Abstract
Despite its endemic status in the Middle East, key knowledge gaps persist regarding the prevalence, transmission rate, and geographical distribution of both human and livestock brucellosis in Jordan. This study aimed to investigate the seroprevalence of human and livestock brucellosis as well as the incidence of brucellosis in humans in Jordan. A total of 500 human participants (202 exposed and 296 unexposed to livestock) were enrolled in the study. Sampling was conducted at baseline and 1.5 years later. Additionally, a total of 700 livestock were sampled, comprising 20 animals per taxa (camels, cattle, sheep, goats) per site, at both baseline (N = 350) and the 1.5-year follow-up (N = 350). Human participants were longitudinally followed, whereas livestock sampling was conducted opportunistically. Blood samples obtained from both humans and livestock at baseline and follow-up were tested for Brucella spp. serum antibodies using the Rose Bengal test (RBT) and complement fixation test (CFT). The overall seroprevalence of brucellosis in humans at baseline was 3.4 % (95 % CI: 2.0–5.4). Positive test results in humans were detected from all five sites with no significant regional variation observed. Seroprevalence was higher in individuals regularly exposed to livestock (6.1 %; 95 % CI: 3.5–9.9) compared to those not regularly exposed (0.80 %; 95 % CI: 0.10–2.9). Incidence of human brucellosis was 924 seropositives per 100,000 person-years, with all incident seropositives occurring in the livestock-exposed cohort. In livestock, the overall seroprevalence of brucellosis was 5.4 % (95 % CI: 3.5–8.3) at baseline compared to 2.6 % (95 % CI: 1.4–4.8) at follow-up. Seropositive livestock were detected at all sites apart from Al-Zarqa, and in all species apart from camels. In conclusion: Brucellosis burden was higher among humans regularly exposed to livestock, re-emphasizing the need for disease control in livestock populations to prevent primary infection in humans.
Keywords: Brucellosis, Zoonosis, One Health, Human-animal interface, Jordan, Zoonotic threat reduction
Highlights
-
•
Brucellosis is an important zoonotic disease across Jordan.
-
•
High incidence of brucellosis was observed among individuals at the human-livestock interface.
-
•
No samples from camels were positive for Brucella spp.-specific antibodies.
-
•
Available resources should be directed at the prevention of livestock brucellosis to avoid human transmission.
1. Introduction
Brucellosis is a zoonotic disease which poses significant concerns for human well-being and economic progress in poor and developing nations [1,2]. The disease is caused by members of the Brucella spp. including B. melitensis, B. abortus, B. suis, and B. canis [[3], [4], [5]]. The main routes of transmission from animals to humans involve consuming raw dairy products, contact with animal tissues or fluids, inhaling contaminated droplets, or exposure through the eyes or damaged skin [6,7]. People at risk of contracting brucellosis include farmers, veterinarians, and workers in slaughterhouses [8,9]. Symptoms of human brucellosis typically include fever, profuse sweating, joint discomfort, and overall fatigue [10]. These symptoms closely resemble those of febrile illnesses, which contributes to misdiagnosis and lowers the number of reported cases of brucellosis in humans [11].
In ruminants, Brucella spp. are shed in postpartum vaginal discharge and milk, as well as sporadically in urine and semen, posing a risk of exposure through contact with these bodily fluids [[6], [7], [8]]. The shedding of Brucella spp. in milk and vaginal discharge can persist for weeks to months after parturition, even in asymptomatic carriers, contributing to the maintenance and spread of brucellosis within a population [[12], [13], [14]]. In endemic areas, persistence of brucellosis has been attributed to several factors including low vaccination rates, low case reporting, negative herds turning positive due to inadequate ongoing control measures, reluctance of farmers to remove positive livestock due to the lack of compensation from regulatory agencies, discontinuation of eradication projects due to funding constraints or other reasons, and recurrence of infection due to a lack of sustained monitoring efforts [15,16].
To date, research on various zoonotic diseases in Jordan, including brucellosis, has mainly focused on specific geographical areas and has relied predominantly on survey or hospital-based methodologies [[17], [18], [19], [20], [21], [22], [23]], leaving a critical gap in our understanding of the distribution of zoonotic diseases across diverse human-animal interface settings. In response, a four-year (2020–2024) multidisciplinary One Health study on avian influenza, Middle East respiratory syndrome (MERS-CoV), brucellosis, and leptospirosis is being conducted across five geographically representative regions across Jordan. The study seeks to enhance our knowledge of the frequency, spread patterns, and related risk elements of these diseases at the human-animal interface. In this paper, we present a subset of the parent study's data: the seroprevalence of brucellosis in livestock and humans as well as the incidence in humans from the first half of longitudinal data collection.
2. Materials and methods
2.1. Study location and site selection
Study sites were selected in five geographically representative regions across Jordan: Northern Jordan (Al Ramtha), Middle Jordan (Al Zarqa), and Southern Jordan (Al Karak, Ma'an, and Aqaba) (Fig. 1). The selection of these regions was guided by previous surveillance activities of the study team (unpublished data) and the presence of livestock production activities in these areas, which were suspected to be potential sites for zoonotic pathogen transmission. In each of the five study regions, sampling sites were selected to ensure representation of interfaces with camels, poultry or cattle/sheep/goats. Sites with a combination of multiple interface types were also included. Sites were randomly selected using a team-developed randomized sampling generator, in an R Studio “Shiny App”, where each region had one randomly selected point. The three closest interface sites were chosen within 10 km of the randomly selected point within the region. If the field team was unable to find all three interface sites within the 10 km grid, then the grid was expanded every 10 km until all three interface sites are found. Selected sites were visited in a random order during each region's first enrollment visit until all interface types were covered, and the site enrollment goal was achieved.
