be <1 mm, but up to 4 mm in some cases. 9 The cumulative effect of these distortions could lead to inaccurate target contouring and result in a geometric treatment miss, albeit in a minority of cases, but with clear clinical implications when they arise. 10
Due to the critical role of geometric accuracy in MRIs, significant efforts have been made to reduce the effects of spatial distortions. One approach is to acquire a second scan with the phase‐encoded gradient polarity reversed and averaging the scans to reduce distortion caused by non‐linear gradients and the utilization of 3D acquisition sequences. 11 Although efforts have been made to minimize the inherent distortion in MRI scanners, the residual inherent distortion combined with the patient‐specific distortion can be of the same order of magnitude as the target sizes treated in cranial SRS and are larger than typical alignment errors. 12 , 13 To address distortion and other uncertainties in SRS treatment paradigms, a uniform margin can be added to target volumes; however, this would not account for the fact that distortion tends to be non‐uniform and increase in magnitude with distance from the image center. 14 Distortions may have more significant dosimetric effects on small targets, where geometric shifts place a higher percentage of the target outside the treatment volume compared to larger targets. 15 Therefore, additional margins to reduce the risk of geometric misses in the absence of patient‐specific distortion corrections may need to be determined based on target size and location.
Given the non‐uniform nature of distortion within the image and the variable effects of distortion on different target sizes, patient‐specific distortion corrections are an attractive option for improving geometric accuracy as this would account for distortion based on each target on any given MRI. One solution would be to allow software to correct the distortion inherent to the planning MRI by adapting it to a planning CT. The Elements Cranial Distortion Correction (CDC) software (Brainlab AG, Munich, Germany) used in this study assumes the anatomy within the CT is free from geometric distortion and adjusts the MRI anatomy to create a distortion‐corrected MRI that corresponds to the CT. Previous research has demonstrated that this algorithm can reduce the maximum distortion in an MRI to approximately 1.0 mm, with 96% of the voxels having distortion < 0.5 mm. 16 In this study, the CDC algorithm was utilized to investigate the dosimetric impact of residual MRI distortions on frame‐based treatment plans for brain metastases that used a clinical stereotactic‐commissioned MRI for target delineation.
2. METHODS
To investigate the impact of MRI distortion on SRS plans, the CDC software was retrospectively applied to correct clinical planning MRs used for target delineation. The corrected MRs were used to re‐contour the targets, and the differences in dose between the original planning targets and the corrected targets were quantified. Additionally, the MRI targets were re‐contoured on the original MRs and compared with the original planning targets to evaluate the extent to which changes in corrected versus original targets were due to intra‐contouring variability.
2.1. Initial patient treatments
The study included 18 patients with a total of 80 previously treated brain metastases using frame‐based radiosurgery. Target sizes ranged from 0.01 to 12.38 cc and were located throughout different regions of the brain. All treatments consisted of a single fraction with doses ranging from 15 to 20 Gy. Prior to treatment, all patients underwent an MRI and CT scan for planning purposes. MRIs were acquired on a Siemens Symphony TIM 1.5T MR scanner using a gadolinium‐enhanced T1 fast low angle shot (FLASH) scanning protocol with 2D distortion correction applied. The FLASH sequence served as the institution's standard clinical MRI protocol for brain metastases, chosen for its historical usage and physician's familiarity with delineating targets using this MRI sequence. The axial MR images were obtained using the following scanning parameters: a field‐of‐view of 256 mm, matrix size of 256 × 256, TR of 1.1 ms, TE of 3.5 ms, bandwidth of 130 Hz/pixel, flip angle of 18.14, and a slice thickness of 1 mm. The MR scanner was commissioned for cranial SRS within the hospital and followed the AAPM and ACR recommendations for rigorous quality assurance. 17 , 18 CT images were acquired immediately after the MRI using the Radiation Oncology department's Philips Big Bore CT scanner or Radiology's Siemens Somatom Sensation 16 CT with a head scanning protocol. The CT scanning parameters included a field‐of‐view of 278 mm, axial pixel size of 1 mm, slice thickness of 1 mm, a kVp of 120 kV, and a focal spot size of 1.2 mm. The CT quality assurance of both machines followed AAPM TG‐66 guidelines.
After acquiring CT and MR images, the patient was moved to a holding area, and the images were imported into the Leksell Gamma Plan (LGP) software (Elekta AB, Stockholm, Sweden) for contouring and treatment design. CT and MRI images were co‐registered, and targets were contoured using the gadolinium‐enhanced regions of the MRI without any additional margin. The accuracy of the co‐registration and target contours was confirmed by the neurosurgeon, radiation oncologist, and medical physicist before initiating treatment. Dose prescriptions were based on target size. All patients were treated using a Gamma Knife Icon, which is composed of a hemisphere of 192 radioactive Cobalt‐60 sources that are precisely focused on a specific location within the unit, known as isocenter with a frame‐based treatment. 19 The TMR 10 algorithm was utilized to calculate dose, with a voxel size of 0.5 mm on the planning CT. Sector blocking, various collimators, and inverse and forward planning were all utilized based on the individual target to create an optimal dose plan in terms of dose, gradient, and conformity.