Fig. 1.
Study sites in Jordan at which humans and livestock were sampled.
2.2. Study population
Eligibility criteria for human participants included adults (18 years or older) who were able to provide written informed consent and children (12 years or older) who were able to provide verbal assent, along with a parent or guardian able to offer written informed consent. Participants could withdraw their consent or assent at any point during the study. Enrolled participants completed a pre-screening checklist to determine their exposure category (exposed vs non-exposed). Exposure was defined as regularly (once a month or more) working with or sharing living areas with any livestock animals and/or poultry.
The livestock study population included cattle, sheep, goats, and camels raised in the selected study regions. All livestock sampled belonged to human participants of the study. Selected animals were subjected to a brief physical examination to determine their basic characteristics (i.e., age, gender, recent pregnancy) and overall health (i.e., body condition, presence of nasal discharge and/or diarrhea).
2.3. Human sample calculation
The One Health parent study determined a minimum sample size of 200 unexposed and 200 exposed human participants, where poultry and livestock were included in categorizing an individual as exposed. These figures were determined by a simulation that incorporated random effects for interface and site, powered to detect a minimum odds ratio of 5 [24]. This power analysis was based off of the assumption that 1 % of the unexposed population would test positive for one of four assayed zoonotic diseases at least once throughout the study. To account for a potential 20 % loss to follow-up, a correction factor of 1.25 (reflecting 80 % retention) was applied, resulting in a total of 500 participants at study initiation. These targets were then distributed across five regions, with each region aiming to enroll 50 unexposed and 50 exposed individuals.
2.4. Livestock sample calculation
Sampling targets were set to 400 livestock per sampling visit, made up of 20 animals per taxa (camels, sheep, cattle, and goats) per site. Livestock were opportunistically sampled and not followed longitudinally for study.
2.5. Study procedures
All interactions with potential participants were conducted in Arabic by native-speaking team members. Participants were administered a standardized questionnaire at baseline (January – March 2022) and follow-up (May – July 2023) focusing on demographic and behavioral data. Participants were considered lost to follow-up if they missed their follow-up visit and were unreachable by telephone to schedule a follow-up visit, or if they withdrew their consent.
2.6. Human sample collection
Blood samples were obtained at baseline and at follow-up. Approximately 5–8 mL of whole blood was collected using vacutainer needle and plain clotting tubes from each participant at each time point. Blood was drawn by a registered nurse.
2.7. Livestock sample collection
Blood samples were collected from livestock in the baseline and follow-up groups. Approximately 5–8 mL of whole blood was collected via jugular venipuncture in cattle, camels, sheep, and goats using vacutainer needle and plain clotting tubes.
2.8. Sample handling and transportation
Human and livestock blood samples were immediately transferred to an icebox with ice packs and transported to the laboratory (2–24 h depending on site location). At the laboratory, samples were centrifuged at 10000g for 10 min to collect serum. Serum was stored short-term at 2 to 8 °C and long-term −70 to −80 °C.
2.9. Laboratory testing
Serum samples were used to detect antibodies against Brucella spp. using the Rose Bengal test (RBT) (JOVAC, Amman, Jordan) for screening [25]. Positive samples were subjected to complement fixation test (CFT) (Bio Industries Center, Amman, Jordan) for confirmation [26]. These tests were selected and performed in series due to the high sensitivity of RBT and the high specificity of CFT in both humans and livestock, thus reducing the likelihood of false positive and negative results [27,28].
2.10. Data analysis and statistical analysis
All statistical analyses were conducted using R software version 4.3.2 (R Core Team, 2023). A sample was considered seropositive if it was positive by both RBT and CFT. The seroprevalences for both livestock and humans were calculated as the percentage of seropositives in a given sampling round and are reported alongside their respective 95 % confidence intervals. In animals, seroprevalences were calculated for the baseline and follow-up rounds. In humans, seroprevalence was calculated at baseline, and incidence was calculated at follow-up. Seroprevalence was calculated for the entire human cohort as well as for exposed and unexposed cohorts. A seropositive was considered incident if the individual was negative by RBT and CFT at baseline and positive on both tests at follow-up. To calculate incidence, an intercept-only Poisson model including an offset term to account for varying follow-up durations of participants was fit to the data, where the outcome was the occurrence of serological evidence of brucellosis. The incidence rate per 100,000 person-years was obtained by exponentiating the intercept term and is reported alongside its corresponding 95 % confidence interval.
To investigate pairwise associations between brucellosis seropositivity and human demographic information collected at baseline, univariable logistic regression models were used. Variables with perfect separation were not included in univariable or multivariable analyses. Variables identified as significant in univariable analysis, where significance was assessed at the p ≤ 0.05 level, were included in the final multivariable regression model and evaluated using likelihood ratio tests (LRTs). Age and sex were included regardless of significance.
3. Results
3.1. Study population
A total of 500 human participants were enrolled in the study at baseline and 422 were retained through follow-up, meeting study enrollment and retention goals. Basic demographic information from participants at baseline is presented in Table 1. Forty-one percent of the population was regularly exposed to camels, sheep, goats, and/or cattle. The study population was overwhelmingly male (78 %). Participants ranged from 13 to 77 years of age, with a median age of 33 years. Regarding monthly income, the majority (97 %) fell within the 250–500 JD range (350–700 USD), with only 2.8 % earning between 501 and 1000 JD (705–1400 USD). The level of education of the study participants varied with 7.0 % having no formal education, 19 % having primary education, 55 % having secondary education, and 19 % having attained a university degree.
Table 1.
Demographic characteristics of human participants at baseline.
| Variable | N = 498 | % |
|---|---|---|
| Livestock exposure | ||
| Exposed | 202 | 41 |
| Unexposed | 296 | 59 |
| Gender | ||
| Female | 110 | 22 |
| Male | 338 | 78 |
| Age | ||
| < 18 | 40 | 8.0 |
| 18–29 | 180 | 36 |
| 30–49 | 208 | 42 |
| 50 + | 70 | 14 |
| Monthly Income | ||
| 250–500 JD | 484 | 97 |
| 501–1000 JD | 13 | 2.8 |
| Highest Education | ||
| None | 35 | 7.0 |
| Primary | 95 | 19 |
| Secondary | 272 | 55 |
| University | 95 | 19 |
While sampling targets were achieved for camels, goats, and sheep, fewer cattle samples were obtained due to their relative scarcity in the south of Jordan. Descriptive information and blood samples were successfully obtained for the 350 livestock sampled per visit (Table 2). Overall, the distribution of herd sizes and age groups represented differed between baseline and follow-up periods. Most notably, the sheep and goats sampled at follow-up were on average younger and belonged to smaller herds in comparison to the baseline period.
Table 2.
Characteristics of livestock sampled at baseline and follow-Up.
| Variable | Sheep |
Goat |
Cattle |
Camel |
||||
|---|---|---|---|---|---|---|---|---|
| Baseline N = 100 | Follow-up N = 100 | Baseline N = 100 | Follow-up N = 100 | Baseline N = 50 | Follow-up N = 50 | Baseline N = 100 | Follow-up N = 100 | |
| Site | ||||||||
| Al-Karak | 20 | 20 | 20 | 20 | 10 | 0 | 20 | 20 |
| Al-Ramtha | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Al-Zarqa | 20 | 20 | 20 | 20 | 15 | 20 | 20 | 20 |
| Aqaba | 20 | 20 | 20 | 20 | 0 | 0 | 20 | 20 |
| Ma'an | 20 | 20 | 20 | 20 | 5 | 10 | 20 | 20 |
| Herd Size | ||||||||
| 1–25 | 27 | 30 | 27 | 30 | 5 | 10 | 78 | 68 |
| 25–100 | 45 | 56 | 45 | 56 | 45 | 40 | 22 | 12 |
| 100–300 | 13 | 14 | 13 | 14 | 0 | 0 | 0 | 5 |
| 300+ | 15 | 0 | 15 | 0 | 0 | 0 | 0 | 15 |
| Age | ||||||||
| <6 months | 9 | 11 | 9 | 11 | 24 | 34 | 24 | 21 |
| 6 mo. - <1 yr. | 14 | 16 | 14 | 16 | 1 | 7 | 5 | 7 |
| 1–5 years | 50 | 66 | 50 | 66 | 21 | 8 | 40 | 41 |
| 5+ years | 27 | 7 | 27 | 7 | 4 | 0 | 31 | 31 |
| Sex | ||||||||
| Female | 87 | 88 | 87 | 88 | 31 | 28 | 74 | 61 |
| Male | 13 | 12 | 13 | 12 | 19 | 22 | 26 | 39 |
3.2. Brucellosis in humans
At baseline, 17 of the 500 individuals sampled were positive for Brucella spp., compared to 10 of 422 individuals sampled at follow-up (Table 3). Five of the individuals positive at follow-up were also positive at baseline.
Table 3.
Number of human samples positive for brucellosis by site.
| Sampling round | Site | No. positive / no. tested | % positive |
|---|---|---|---|
| Baseline | Al-Karak | 4/100 | 4 |
| Al-Ramtha | 2/100 | 2 | |
| Al-Zarqa | 3/100 | 3 | |
| Aqaba | 3/100 | 3 | |
| Ma'an | 5/100 | 5 | |
| All Sites | 17/500 | 3.4 | |
| Follow-up | Al-Karak | 2⁎/81 | 2.5 |
| Al-Ramtha | 0/93 | 0 | |
| Al-Zarqa | 3/84 | 3.6 | |
| Aqaba | 2†/82 | 2.4 | |
| Ma'an | 3‡/82 | 3.7 | |
| All Sites | 10§/422 | 2.37 |
Two individuals also positive in round 1.
One individual also positive in round 1.
Two individuals also positive in round 1.
Five individuals also positive in round 1.
Overall seroprevalence of brucellosis in humans at baseline was 3.40 % (95 % CI: 1.99–5.39). Seropositive participants were detected at all five sampling sites with no significant regional variation observed. The highest percentage of seropositives was observed in Ma'an (5 %), followed by Al-Karak (4 %). Both Al-Zarqa and Aqaba each reported 3 % seroprevalence, while in Al-Ramtha (2 %), the lowest percentage of seropositives was noted. Seroprevalence was notably higher among individuals regularly exposed to livestock (6.10 %; 95 % CI: 3.45–9.86) compared to individuals not regularly exposed to livestock (0.80 %; 95 % CI: 0.097–2.85).