The planner's goal was to achieve 100% target coverage with the prescription isodose line, which was typically set at 50% of maximum dose, while minimizing radiation dose to the surrounding normal brain tissue.
2.2. Distortion investigation
To initiate the investigation of MR distortion, MR and CT planning images, MR‐CT registration, target structures, and dose DICOM files were exported from LGP to the MIM software (MIM Software Inc., Beachwood, OH). MIM was used for all structure and dose analysis in the study. Once all the LGP data were imported into MIM, MR and CT images were exported into the CDC software. An example CDC correction, along with the PTVORIG and PTVCORR on the original and corrected MRIs, is presented in Figure 1. Co‐registration of MR and CT images was performed, and the CDC algorithm was applied, resulting in a corrected MRI that was co‐registered to the CT. The CDC algorithm employs a 5‐step process to address distortion within the MRI. First, the MRI is co‐registered to the CT. Next, the MRI is subdivided into overlapping sections, and an optimal affine transformation is calculated between individual MRI sections and the CT. A global deformation vector field is computed based on these transformations. Lastly, the deformation vector field is applied to the MRI to generate a corrected MRI. Further algorithm specifics can be found in a Brainlab technical note. 20 After running the CDC software, the unmodified CT, corrected MRI, and co‐registration DICOM files were exported to MIM. Finally, the physician re‐contoured targets on both corrected and uncorrected MRIs while blinded to the original contour.
FIGURE 1.
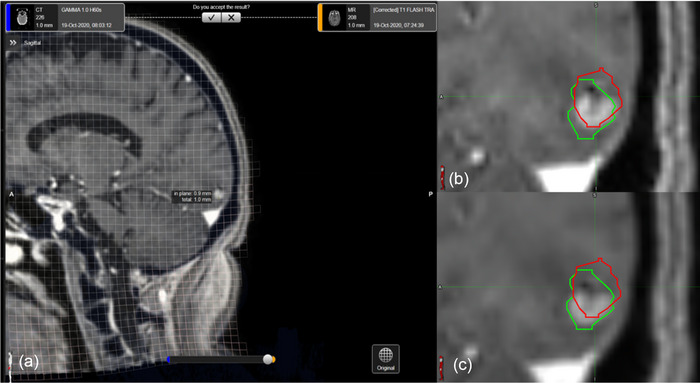
Cranial distortion correction of an MRI using the CDC algorithm. (a) shows the CDC interface displaying the distortion grid. (b) shows the original (red) and corrected (green) contours overlaid on the uncorrected MRI. (c) displays the final corrected MRI.
Data analysis of contours was broken into three parts: 1) cross‐comparison of target contours, 2) target dosimetric analysis, and 3) whole brain—target dosimetric analysis. Data analysis began with a comparison of the re‐contoured targets (PTVRECON), or corrected targets (PTVCORR) and the original planning targets (PTVORIG). All comparisons will be expressed using the following notation: , where “TARGET” represents either PTV or NT (normal tissue), “SOURCE” indicates either re‐contour (RECON) or corrected (CORR), and metric is either volume, minimum dose (D min), dose to 95% of the PTV (D95%), or volume receiving a given dose (V dose) (e.g., ). When referencing a given metric for both sources, the “SOURCE” will be listed as “RECON, CORR” (e.g., ). It is implied that for a given source represents the normalized difference relative to the corresponding PTVORIG statistic.
Cross comparison of targets using similarity coefficients will be presented as: , where “METRIC” is either DICE or JACCARD, and “SOURCE” is either RECON, CORR, or “RECON, CORR” when referring to both. Likewise, to , these RECON and CORR similarity metrics are all calculated relative to the original PTVs.
Volumes of PTVs were quantified by counting the number of voxels in each contour. Changes in number of voxels for individual PTVRECON and PTVCORR were calculated as the percentage change in number of voxels compared to PTVORIG, which was normalized to the voxels in PTVORIG. In addition to calculating changes in contour size, degree of overlap between contours was quantified by calculating DICE and JACCARD similarity coefficients between the new PTVRECON and PTVCORR contours, and the original PTVORIG. 21 , 22 Finally, to evaluate the potential benefit of adding uniform margins to the PTVORIG contours to account for distortion, a 1 mm margin was introduced and the percentage of PTVCORR and NT covered by the expanded PTVORIG were calculated as a normalized ratio of PTVORIG.
To assess dosimetric plan quality for the various targets, minimum target dose and dose covering 95% of the individual targets was recorded. Changes in target doses for PTVRECON and PTVCORR were calculated as a normalized percent change from PTVORIG.
To investigate changes in normal tissue (NT) dose, the dose delivered to the whole brain, excluding the PTV structures, was evaluated. The whole brain was contoured on the CT in MIM, and target contours were subtracted to create three NT structures, one for each set of targets (ORIG, RECON, CORR). The volume of the NT structures receiving 12 Gy (V 12Gy), 10 Gy (V 10Gy), and 5 Gy (V 5Gy), were recorded. Changes NT dose metrics for NTRECON and NTCORR were calculated as the percentage change from NTORIG, normalized to metrics in NTORIG.
For each calculation, two sets of changes or metrics were generated for the PTVRECON and PTVCORR targets. To discern whether changes in these targets, as compared to PTVORIG, were primarily attributed to variability in contouring or MRI distortion, two‐tailed Student's t‐tests were performed between corresponding sets of data. 23
3. RESULTS
3.1. Volume changes and similarity comparisons
Total PTVCORR volumes were 1.48% ± 32.92% larger, on average, than PTVORIG volumes, but there was a large standard deviation across changes in individual PTVCORR volumes. In contrast, recontoured PTVRECON volumes were 6.19% ± 10.95% smaller than PTVORIG volumes, indicating that recontouring resulted in smaller volumes, on average, compared to contouring in the original planning software. Therefore, similarity in sizes between the PTVCORR and PTVORIG volumes suggests that enhancement regions on the corrected MRIs increased relative to uncorrected MRIs to account for smaller contour sizes in MIM. Table 1 summarizes average and standard deviations of the ΔPTVs and similarity metrics, along with the p‐values from the Student's t‐test. Figure 2 presents a histogram comparison of and .
TABLE 1.
Normalized change in PTV volumes, minimum dose to PTV (ΔD min), and dose to 95% of the PTV (ΔD95%) resulting from re‐contouring (RECON) and after distortion corrections (CORR), with respect to the original (ORIG) volumes.
| RECON and CORR PTV volumes and dose relative to ORIG | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Δ PTV volume | DICE | JACCARD | Δ D min | Δ D95% | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| RECON | −6.18 | 10.95 | 0.89 | 0.08 | 0.82 | 0.12 | −0.88 | 8.77 | −0.34 | 5.88 |
| CORR | 1.48 | 32.92 | 0.73 | 0.16 | 0.60 | 0.18 | −12.90 | 17.29 | −8.68 | 13.21 |
| p‐values | p = 0.04 | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | |||||
Note: p‐values comparing RECON and CORR changes calculated using Student's t‐test.
FIGURE 2.
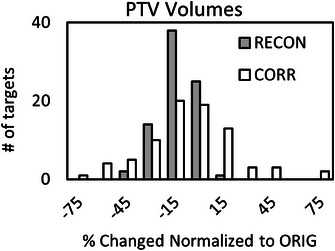
Normalized change in PTV volumes resulting from re‐contouring (RECON) and distortion corrections (CORR), with respect to the original (ORIG).
Although PTVCORR targets had similar average sizes as PTVORIG, there was a significant reduction in similarity metrics compared to PTVRECON (p < 0.01). This is consistent with the large standard deviation in , indicating that MRI distortion can cause substantial changes in individual target volumes. Moreover, the large standard deviation in with an average close to zero suggests that targets both increased and decreased in size due to distortion. Figures 3 and 4 show histograms of DICERECON, CORR and JACCARDRECON, CORR similarity metrics, respectively.
FIGURE 3.
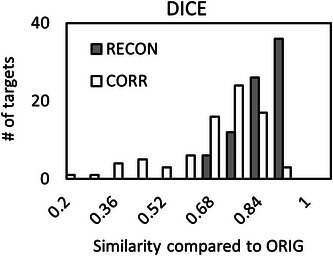
Histograms of DICE similarity coefficients between re‐contoured PTVs (RECON) and distortion‐corrected PTVs (CORR) compared to the original (ORIG) PTVs.
FIGURE 4.
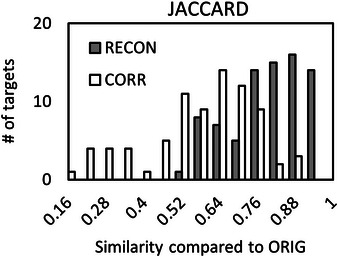
Histograms of JACCARD similarity coefficients between re‐contoured PTVs (RECON) and distortion‐corrected PTVs (CORR) compared to original (ORIG) PTVs.
3.2. Dosimetric impacts of distortion
Average reduction in (−12.9 ± 17.3%) was significantly lower than reduction in (−0.88% ± 8.77%) due to intra‐contouring variability (p < 0.01). Additionally, average reduction in (−8.68% ± 13.21%) was significantly lower than reduction in (−0.34% ± 5.89), with a p < 0.01. Figure 5 displays box plots of and . Notably, out of 88 PTVCORR targets, 14 still exhibit more than 5% of their volume that remains uncovered by PTVORIG, even with the inclusion of a 1 mm expansion. In contrast, all PTVRECON targets had 98.5% or more of their volumes covered by PTVORIG targets with a 1 mm expansion.