The incidence rate of brucellosis among this study population was 947.5 seropositives per 100,00 people per year (95 % CI: 339.4–2036). No seropositives were detected among individuals without regular livestock contact. Among the livestock-exposed cohort, incidence was 2036 seropositives per 100,000 people per year (95 % CI: 730.2–4377).
Only exposure to livestock (OR: 5.39; 95 % CI: 1.78–14.9, p < 0.01) was identified as significantly associated with brucellosis seropositivity in univariable analysis (Table 4). The final multivariable model included gender, age, and exposure to livestock (Table 4). In multivariable analysis, exposure to livestock remained significantly associated with an increase in risk of brucellosis seropositivity at baseline (LRT: X2 = 12.6 df = 3, p < 0.01).
Table 4.
Univariable and Multivariable Analyses for Brucellosis Seropositivity in Human Cohort at Baseline.
| Variable | Case Population n/N (%) |
Non-case population n/N (%) |
Univariable OR (95 % CI) |
Univariable OR p value |
Multivariable aOR (95 % CI) |
Multivariable aOR p value |
|
|---|---|---|---|---|---|---|---|
| Livestock Exposure | No | 11/17 (64.7) | 435/479 (90.8) | Ref | Ref | – | |
| Yes | 6/17 (35.3) | 44/479 (9.2) | 5.39 (1.78–14.9) | 0.002 | 5.51 (1.80–15.5) | 0.002 | |
| Gender | M | 16/17 (94.1) | 371/479 (77.5) | Ref | – | Ref | – |
| F | 1/17 (5.9) | 108/479 (22.5) | 0.215 (0.012–1.07) | 0.138 | 0.25 (0.01–1.31) | 0.190 | |
| Age | – | – | 0.978 (0.938–1.01) | 0.265 | 0.98 (0.94–1.02) | 0.289 | |
| Highest Education | None | 1/17 (5.9) | 34/479 (7.1) | 0.858 (0.046–4.77) | 0.885 | – | – |
| Primary | 6/17 (35.3) | 89/479 (18.6) | 1.96 (0.642–5.59) | 0.213 | – | – | |
| Secondary | 9/17 (52.9) | 262/479 (54.7) | Ref | – | – | ||
| University | 1/17 (5.9) | 94/479 (19.6) | 0.310 (0.017–1.86) | 0.269 | – | – | |
| Monthly Income (JD) | 250–500 | 17/17 (100) | 465/479 (97.1) | – | – | – | – |
| 500+ | 0/17 (0) | 14/479 (2.9) | – | – | – | – | |
3.3. Brucellosis in livestock
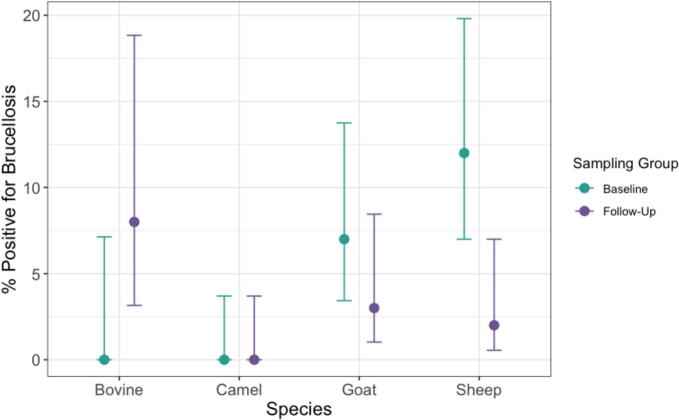
The overall prevalence of Brucella spp. serum antibodies in livestock was 5.42 % (95 % CI: 3.50–8.32) in the baseline group and 2.57 % (95 % CI: 1.25–4.81) in the follow-up group (Table 5). No seropositives were detected in camels at either visit. Seroprevalence varied by site, with livestock in Al-Ramtha consistently exhibiting the highest proportion of brucellosis seropositives and none being detected in either sampling period in Al-Zarqa (Table 5). At baseline, sheep had the highest seroprevalence (12 %) compared to other taxa, whereas at follow-up, the highest seroprevalence was observed in cattle (8 %) (Fig. 2).
Table 5.
Number of livestock samples positive for brucellosis by site.
| Sampling round | Site | No. positive / no. tested | % positive |
|---|---|---|---|
| Baseline | Al-Karak | 5/70 | 7.1 |
| Al-Ramtha | 7/80 | 8.8 | |
| Al-Zarqa | 0/75 | 0 | |
| Aqaba | 3/60 | 5.0 | |
| Ma'an | 4/65 | 6.2 | |
| All Sites | 19/350 | 5.4 | |
| Follow-up | Al-Karak | 1/60 | 1.7 |
| Al-Ramtha | 5/80 | 6.3 | |
| Al-Zarqa | 0/80 | 0 | |
| Aqaba | 0/60 | 0 | |
| Ma'an | 3/70 | 4.3 | |
| All Sites | 9/350 | 2.6 |
Fig. 2.
Percentage of livestock positive for brucellosis by species with 95 % confidence intervals.
4. Discussion
This is the first study in Jordan designed to investigate the human-animal interface as a determining factor in brucellosis and to collect data and samples concurrently (both spatially and temporally) from humans and animals. This approach is particularly crucial for countries like Jordan, where livestock play a significant role in the economy and livelihoods of communities, and where zoonotic diseases pose substantial public health risks.