FIGURE 5.
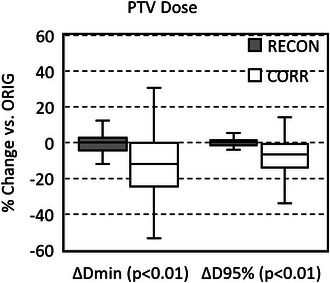
Box‐and‐whisker plots of normalized changes in minimum PTV dose (ΔD min) and dose to 95% of the PTV (ΔD95%) resulting from re‐contouring (RECON) and after distortion corrections (CORR), with respect to original (ORIG) PTVs. The x‐axis labels show p‐values from the Student's t‐test comparing RECON and CORR data.
Table 2 reports average and standard deviation ΔNTs, as well as p‐values from the Student's t‐test. Figure 6 shows box plots of , , and .
TABLE 2.
Normalized changes in the volume of normal tissue (brain‐PTVs) receiving 12 Gy (ΔV12Gy), 10 Gy (ΔV10Gy), and 5 Gy (ΔV5Gy) dose due to re‐contouring (RECON) and after distortion corrections (CORR), with respect to original (ORIG) normal tissue volumes.
| Normal tissue (brain—PTVs) dose relative to ORIG | ||||||
|---|---|---|---|---|---|---|
| ΔV12Gy | ΔV10Gy | ΔV5Gy | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| RECON | −0.09 | 7.39 | −0.38 | 5.67 | −0.08 | 2.04 |
| CORR | −2.14 | 7.34 | −1.42 | 5.45 | −0.61 | 1.93 |
| p‐values | p = 0.04 | p = 0.15 | p = 0.02 | |||
Note: p‐values comparing RECON and CORR changes calculated using Student's t‐test.
FIGURE 6.
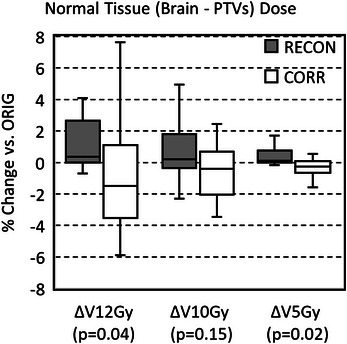
Box‐and‐whisker plots of normalized changes in the volume of normal tissue (brain—PTVs) receiving 12 Gy (ΔV12Gy), 10 Gy (ΔV10Gy), and 5 Gy (ΔV5Gy) resulting from re‐contouring (RECON) and after distortion corrections (CORR), with respect to the original (ORIG) PTVs. The x‐axis labels show p‐values from the Student's t‐test comparing RECON and CORR data.
4. DISCUSSION
The CDC software was used to correct distortions in frame‐based MRIs, and it was observed that errors encountered were significantly larger than intra‐contouring variability. However, current clinical practice relies on historical data that demonstrates favorable outcomes without such corrections. 24 , 25 , 26 In their investigation, Amaral et al. discovered that distortions exceeding 1 mm were present in 17.5% of cranial tumor studies potentially leading to higher incidence of local recurrence. Nonetheless, the entire cohort exhibited overall good rates of local control. 27 Consequently, addressing how the CDC algorithm can be effectively integrated into clinical workflows to enhance normal tissue sparing, decrease geometric misses, while preserving favorable overall outcomes will be explored in three sections. First, the characteristics of the distortions found in this study and their impact on clinical plans will be explored. Second, potential applications of the CDC software in a clinical setting will be proposed. Finally, the limitations of this study and potential avenues for future investigation will be discussed.
4.1. Impacts of distortion
To assess magnitudes of distortion relative to contouring variability within a single observer, recontouring was employed as an internal control and baseline. It is important to note that the contouring observer was blinded to initial contours, hence some changes in tumor volume were expected. Although the PTVRECON were smaller than the PTVORIG targets, and similarity coefficients were in line with interobserver variabilities previously reported in the literature for cranial SRS contouring. 28 , 29 , 30 , 31 The average reduction in size of the PTVRECON compared to the PTVORIG targets indicates that residual distortion in the original uncorrected MRIs caused an underestimation of the contrast‐enhanced regions in patients. On average, PTVCORR increased and PTVRECON decreased in size compared PTVORIG, however volumetric changes occurred in both directions as shown in Figure 2. Moreover, higher degrees of agreement observed between original and recontoured targets through JACCARD and DICE metrics support contour changes based on distortion having a more pronounced impact on target delineation compared to contouring variability.
For both PTVRECON and PTVCORR individual targets that showed an increase in volume, there was either no change or a reduction in D min. This can be attributed to increased volumes causing the exterior of the PTVs to extend further into the dose falloff region, resulting in overlap with a lower isodose line. Instances where the target volume increased, without any change in D min, were likely caused by either shifting of a target without a significant change in conformity or limitations in the significant figures and dose resolution of the software.