In this study, we report an overall seroprevalence of 3.4 % (95 % CI: 2.0–5.4) in humans. This is comparable to a recent seroprevalence estimate of 6.7 % generated by a study of over 900 adults across Jordan [29], which may have reported a slightly higher seroprevalence due to using less strict criteria for defining a positive case (considered a case if positive on one of two different assays), and due to sampling a higher proportion of people living in the north of Jordan, where prevalence is reportedly higher. While most studies note markedly increased seroprevalence in northern Jordan [5,17,29], no significant regional variations were detected in our study. Previous research has suggested that elevated seroprevalence in the north may be linked to higher livestock density and more frequent small ruminant ownership in the region compared to the south [5,17,19,29]. Thus, the lack of regional variation in the present study may be attributable to our sampling framework ensuring an even representation of livestock-exposed and livestock-unexposed individuals and a variety of human-animal interface types at all sites.
Accurate estimates of the incidence of human brucellosis in Jordan and the Middle East are lacking [[30], [31], [32], [33], [34]]. Estimates vary widely even within countries, with reports ranging between 6.00 and 149.54 cases per 100,000 person-years in Saudi Arabia, and 2.6–268.81 cases per 100,000 person-years in Iraq [4]. The incidence rate reported in the present study — 947 seropositives per 100,000 person-years — is substantially higher, which may be accounted for by several reasons. A majority of existing estimates are based on passive surveillance relying on notified cases or hospital-based studies, which are suspected to underestimate the incidence of brucellosis by 12 to 25-fold [30]. Further, our study deliberately enrolled participants in regions where zoonotic pathogen transmission was theorized to be high. Additionally, nearly half of the population in the present study are regularly exposed to livestock, which is a known risk factor for brucellosis. The incidence for this at-risk population has not been determined previously and requires follow-up studies.
Brucellosis is a significant concern in the Middle East, and the high incidence rate among humans is alarming [[30], [31], [32], [33], [34]]. One of the major contributing factors to this issue is the consumption of raw milk and unpasteurized dairy products, as well as undercooked meat [[6], [7], [8], [9]]. These dietary habits facilitate the transmission of Brucella species, which are responsible for the disease. Addressing these eating habits is crucial for controlling and preventing brucellosis in the region. Public health interventions that promote the pasteurization of milk and proper cooking of meat, along with educational campaigns about the risks associated with consuming unpasteurized products, are essential steps in mitigating the impact of brucellosis. Furthermore, strengthening veterinary and agricultural practices to ensure the health of livestock can also play a vital role in reducing the incidence of this disease.
In the present study, exposure to livestock was significantly associated with brucellosis seropositivity at baseline in multivariable analysis. Accordingly, seroprevalence and incidence were notably higher among individuals regularly exposed to livestock in our study (6.10 %) compared to unexposed individuals (0.80 %), with no incident cases being identified in the unexposed cohort. This is consistent with the seroprevalence reported for high risk (8.2 %) and low risk (0.5 %) participants in a study from 1991 in Northern Jordan, which, however, did not describe incidence [19,32]. Further, our results support findings from previous research in the region identifying significantly increased risk of Brucella spp. exposure among dairy factory workers [19], veterinarians [32], and those otherwise indirectly exposed to livestock [5].
Findings of this study indicate widespread presence of brucellosis among livestock across Jordan, with an overall prevalence of 5.4 % (95 % CI: 3.5–8.3) in the baseline group and 2.6 % (95 % CI: 1.4–4.8) in the follow-up group. Though differences were not significant, slightly higher seroprevalence at baseline may be partially attributable to seasonality and climate— baseline sampling occurred during late winter and early spring, whereas follow-up sampling occurred during the summer. While no previous studies in Jordan have reported seasonality as a risk factor for seropositivity of brucellosis, studies from Iran [15,35], Turkey [2,30], Germany [2,30], Greece [33], and Ethiopia [2,30] have reported that the incidence of brucellosis is seasonal, often corresponding to calving or increased rainfall. In the present study, baseline sampling occurred shortly after calving and the rainy season in Jordan.
In this study, the proportion of seropositives detected were highest among sheep (7 %) followed by cattle (4 %) and goats (2 %), with no seropositives detected in camels. Previous research has suggested varying degrees of susceptibility or exposure to Brucella spp. among different livestock species and herd compositions. The most comprehensive, nationwide prevalence study on brucellosis in Jordan among non-camel livestock to date found significantly higher seroprevalence among small ruminant flocks (34.3 %) – particularly in mixed sheep-goat flocks (70.4 %) – compared to cattle-only flocks (18.1 %) [17]. Because Musallam et al. (2015) [17] examined seroprevalence on a herd level, which produces significantly higher seroprevalence estimates than calculating seroprevalence at the individual level, it is difficult to draw comparisons between studies. That said, our seroprevalence estimate among cattle is relatively consistent with that of another study reporting a 6.5 % prevalence among cattle at the individual level and 25.8 % at the herd level [36].
Camel brucellosis is considered an ignored but significant zoonotic threat in countries where camels are raised, with substantial economic implications. A global meta-epidemiological study estimated a worldwide prevalence of camel brucellosis at 9.23 % [37]. It is interesting to note that camels exhibited no positive samples in the current study, contrary to previously published data from Jordan, which reported a 12.1 % true prevalence of seropositive camels, with 35.1 % of herds having at least one positive camel [38]. One factor which may contribute to this discrepancy is herd size. Al Majali et al. (2008) identified large herd size as a significant risk factor for camel brucellosis [38]. In the present study, an overwhelming majority of camels sampled belonged to herds of less than 25 camels.
Although human seropositive samples were detected at all sites sampled, no livestock seropositive samples were detected in Al-Zarqa in either sampling period, nor in Aqaba in the follow-up period. While regional variation was not significant, it is worth noting that small herd sizes in Al-Zarqa and Aqaba may contribute to the lack of seropositives detected at these sites. Moreover, differences in management practices, housing conditions, and migration patterns could play a role in regional variation in the seroprevalence of livestock brucellosis. Livestock population density also varies across Jordan, both by region and species. In the northern, eastern, and central parts of the country, cattle, camels, sheep, and goats are plentiful, whereas cattle are scarce in the southern regions. Additionally, the presence or absence of effective disease control measures, such as vaccination campaigns or quarantine procedures, may vary from one region to another, impacting the prevalence of the disease.
Our findings should be interpreted with several important considerations in mind. We utilized RBT followed by confirmatory CFT, which unlike bacteriological or molecular methods, cannot definitively diagnose brucellosis. Serological tests can produce false positives due to cross-reactivity and may fail to detect active infections during their early or late phases, leading to false negatives. Reported seroprevalence and incidence estimates are thus subject to bias. Furthermore, the sensitivity and specificity of these diagnostic tests can vary by species, potentially skewing reported seroprevalence estimates depending on the species involved. However, by employing RBT, which has high sensitivity, along with CFT, which is highly specific, the likelihood of false positives and false negatives is reduced, thereby enhancing the reliability of our findings. Additionally, the seroprevalence of human brucellosis reported in the present study should not be extrapolated to Jordan at large because our sampling method lacked deliberate stratification to ensure representativeness of Jordan's demographic composition. Further, for human participants testing positive in both sampling periods, it was not possible to differentiate between long-lasting immune response or reoccurrence of infection. Though possible, reinfection with Brucella spp., is rare [39,40]. Therefore, for the purpose of calculating an incidence rate in this study, individuals seropositive in both sampling periods were not treated as new infections at the second round, reducing the likelihood of overestimating incidence and leaving a small chance that the present study underestimates the incidence of human brucellosis in Jordan. In terms of livestock, we were unable to capture a geographically representative sample of cattle given the relative scarcity of cattle in southern Jordan. Moreover, differences in sex and herd size makeup of livestock sampled at baseline compared to follow-up make it difficult to identify whether temporal changes in seroprevalence are genuine or due to sampling methods. Lastly, because livestock were not tagged or followed longitudinally, it is possible that animals sampled at baseline were re-sampled at follow-up, though the probability of this is low due to animal turnover from sale, death, or culling during the 1.5-year time period.
5. Conclusion
Results of this study indicates that humans regularly exposed to livestock show evidence of brucellosis at a higher rate than unexposed individuals. These results underscore the necessity and opportunity to prioritize brucellosis control measures aimed at reducing the disease burden within livestock populations as well as encouraging behavioral changes to reduce animal to human transmission. Moreover, given the high incidence of livestock brucellosis in the Middle East and extensive livestock trade within the region, regional coordinated efforts will be necessary to efficiently combat this disease.
Ethics approval and consent to participate
The study protocols were reviewed and approved by Jordan University of Science and Technology (JUST) Institutional Review Board (IRB) and HML IRB in Washington, DC, USA for research on human subjects. For animal subjects research, the study protocols received approval from Tufts University, EcoHealth Alliance, and JUST Institutional Animal Care and Use Committees (IACUC) (Grant number 32–2021). All study team members underwent training on Collaborative Institutional Training Initiative (CITI) modules for human subject research and ethical research with animals. Informed written and signed consent forms were obtained from human participants and from animal owners before samples were collected.
Funding
The project depicted is sponsored by the U.S. Department of Defense, Defense Threat Reduction Agency. The content of the information does not necessarily reflect the position or the policy of the federal government, and no official endorsement should be inferred.
CRediT authorship contribution statement
Ehab A. Abu-Basha: Supervision, Software, Resources, Project administration, Investigation, Funding acquisition, Conceptualization. Zuhair Bani Ismail: Writing – original draft, Project administration. Lea Widemann: Writing – review & editing, Formal analysis, Data curation. Yasmin Daradkeh: Investigation. Omar Al-Omari: Data curation. Alaa Fahmawi: Data curation. Mais Lakaideh: Data curation. Belal Sha'fout: Data curation. Haia Mellhem: Data curation. Leen Al-Bayari: Data curation. Hani Talafha: Project administration. Zaidoun Hijazeen: Data curation. Bilal Al-Omari: Validation, Software, Methodology, Formal analysis. Jean DeMarco: Project administration, Formal analysis. William B. Karesh: Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thank the study participants, field personnel, and lab personnel for their contributions. We also extend our thanks to Dr. Melinda Rostal and Dr. Jo Halliday for their input on analytical approaches. Thanks to the Deanship of research at Jordan University of Science and Technology for their logistical support of conducting this project (grant number 32-2021).
Data availability
Data will be made available on request.