While applications of the CDC software led to overall average increases in target size, some targets did decrease in size. These decreasing volumes did not always exhibit the trend of increasing the minimum target dose. In fact, there were instances where target size decreased and the minimum dose either increased, decreased, or remained unchanged. This was because distortion corrections did not always result in a simple uniform contraction of target volumes. Instead, reductions in size were sometimes accompanied by a shift of the target centroid as shown in Figure 1. Such a shift could cause targets to extend further into the dose falloff region, like what was observed with increasing target sizes. Overall, the study found that both increasing and decreasing target volumes associated with CDC corrections could result in lower minimum PTV doses, but only a decrease in target volume could potentially lead to an increase in minimum PTV dose.
The impact of distortion on normal tissue would vary on a case‐by‐case basis in terms of clinical relevance; however, bidirectional changes in PTV dose were accompanied by corresponding bidirectional changes in dose to normal brain tissue. On average across all patients, volumetrics used to evaluate normal tissue (V 12Gy, V 10Gy, V 5Gy) decreased after distortion correction. The were significantly different (p < 0.05) than , while no statistically significant difference was observed for V 10Gy. As with standard deviations between changes in PTV contours, there was a large standard deviation in changes observed in normal tissues. This underscores the difficulty in predicting distortion effects on a cohort of MRI data, even when scanners and inter‐patient geometry stay relatively constant between scans.
While this study primarily focused on dosimetric evaluations of distortion within a clinical patient population, other investigations have examined this phenomenon using phantoms or MRIs with artificially induced distortions. In a recent study, Ohira et al. explored the impact of MR distortion through phantom studies involving single‐isocenter multi‐target treatments. 32 They observed that the dosimetric deviations due to distortion were less than 5% of the planned value when the target was within 50 mm of the MR image center but increased as the targets extended away from the image center. Similarly, Pappas et al. noted comparable trends of distortion increasing away from the center in their phantom studies. For smaller targets with diameters less than 2 mm, 1 mm distortions caused 5% changes in dose and as target sizes grew to diameters of 20 mm, a 1.5 mm distortion would produce a similar dosimetric effect. 14 Calvo‐Ortega et al. introduced artificial distortions into MRIs of vestibular schwannoma patients and utilized the CDC algorithm to correct these distortions. 33 Their findings indicated that the algorithm could correct patients within 0.1 ± 0.1 Gy of the undistorted targets. These studies collectively underscore the potential impact of distortion in cranial stereotactic radiosurgery treatments.
4.2. Clinical implementation of the CDC algorithm
The availability of quantifying and correcting patient‐specific MR distortion introduces a possible paradigm shift in target contouring and use of margins for radiosurgery. When considering data on distortion in conjunction with acceptable clinical outcomes, radiosurgeons have several viable courses of action. The first option is to continue common clinical practices of not adding a margin to PTVs for some frame‐based systems, while loosely conforming prescription isodose lines to targets to ensure 100% coverage. The second option is to introduce a planning margin during PTV generation to account for planning and treatment uncertainties, including distortion, while more tightly conforming dose to the contour. However, previous investigations have shown that magnitudes of distortion and the effect on each target is non‐uniform, rendering a uniform distortion‐correction margin inappropriate. 14 , 15 , 34 Despite the implementation of a 1 mm margin in this study, some tumors still exhibited distortion beyond this expansion, indicating this approach may mitigate some, but not all distortion‐related risks. Consequently, adding additional planning margins alone would not eliminate the potential for a geometric miss caused by distortion, while increasing the risk of radionecrosis by exponentially enlarging treated tissue volumes. An alternative approach would be to generate a composite target that includes both initial targets without distortion correction and distortion‐corrected targets, resulting in a likely lenticular volume of normal tissue included in treatment volumes. This approach may potentially avoid the aforementioned exponential treatment volume increase. Lastly, a target may be treated solely based on algorithmic estimation and correction of distortion, with or without the inclusion of a planning margin and its associated risks and benefits. There may be instances where a combination of these approaches is considered, rather than uniformly implementing a single approach based on target size, location, and physician comfort when presented with patient‐specific distortion correction calculations.
While the CDC software has been investigated for correcting patient‐specific distortion, system‐level MR distortion may be present in treatment planning MRIs. These system‐level distortions can stem from machine‐related issues, such as inadequate magnetic shimming and eddy current compensation. 17 , 35 Moreover, significant distortion errors may occur if correction options in the scanning protocol are unintentionally disabled, resulting in geometric errors up to 5.0 mm that may go unnoticed by certain observers. 36 System‐level distortions are more likely to occur when an SRS program utilizes MRIs from external radiology departments for planning, as scanner quality assurance in such cases tends to prioritize diagnostic contrast rather than sub‐millimeter geometric accuracy. Additionally, system‐level distortions generally exhibit larger magnitudes than patient distortions, thereby increasing the likelihood of substantial geometric errors. 5 In such instances, the utilization of the CDC software would offer the advantage of allowing a practice to use images from different MRI scanners, while preserving high‐level spatial accuracy required for SRS deliveries.
4.3. Future directions and study limitations
While CTs can be acquired for planning and stereotactic frame definition, current research is exploring generation of synthetic CTs from planning MRIs, which would eliminate the requirement for a separate CT scan for treatments. 37 However, one limitation of this technique is that CTs would inherit the geometric distortion present in source MRIs. To mitigate this effect, the CDC software can be employed to correct planning MRs using a recent diagnostic cranial CT, thereby reducing geometric uncertainty within the cranial region of synthetic CTs.
Although the CDC algorithm shows promise in improving geometric accuracy, like any distortion correction algorithm, it poses challenges when it comes to in vivo validation. Retif et al. examined the precision of the CDC algorithm in rectifying distortions in a cranial phantom and found the algorithm corrected 96% of voxels to within 0.5 mm. 16 However, the CDC algorithm is designed to use anatomy contained with the patient's CT for its correction, therefore validation in anthropomorphic phantoms may not sufficiently account for the full range of CT and MRI densities and settings within the clinical framework. Like other clinical anatomical algorithms, the acceptance of a solution requires close evaluation on a case‐by‐case basis. This holds true for all imaging fusions, whether they involve distortion, deformation corrections, or fixed‐based fusion solutions.
While the inability to validate the distortion corrections in situ represents a limitation of this study, another constraint lies in the incapacity to differentiate between distortion and registration errors between the planning CT and MR images. Although the co‐registration of the images was confirmed by a neurosurgeon, radiation oncologist, and physicist during the planning process, distortion in the initial MRIs could introduce unavoidable registration errors when attempting to co‐register targets throughout the brain, especially where geometric spacing was inconsistent between the CT and MR images. The CDC algorithm's initial step of subdividing the brain into smaller sections before deformable registration would help mitigate the effects of these global registration errors. From a therapeutic perspective, these registration errors would impact treatment whenever the CT was used for patient alignment. This CT could be employed to define the stereotactic frame, or the CT anatomy of the skull could be used to validate patient positioning through co‐registration to a cone‐beam CT acquired immediately before dose delivery on the treatment machine.
Another constraint in this study was the restricted scope of analysis, which focused solely on brain metastasis from a single institution. Various factors, including target size and location, magnetic field strength, acquisition parameters, and MR scanner‐specific distortion correction algorithms, could influence distortion magnitudes. Furthermore, the added complexities of physician variability in contouring MR‐enhancing regions and institutional variations in required dose conformality further impact distortion effects. Consequently, further validation of the CDC software across multiple institutions for various treatment indications is imperative to enhance the global significance of the findings. In addition to its application across multiple institutions to quantify overall impact of MRI distortions, the CDC software can also improve dosimetric reporting in clinical trials, where data errors can impede meaningful conclusions during meta‐analysis. Therefore, integrating the CDC software into the planning process presents an opportunity to adopt a more robust approach that minimizes distortion errors arising from both machines and patients of a large cohort of centers participating in clinical trials.
5. CONCLUSION
This study retrospectively applied the CDC software to correct distortion in 18 MRIs used for target delineation in frame‐based SRS plans. The objective was to evaluate the effect of distortion‐induced changes in target shapes, target dose, and normal tissue dose. The results revealed that the distortion in MRIs used for SRS caused significant changes in most metrics used for evaluating quality and efficacy of SRS plans. These findings suggest that accounting for MRI distortions is critical to ensure accurate SRS treatment planning and delivery. To reduce residual distortion in treatment planning MRIs, which may persist even after scanner‐specific corrections, additional patient‐specific correction methods such as the CDC software can be implemented.
AUTHOR CONTRIBUTION
All authors have made significant contributions to the research and writing of this article. They have all reviewed this manuscript and have approved it for submission to this journal.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
ACKNOWLEDGMENTS
The authors have nothing to report.