References
- 1.Godfroid J. Brucellosis in livestock and wildlife: zoonotic diseases without pandemic potential in need of innovative one health approaches. Arch. Public Health. 2017;75(1) doi: 10.1186/s13690-017-0207-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lai S., Chen Q., Li Z. Human brucellosis: an ongoing Global Health challenge. China CDC Week. 2021;3(6):120–123. doi: 10.46234/ccdcw2021.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan M., Zahoor M. An overview of brucellosis in cattle and humans, and its serological and molecular diagnosis in control strategies. Trop. Med. Infect. Dis. 2018;3(2):65. doi: 10.3390/tropicalmed3020065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagheri Nejad R., Krecek R.C., Khalaf O.H., Hailat N., Arenas-Gamboa A.M. Brucellosis in the Middle East: current situation and a pathway forward. PLoS Negl. Trop. Dis. 2020;14(5) doi: 10.1371/journal.pntd.0008071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Amr M., Abasi L., Khasawneh R., Almharat S., Al-Smadi R., Abbasi N., Oudat R. Epidemiology of human brucellosis in military hospitals in Jordan: a five-year study. J. Infect. Dev. Ctries. 2022;16(12):1870–1876. doi: 10.3855/jidc.16861. [DOI] [PubMed] [Google Scholar]
- 6.Jiang W., Chen J., Li Q., Jiang L., Huang Y., Lan Y., Li Y., et al. BMC Infect. Dis. 2019;19(1) doi: 10.1186/s12879-019-4081-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.González-Espinoza G., Arce-Gorvel V., Mémet S., Gorvel J.P. Brucella: reservoirs and niches in animals and humans. Pathogens. 2021;10(2):186. doi: 10.3390/pathogens10020186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.O’Callaghan D. Human brucellosis: recent advances and future challenges. Infect. Dis. Poverty. 2020;9(1) doi: 10.1186/s40249-020-00715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Z., Zhang X., Chen X., Cui X., Cai M., Yang L., Zhang Y. Clinical features of human brucellosis and risk factors for focal complications: a retrospective analysis in a tertiary-Care Hospital in Beijing, China. Int. J. Gen. Med. 2022;15:7373–7382. doi: 10.2147/IJGM.S380328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin M., Fan Z., Gao R., Li X., Gao Z., Wang Z. Research progress on complications of brucellosis. Front. Cell. Infect. Microbiol. 2023;13 doi: 10.3389/fcimb.2023.1136674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi C., Wang L., Lv D., Wang G., Mengist H.M., Jin T., et al. Epidemiological, clinical and laboratory characteristics of patients with Brucella infection in Anhui Province, China. Infect. Drug Resist. 2021;14:2741–2752. doi: 10.2147/IDR.S319595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Narnaware S.D., Dahiya S.S., Kumar S., Tuteja F.C., Nath K., Patil N.V. Pathological and diagnostic investigations of abortions and neonatal mortality associated with natural infection of Brucella abortus in dromedary camels. Comp. Clin. Pathol. 2016;26(1):79–85. doi: 10.1007/s00580-016-2348-4. [DOI] [Google Scholar]
- 13.Pinn-Woodcock T., Frye E., Guarino C., Franklin-Guild R., Newman A.P., Bennett J., Goodrich E.L. A one-health review on brucellosis in the United States. J. Am. Vet. Med. Assoc. 2023:1–12. doi: 10.2460/javma.23.01.0033. [DOI] [PubMed] [Google Scholar]
- 14.Adel M. Brucella transmission from domestic and wild animals to dromedary camel: diagnostic methods and zoonotic threats – a review. Open Vet. Sci. 2022;3(1):1–12. doi: 10.1515/ovs-2022-0113. [DOI] [Google Scholar]
- 15.Golshani M., Buozari S. A review of brucellosis in Iran: epidemiology, risk factors, diagnosis, control, and prevention. Iran. Biomed. J. 2017;21:349–359. doi: 10.18869/acadpub.ibj.21.6.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang N., Huang D., Wu W., Liu J., Liang F., Zhou B., et al. Animal brucellosis control or eradication programs worldwide: a systematic review of experiences and lessons learned. Prev. Vet. Med. 2018;160:105–115. doi: 10.1016/j.prevetmed.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 17.Musallam I., Abo-Shehada M., Omar M., Guitian J. Cross-sectional study of brucellosis in Jordan: prevalence, risk factors and spatial distribution in small ruminants and cattle. Prev. Vet. Med. 2015;118(4):387–396. doi: 10.1016/j.prevetmed.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Abo-Shehada M.N., Abu-Halaweh M. Risk factors for human brucellosis in northern Jordan. East Mediterr. Health J. 2013;19(2):135–140. https://pubmed.ncbi.nlm.nih.gov/23516823/ [PubMed] [Google Scholar]
- 19.Abo-Shehada M.N., Rabi A., Abuharfeil N. The prevalence of brucellosis among veterinarians in Jordan. Ann. Saudi. Med. 1991;11(3):356–357. doi: 10.5144/0256-4947.1991.356. [DOI] [PubMed] [Google Scholar]
- 20.Abo-Shehada M.N., Odeh J.S., Abu-Essud M., Abuharfeil N. Seroprevalence of brucellosis among high risk people in northern Jordan. Int. J. Epidemiol. 1996;25(2):450–454. doi: 10.1093/ije/25.2.450. [DOI] [PubMed] [Google Scholar]