Knill C, Halford R, Sandhu R, et al. Evaluating stereotactic accuracy with patient‐specific MRI distortion corrections for frame‐based radiosurgery. J Appl Clin Med Phys. 2024;25:e14472. 10.1002/acm2.14472
REFERENCES
- 1. Schmidt MA, Payne GS. Radiotherapy planning using MRI. Phys Med Biol. 2015;60(22):R323‐R32361. doi: 10.1088/0031-9155/60/22/R323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanssens P, Karlsson B, Yeo TT, Chou N, Beute G. Detection of brain micrometastases by high‐resolution stereotactic magnetic resonance imaging and its impact on the timing of and risk for distant recurrences. J Neurosurg. 2011;115(3):499‐504. doi: 10.3171/2011.4.JNS101832 [DOI] [PubMed] [Google Scholar]
- 3. Khoo VS, Dearnaley DP, Finnigan DJ, Padhani A, Tanner SF, Leach MO. Magnetic resonance imaging (MRI): considerations and applications in radiotherapy treatment planning. Radiother Oncol. 1997;42(1):1‐15. doi: 10.1016/s0167-8140(96)01866-x [DOI] [PubMed] [Google Scholar]
- 4. Wang D, Doddrell DM. Geometric distortion in structural magnetic resonance imaging. Current Medical Imaging. 2005;1(1):49‐60. [Google Scholar]
- 5. Weygand J, Fuller CD, Ibbott GS, et al. Spatial precision in magnetic resonance imaging‐guided radiation therapy: the role of geometric distortion. Int J Radiat Oncol Biol Phys. 2016;95(4):1304‐1316. doi: 10.1016/j.ijrobp.2016.02.059 [DOI] [PubMed] [Google Scholar]
- 6. Walker A, Liney G, Metcalfe P, Holloway L. MRI distortion: considerations for MRI based radiotherapy treatment planning. Australas Phys Eng Sci Med. 2014;37(1):103‐113. doi: 10.1007/s13246-014-0252-2 [DOI] [PubMed] [Google Scholar]
- 7. Alzahrani M, Broadbent DA, Chuter R, et al. Audit feasibility for geometric distortion in magnetic resonance imaging for radiotherapy. Phys Imaging Radiat Oncol. 2020;15:80‐84. doi: 10.1016/j.phro.2020.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moutsatsos A, Karaiskos P, Petrokokkinos L, et al. Assessment and characterization of the total geometric uncertainty in Gamma Knife radiosurgery using polymer gels. Med Phys. 2013;40(3):031704. doi: 10.1118/1.4789922 [DOI] [PubMed] [Google Scholar]
- 9. Wang H, Balter J, Cao Y. Patient‐induced susceptibility effect on geometric distortion of clinical brain MRI for radiation treatment planning on a 3T scanner. Phys Med Biol. 2013;58(3):465‐477. doi: 10.1088/0031-9155/58/3/465 [DOI] [PubMed] [Google Scholar]
- 10. Seibert TM, White NS, Kim GY, et al. Distortion inherent to magnetic resonance imaging can lead to geometric miss in radiosurgery planning. Pract Radiat Oncol. 2016;6(6):e319‐e328. doi: 10.1016/j.prro.2016.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Karaiskos P, Moutsatsos A, Pappas E, et al. A simple and efficient methodology to improve geometric accuracy in gamma knife radiation surgery: implementation in multiple brain metastases. Int J Radiat Oncol Biol Phys. 2014;90(5):1234‐1241. doi: 10.1016/j.ijrobp.2014.08.349 [DOI] [PubMed] [Google Scholar]
- 12. Brezovich IA, Wu X, Duan J, et al. End‐to‐end test of spatial accuracy in Gamma Knife treatments for trigeminal neuralgia. Med Phys. 2014;41(11):111703. doi: 10.1118/1.4896819 [DOI] [PubMed] [Google Scholar]
- 13. Dorenlot A, Champoudry J. End‐to‐end tests of the new Elekta gamma knife ICON. Physica Medica: Eur J Med Phys. 2016;32:353. 25. [Google Scholar]
- 14. Pappas EP, Alshanqity M, Moutsatsos A, et al. MRI‐related geometric distortions in stereotactic radiotherapy treatment planning: evaluation and dosimetric impact. Technol Cancer Res Treat. 2017;16(6):1120‐1129. doi: 10.1177/1533034617735454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jacobson S, Jones C, Lusk R, et al. Clinical impact of magnetic resonance imaging distortions on gamma knife radiosurgery. J Med Radiat Sci. 2021;68(3):274‐281. doi: 10.1002/jmrs.472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Retif P, Djibo Sidikou A, Mathis C, et al. Evaluation of the ability of the Brainlab elements cranial distortion correction algorithm to correct clinically relevant MRI distortions for cranial SRT. Strahlenther Onkol. 2022;198(10):907‐918. doi: 10.1007/s00066-022-01988-1 [DOI] [PubMed] [Google Scholar]
- 17. Jackson E, Bronskill M, Drost D, et al. Acceptance testing andquality assurance procedures for magnetic resonance imagingfacilities. MD: College Park; 2010.