- 21.Al-Majali A.M., Shorman M. Childhood brucellosis in Jordan: prevalence and analysis of risk factors. Int. J. Infect. Dis. 2009;13(2):196–200. doi: 10.1016/j.ijid.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 22.Al-Sanouri T., Khader Y., Hailat E., Iweir S., Abu Khudair M., Al Nsour M. Seroprevalence of human brucellosis among Syrian refugees in Jordan, 2022. J. Pathog. 2023;5885316 doi: 10.1155/2023/5885316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kheirallah K.A., Al-Mistarehi A.H., Alsawalha L., Hijazeen Z., Mahrous H., Sheikali S., Al-Ramini S., Maayeh M., Dodeen R., Farajeh M., Masadeh N., Alemam A., Alsulaiman J., Samhouri D. Prioritizing zoonotic diseases utilizing the one health approach: Jordan’s experience. One Health. 2021;13 doi: 10.1016/j.onehlt.2021.100262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson P.C., Barry S.J., Ferguson H.M., P. & Müller. Power analysis for generalized linear mixed models in ecology and evolution. Methods Ecol. Evol. 2015;6(2):133–142. doi: 10.1111/2041-210X.12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose J.E., Roepke M.H. An acidified antigen for detection of nonspecific reactions in the plate-agglutination test for bovine brucellosis. Am. J. Vet. Res. 1957;18(68):550–555. (PMID: 13444570) [PubMed] [Google Scholar]
- 26.Taylor L.H. OIE Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. 2014. https://api.semanticscholar.org/CorpusID:59815459
- 27.OIE . 13th ed., Chapter 3.1.4. Office Internationale des Épizooties; 2022. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals. [Google Scholar]
- 28.Corbel M.J., Food and Agriculture Organization of the United Nations, World Health Organization, & World Organisation for Animal Health . World Health Organization; 2006. Brucellosis in Humans and Animals.https://iris.who.int/handle/10665/43597 [Google Scholar]
- 29.Obaidat M.M., Malania L., Arner R.J., Roess A.A. Seroprevalence and risk factors for Brucella infections in Jordan. Am. J. Trop. Med. Hyg. 2022;107(3):576–580. doi: 10.4269/ajtmh.21-0952. [DOI] [Google Scholar]
- 30.Laine G.G., Johnson V.E., Scott M.M., Arenas-Gamboa A.M. Global Estimate of Human Brucellosis Incidence. Emerg. Infect. Dis. 2023;29(9):1789–1797. doi: 10.3201/eid2909.230052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z., Gao L., Wang M., Yuan M., Li Z. Long ignored but making a comeback: a worldwide epidemiological evolution of human brucellosis. Emerg. Microb. Infect. 2024;13(1):2290839. doi: 10.1080/22221751.2023.2290839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alshehabat M., Obaidat M., Hayajneh W. Seroprevalence of Brucella canis in dogs and at-risk humans in Jordan. Vet. Med. 2019;64:260–265. doi: 10.17221/67/2018-VETMED. [DOI] [Google Scholar]
- 33.Minas M., Minas A., Gourgulianis K., Stournara A. Epidemiological and clinical aspects of human brucellosis in Central Greece. Jpn. J. Infect. Dis. 2007;60:362. (PMID: 18032835) [PubMed] [Google Scholar]
- 34.Buzgan T., Karahocagil M.K., Irmak H., Baran A.I., Karsen H., Evirgen O., Akdeniz H. Clinical manifestations and complications in 1028 cases of brucellosis: a retrospective evaluation and review of the literature. Int. J. Infect. Dis. 2010;14(6):e469–e478. doi: 10.1016/j.ijid.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 35.Moosazadeh M., Abedi G., Kheradmand M., Safiri S., Nikaeen R. Seasonal pattern of brucellosis in Iran: a systematic review and meta-analysis. Iran. J. Health Sci. 2016;4(1):62–72. doi: 10.18869/acadpub.jhs.4.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Al-Majali A.M., Talafha A.Q., Ababneh M.M., Ababneh M.M. Seroprevalence and risk factors for bovine brucellosis in Jordan. J. Vet. Sci. 2009;10(1):61–65. doi: 10.4142/jvs.2009.10.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dadar M., Omar S.S., Shahali Y., Fakhri Y., Godfroid J., Mousavi Khaneghah A. The prevalence of camel brucellosis and associated risk factors: a global meta-epidemiological study. Qual. Assur. Saf. Crops Foods. 2022;14(3):55–93. doi: 10.15586/qas.v14i3.1088. [DOI] [Google Scholar]
- 38.Al-Majali A.M., Al-Qudah K.M., Al-Tarazi Y.H., Al-Rawashdeh O.F. Risk factors associated with camel brucellosis in Jordan. Trop. Anim. Health Prod. 2008;40(3):193–200. doi: 10.1007/s11250-007-9080-7. [DOI] [PubMed] [Google Scholar]
- 39.Qureshi K.A., Parvez A., Fahmy N.A., Abdel Hady B.H., Kumar S., Ganguly A., Atiya A., Elhassan G.O., Alfadly S.O., Parkkila S., Aspatwar A. Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann. Med. 2023;55(2):2295398. doi: 10.1080/07853890.2023.2295398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.López-Santiago R., Sánchez-Argáez A.B., De Alba-Núñez L.G., Baltierra-Uribe S.L., Moreno-Lafont M.C. Immune response to mucosal Brucella infection. Front. Immunol. 2019;10:1759. doi: 10.3389/fimmu.2019.01759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.