- 18. Chen CC, Wan YL, Wai YY, Liu HL. Quality assurance of clinical MRI scanners using ACR MRI phantom: preliminary results. J Digit Imaging. 2004;17(4):279‐284. doi: 10.1007/s10278-004-1023-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zeverino M, Jaccard M, Patin D, et al. Commissioning of the Leksell Gamma Knife((R)) icon. Med Phys. 2017;44(2):355‐363. doi: 10.1002/mp.12052 [DOI] [PubMed] [Google Scholar]
- 20. Hiepe P, Cranial distortion correction—Technical background. 2017. Accessed July 21, 2022. https://www.researchgate.net/publication/317359164_Cranial_Distortion_Correction_‐_Technical_Background?channel=doi&linkId=593694aeaca272fc55739a28&showFulltext=true
- 21. Dice LR. Measures of the amount of ecologic association between species. Ecology. 1945;26(3):297‐302. [Google Scholar]
- 22. Jaccard P. Étude comparative de la distribution florale dans une portion des Alpes et des Jura. Bull Soc Vaudoise Sci Nat. 1901;37:547‐579. [Google Scholar]
- 23. Student . The probable error of a mean. Biometrika. 1908;6(1):1‐25. [Google Scholar]
- 24. Sneed PK, Lamborn KR, Forstner JM, et al. Radiosurgery for brain metastases: is whole brain radiotherapy necessary? Int J Radiat Oncol Biol Phys. 1999;43(3):549‐558. doi: 10.1016/s0360-3016(98)00447-7 [DOI] [PubMed] [Google Scholar]
- 25. Ilyas A, Chen CJ, Abecassis IJ, et al. Stereotactic radiosurgery for a randomized trial of unruptured brain arteriovenous malformations‐eligible patients: a meta‐analysis. Neurosurgery. 2022;91(5):684‐692. doi: 10.1227/neu.0000000000002115 [DOI] [PubMed] [Google Scholar]
- 26. Kondziolka D, Zorro O, Lobato‐Polo J, et al. Gamma knife stereotactic radiosurgery for idiopathic trigeminal neuralgia. J Neurosurg. 2010;112(4):758‐765. doi: 10.3171/2009.7.JNS09694 [DOI] [PubMed] [Google Scholar]
- 27. Amaral T, Kiecker F, Schaefer S, et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: a DeCOG* study in 380 patients. J Immunother Cancer. 2020;8(1):e000333. doi: 10.1136/jitc-2019-000333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lu SL, Xiao FR, Cheng JC, et al. Randomized multi‐reader evaluation of automated detection and segmentation of brain tumors in stereotactic radiosurgery with deep neural networks. Neuro Oncol. 2021;23(9):1560‐1568. doi: 10.1093/neuonc/noab071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stanley J, Dunscombe P, Lau H, et al. The effect of contouring variability on dosimetric parameters for brain metastases treated with stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2013;87(5):924‐931. doi: 10.1016/j.ijrobp.2013.09.013 [DOI] [PubMed] [Google Scholar]
- 30. Yamazaki H, Shiomi H, Tsubokura T, et al. Quantitative assessment of inter‐observer variability in target volume delineation on stereotactic radiotherapy treatment for pituitary adenoma and meningioma near optic tract. Radiat Oncol. 2011;6:10. doi: 10.1186/1748-717X-6-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Growcott S, Dembrey T, Patel R, Eaton D, Cameron A. Inter‐Observer variability in target volume delineations of benign and metastatic brain tumours for stereotactic radiosurgery: results of a national quality assurance programme. Clin Oncol (R Coll Radiol). 2020;32(1):13‐25. doi: 10.1016/j.clon.2019.06.015 [DOI] [PubMed] [Google Scholar]
- 32. Ohira S, Suzuki Y, Washio H, et al. Impact of magnetic resonance imaging‐related geometric distortion of dose distribution in fractionated stereotactic radiotherapy in patients with brain metastases. Strahlenther Onkol. 2024;200(1):39‐48. doi: 10.1007/s00066-023-02120-7 [DOI] [PubMed] [Google Scholar]
- 33. Calvo‐Ortega JF, Mateos J, Alberich A, et al. Evaluation of a novel software application for magnetic resonance distortion correction in cranial stereotactic radiosurgery. Med Dosim. 2019;44(2):136‐143. doi: 10.1016/j.meddos.2018.04.002 [DOI] [PubMed] [Google Scholar]
- 34. Pappas EP, Seimenis I, Moutsatsos A, Georgiou E, Nomikos P, Karaiskos P. Characterization of system‐related geometric distortions in MR images employed in Gamma Knife radiosurgery applications. Phys Med Biol. 2016;61(19):6993‐7011. doi: 10.1088/0031-9155/61/19/6993 [DOI] [PubMed] [Google Scholar]
- 35. Glide‐Hurst CK, Paulson ES, McGee K, et al. Task group 284 report: magnetic resonance imaging simulation in radiotherapy: considerations for clinical implementation, optimization, and quality assurance. Med Phys. 2021;48(7):e636‐e670. doi: 10.1002/mp.14695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lu L, Yang X, Raterman B, et al. Assessment of MRI image distortion based on 6 consecutive years of annual QAs and measurements on 14 MRI scanners used for radiation therapy. J Appl Clin Med Phys. 2023;24(1):e13843. doi: 10.1002/acm2.13843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hsu SH, Cao Y, Huang K, Feng M, Balter JM. Investigation of a method for generating synthetic CT models from MRI scans of the head and neck for radiation therapy. Phys Med Biol. 2013;58(23):8419‐8435. doi: 10.1088/0031-9155/58/23/8419 [DOI] [PMC free article] [PubMed] [Google Scholar]


